Abstract
65Na2B4O7–25SiO2–(10 − x) CaO–x Cr2O3 x = (0 ≤ x ≤ 5 mol.%) glass system was synthesized and optical, structural, and radiation shielding characteristics are examined. The glass density increased as Cr2O3 was added, while the molar volume decreased. FT-IR bands are correlated with the vibrations of BO3 and BO4. Cr3+ appears to convert BO3 into BO4, according to preliminary FT-IR results. \(E_{{{\text{opt}}}}\) values are in the range of 2.57–3.31 eV. Increases in the \(E_{{{\text{opt}}}}\) can be linked to density and N4 variation. (Zeq) increased then decreased as the incident photon energy increased with the replacement of CaO by Cr2O3. \(\sum R\) enhances as the Cr2O3 content of the glasses increases. G 5 is a better absorber of fast neutron when the FNRC of glass samples is compared. For neutron attenuation applications, the glass sample G 5 is the best option.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Kodama noticed that the stoichiometric ratio of a wide range of binary sodium borate glasses Na2B4O7 is established BO3 is transformed into BO4 [1,2,3,4]. At this ratio, Na2O surrounds the network, preventing the surrounding soft B2O3 from deforming [5,6,7,8,9,10,11,12]. SiO2 incorporated into alkali borate glasses improved their radiation and UV transmittance. As a result, alkali borosilicate glasses show promise for incorporating a variety of modifying oxides [13,14,15,16,17]. In technology, borosilicate glasses are extremely important. High-mechanical-stability household and laboratory glasses, as well as high-performance optical glasses, are some of their applications [15, 18,19,20].
Oxide glasses doped with transition metal ions (TMI) have recently attracted a lot of attention due to their appealing combination of physical and chemical characteristics. In recent years, TMI-doped borate, phosphate, silicate, and borosilicate glasses have been investigated physically, optically, and radiationally [21,22,23,24,25,26,27,28,29,30]. Glasses with semiconducting properties, such as Cr2O3, CdO, CuO, and others, are known as (TMO) glasses. There has been a lot of focus in recent years on borosilicate glasses containing various TMO, such as CoO, NiO, V2O5, and Cr2O3 [4, 31].
TMO-based glasses, such as Cr2O3, also have appealing optical properties, making them ideal for use as non-linear optical (NLO) materials. According to previous research, TMO-containing borosilicate glasses have a variety of intriguing physical, structural, radiation, and spectroscopic characteristics [16, 17, 32]. As a result of the above existing literature, we have expanded our investigations into TM in calcium borosilicate glasses. As a result, we’ve developed calcium borosilicate glasses in a variety of compositions with Cr2O3 substituted. The synthesized glasses are characterized using XRD, FT-IR, and UV–Vis–NIR spectroscopy. The optical of these glasses with the composition 65Na2B4O7–25SiO2–(10 − x) CaO–x Cr2O3, x = (0 ≤ x ≤ 5 mol.%) are investigated in this study. In addition to the aforementioned studies, photons, neutrons, characteristics of Cr3+ doped calcium borosilicate glasses will be investigated using theoretical code in this work.
2 Materials and methodology
Glasses with the following composition were formed using the traditional melt-quench procedure: 65Na2B4O7–25SiO2–10CaO, 65Na2B4O7–25SiO2–9CaO–1Cr2O3, 65Na2B4O7–25SiO2–8CaO–2Cr2O3, 65Na2B4O7–25SiO2–6CaO–4Cr2O3, 65Na2B4O7–25SiO2–5CaO–5Cr2O3. Starting materials for the fabrication of glasses included high purity (Aldrich) CaO (99.5%), Cr2O3 (99.9%), SiO2 (99.5%), and Na2B4O7 (99.5%). The mixtures were then homogenized using a magnetic stirrer for 10 min. Glass was melted at 1100 °C in a platinum crucible. A random error in the melting temperature is ±10 °C. The glasses were annealed at 375 °C for 2 h. To calculate densities in toluene, the Archimedes model was used. On a Perkin Elmer Frontier FT-IR measurements in the 400–1800 cm−1 range were obtained using the KBr procedure. A random error in the center of FT-IR bands was found as ± 2 cm−1. To establish a baseline and correct for noise, spectrum software was used. The optical description of these glasses was obtained in the wavelength range of 250–2500 nm by (JASCO V-670, Japan). The absorption coefficient (α), optical bandgap (\(E_{{{\text{opt}}{.}}}^{{{\text{indir}}}}\)), and refractive index (\(n_{{\text{D}}}\)) were all calculated using absorption spectra. As physical parameters related to optical energy, the following physical parameters were calculated: molar refractivity \(R_{{\text{m}}}\), molar polarizability \(\propto_{{\text{m}}}\), reflection loss \(R_{{\text{L}}}\), metallization \(M\), electronegativity \(\chi\), electron polarizability \(\propto^\circ\), optical basicity \(\wedge\), Bulk module (K) and glass transition temperature \(\left( {T_{{g\left( {thero.} \right)}} } \right){ }\):\(R_{{\text{m}}} = Vm\left( {1 - \sqrt {Eopt./20} } \right), \propto_{{\text{m}}} = \left( {\frac{3}{4\pi N}} \right)R_{{\text{m}}}\). \(R_{{\text{L}}} = \left( {\frac{{R_{{\text{m}}} }}{Vm}} \right)\) \(M = 1 - \frac{{R_{{\text{m}}} }}{Vm}\), \(\chi = 0.2688E_{{{\text{opt}}{.}}} \propto^\circ = - 0.9 \chi + 3.5\) and \(\wedge = - 0.5\chi + 1.7, K_{{{\text{th}}}} = - 478.93 + 200.13 E_{{{\text{opt}}{.}}}\), \(T_{{{\text{g}}\left( {{\text{thero}}{.}} \right)}} = - 701.87 + 403.33 E_{{{\text{opt}}{.}}}\) As physical parameters related to the refractive index \(n_{{\text{D}}}\), \(R_{m}\), \(\propto_{m}\), \(\propto_{0}^{2 - }\), and \(\Lambda\) were calculated: \(R_{m}\), \(\propto_{m}\), \(\propto_{0}^{2 - }\), and \(\Lambda .\) \(R_{{\text{m}}} = \left\langle {n^{2} - 1} \right.|n^{2} + \left. 2 \right\rangle Vm\), \(\propto_{{\text{m}}} \left( {3|4\pi N} \right)R_{{\text{m}}} , \propto_{0}^{2 - } = \frac{{[\frac{Vm}{{2.52}}\left( {\frac{{n^{2 } - 1}}{{n^{2} + 2}}} \right) - \sum \propto_{{{\text{cat}}}} ]}}{{N_{o}^{2 - } }}\), and \(\Lambda = 1.67\left( {1 - \frac{1}{{ \propto_{0}^{2 - } }}} \right)\).
Using the online version of the Phy-X/PSD software, the study’s goal was met by calculating all effective parameters that judge the prepared glasses shielding effectiveness [33]. Zeq predictable with \(eq = \frac{{{\text{Z}}1\left( {{\text{logR}}2 - {\text{logR}}} \right) + {\text{Z}}2\left( {{\text{logR}} - {\text{logR}}1} \right)}}{{{\text{logR}}2 - {\text{logR}}1}}\), The following parameters were calculated for G–P fitting: \(P = \frac{{{\text{P}}1\left( {{\text{logZ}}2 - {\text{logZeq}}} \right) + {\text{Z}}2\left( {{\text{logZeq}} - {\text{logZ}}1} \right)}}{{{\text{logZ}}2 - {\text{logZ}}1}},\) EABF and EBF were calculated using G–P fitting. \(B\left( {E,X} \right) = 1 + \frac{b - 1}{{K - 1}}\)\((K^{x} - 1)\) for \(K \ne 1\), \(B\left( {E,X} \right) = 1 + \left( {b - 1} \right)x\) \(K = 1\) where \(K\left( {E,X} \right) = cx^{a} + d\frac{{\tanh \left( {\frac{x}{Xk} - 2} \right) - \tanh \left( { - 2} \right)}}{{1 - \tanh \left( { - 2} \right)}}\) for x ≤ 40.[30] and [40]
3 Results and discussion
3.1 Physical examinations
Figure 1 depicts the XRD of the samples under investigation. XRD shows no sharp peaks in these samples have a significant level of glassiness [29, 34,35,36].
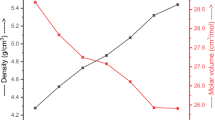
The density (\(\rho\)) and molar volume (\(V_{{\text{m}}}\)), are exemplified in Fig. 2. (\(\rho\)) of samples increased while (\(V_{{\text{m}}}\)), declined when Cr2O3 was added to them. The exchange of smaller CaO molecules (M = 56.077 g/mol, = 3.34 g/cm3) with thicker and heavier Cr2O3 (M = 151.99 g/mol, = 5.2 g/cm3) is the predominant cause of the increase in density. BO3 have been transformed into BO4 elements by the addition of modifier oxides like Cr2O3. The fact that BO4 units are denser than BO3 units demonstrates an increase in sample density [34, 37, 38].
3.2 FT-IR investigations
FT-IR spectrum of fabricating samples is exhibited in Fig. 3. In Fig. 3, the broad bands are the result of several distinct bands overlapping. Therefore, a deconvoluted process is used to obtain exact band positions. The difference between experimental and simulated Fig. is small than 0.02%. As a result, FT-IR of glasses was deconvoluted with (Peak Fit v4.12) using Gaussian distribution to determine the exact band positions and structural unit concentration. Figure 4 depicts a typical glass deconvoluted FT-IR spectrum including band center (C), and relative area (A) as Table 1[8, 39,40,41,42]. (BO3) is converted to (BO4) in borosilicate glasses with low modifier oxide inclusions. For the borosilicate glasses, Si–O–B bonds must be established. The band at 442 cm−1 is combined with the deformation modes of the network structure. This band could be attributed to the cationic vibration of Ca-O in (CaO6). The (NaO6), (CrO6), SiO4, and Si–O–B vibrations are linked to bands in the 507–534 cm−1 range, which overlap with the O–Si–O. B–O–B bending vibrations of (BO3) may be responsible for the bands observed at ~ 703 cm−1. In borosilicate glasses with a large amount of alkaline earth oxides, SiO4 tetrahedra with two NBOs can be found. Absorptions due to stretching vibrations of B–O–Si linkages between 952 and 1118 cm−1 must be noted, indicating a possible linkage between silicate and borate structural units [45]. The bands of 1000–1080 cm−1 due to the vibrations of the asymmetric stretching vibrations of Si–O–Si, O–Si–O− [5]. The broadband between 836 and 1118 cm−1 is designated to B–O bond stretching vibrations of BO4. The band at 1226–1262 cm−1 is recognized to asymmetric stretching vibrations of B–O bonds in (BO3) units (NBO). The peaks at 1302–1357 cm−1 explained by asymmetric stretching modes of borate triangles with NBOs. B–O− stretching vibrations in (BO3) related to1440 cm−1. The O–H and H2O bending vibrations in the sample may be responsible for 1534 and 1686 cm−1. The assignments of glass samples are listed in Table 2.
\(N_{4}\) fraction is defined as \(N_{4} = \frac{{concentration of \left( {BO4} \right)}}{{concentration of\left( {BO4} \right) + concentration of\left( {BO3} \right)}}\) Table 1 shows the \(N_{4}\) values for each of the glasses examined [46, 47]. The concentration of (BOs) increases with increasing Cr2O3 content, according to the Perusal of calculated values for \(N_{4}\). After Cr2O3 is incorporated, BO3 transforms into BO4 tetrahedra. The glass network’s coherence improves, and the structure stiffens as a result.
3.3 Optical investigations
In the wavelength range 250–2500 nm, the UV–Vis absorption spectra of Cr2O3 doped calcium borosilicate glasses are shown in Fig. 5. In comparison to the G1 glass, the absorption edge of all doped glasses has shifted to a lower wavelength. Figure 6 depicts the absorption coefficient (α) of these glasses. (α) calculated as \(\propto = \left( {2.303|d} \right) \times A\). Their intensities have also differed slightly. With Cr2O3 doping, the band’s absorption intensity has increased. The (Cr3+) is correlated to the identified absorption peaks ranging from 550 to 700 nm.
The determination of the energy \(E_{{{\text{opt}}{.}}}\) of the glasses is used Tauc's relation: \(\alpha h\nu = C(h\nu - E_{{{\text{opt}}{.}}} )^{{\text{s}}} .\) The relationship between (\(\alpha h\nu\))1/2 and (\(h\nu\)) is depicted in Fig. 7. Table 3 demonstrates that the \(E_{{{\text{opt}}{.}}}\) values are in the range of 2.57–3.31 eV [9, 43,44,45,46,47,48,49,50]. According to Babu and Cole, structural changes, and the formation of BOs cause differences in \(E_{{{\text{opt}}{.}}}\) values. Increases in the \(E_{{{\text{opt}}{.}}}\) can be linked to density and N4 variation. Cr-ions occupy interstitial site positions, resulting in increased network compactness and \(E_{{{\text{opt}}{.}}}\).
Calculating (\(R_{{\text{m}}}\)), (\(\propto_{{\text{m}}}\)), (\(R_{{\text{L}}}\)), (χ),\(M, K_{{{\text{th}}}}\) and \(T_{{{\text{g}}\left( {{\text{thero}}{.}} \right)}}\) using values from the (\(E_{{{\text{opt}}{.}}}\)). The values of (\(R_{{\text{m}}}\)), (\(\propto_{{\text{m}}}\)), and (\(R_{{\text{L}}}\)) decrease, although (χ),\(M\) increment. Because \(V_{{\text{m}}}\) has decreased, these observations have been reduced. Because the values of (\(\propto^\circ\) and \(\wedge\)) are different, they both decline. The increment in \(K_{{{\text{th}}}}\) and \(T_{{{\text{g}}\left( {{\text{thero}}{.}} \right)}}\) as Cr2O3 is thought to be caused by an increase in the bandgap. Table 3 displays the data values obtained.
(\(n_{{\text{D}}}\)) was determined as \(nD = \frac{{\left( {1 - R} \right)^{2} + k^{2} }}{{\left( {1 + R} \right)^{2} + k^{2} }}\). The investigated sample’s \(n_{{\text{D}}}\) increases, as shown in Fig. 8. The relationship between \(n_{{\text{D}}}\) and \(\rho\) is similar. As a result, \(n_{{\text{D}}}\) increases as Cr2O3, which is thought to be due to a density increase. Calculating \(R_{{\text{m}}}\), \(\propto_{0}^{2 - }\), and \(\Lambda\) using values from the (\(n_{{\text{D}}}\)). The values of \(R_{{\text{m}}}\), \(\propto_{0}^{2 - }\), and \(\Lambda\) are shown in Figs. 9, 10, 11. \(R_{{\text{m}}}\), \(\propto_{0}^{2 - }\), and \(\Lambda\) have the same value as the refractive index.
3.4 Radiation attenuation capacities
Figure 12 shows the (Zeq) value of glass samples. (Zeq) increased then decreased as the incident photon energy was increased and CaO was replaced with Cr2O3. This result is due to the Compton scattering interaction. At energies greater than 1 MeV, the (Zeq) value decreases due to the pair creation interaction [22, 48, 51,52,53,54].
The variation of EAB and EBAF are presented in Fig. 13. EAF and EABF decrease as the density and Cr2O3 in the glasses increase. This also confirms that the photon energy and chemical structure of a material determines its EAF and EABF.
Finally, we use effective removal cross-sections \(\sum R\) to investigate the neutron attenuation behaviors of glasses. Figure 14 exemplifies the \(\sum R\) of synthesized glasses. As shown in Fig. 15, \(\sum R\) is a density-dependent parameter. \(\sum R\) enhances as the Cr2O3 content of the glasses increases. G 5 is a better fast neutron absorber when the FNRC of glass samples is compared. For neutron attenuation applications, the glass sample G 5 is the best option [22, 48, 51,52,53,54].
4 Conclusions
Melt quenching was used to successfully fabricate chromium-modified calcium borosilicate glasses. The optical, structural, and γ shielding characteristics of these glasses are investigated. With CrO6 octahedral structural units, Cr2O3 acts as network modifiers. With the addition of Cr2O3, the glass density increased while molar volume reduces. All glass compositions contain B2O3 in the form of BO3 and BO4 units, as well as SiO2 in the SiO4. The concentration of (BOs) increases with increasing Cr2O3 content, according to the Perusal of calculated values for N4. After Cr2O3 is incorporated, BO3 transforms into BO4 tetrahedra. The glass network's coherence improves, and the structure stiffens as a result. \(E_{{{\text{opt}}}}\) values are in the range of 2.42–3.18 eV. Increases in the \(E_{{{\text{opt}}}}\) can be linked to density and N4 variation. (Zeq) increased then decreased as the incident photon energy was increased and CaO was replaced with Cr2O3. \(\sum R\) enhances as the Cr2O3 content of the glasses increases. G 5 is a better fast neutron absorber when the FNRC of glass samples is compared. For neutron attenuation applications, the glass sample G 5 is the best option. Incorporating Cr2O3 into the investigated glass compositions has a significant impact on their optical, structural, and γ shielding characteristics, according to the findings of this study. This research could be used in the future to improve optical efficiency and reduce radiation's harmful effects on organisms.s
Data availability
My manuscript and associated personal data.
References
M. Kodama, Ultrasonic velocity in sodium borate glasses. J. Mater. Sci. 26, 4048–4053 (1991). https://doi.org/10.1007/bf02402945
M.S.I. Koubisy, K.S. Shaaban, E.A.A. Wahab, M.I. Sayyed, K.A. Mahmoud, Synthesis, structure, mechanical and radiation shielding features of 50SiO2–(48 + X) Na2B4O7–(2 − X) MnO2 glasses. Eur. Phys. J. Plus (2021). https://doi.org/10.1140/epjp/s13360-021-01125-4
K.H.S. Shaaban, Y.B. Saddeek, K. Aly, Physical properties of pseudo quaternary Na2B4O7 – SiO2 – MoO3 – Dy2O3 glasses. Ceram. Int. 44, 3862–3867 (2018). https://doi.org/10.1016/j.ceramint.2017.11.175
E.A. Abdel Wahab, M.S.I. Koubisy, M.I. Sayyed, K.A. Mahmoud, A.F. Zatsepin, S.A. Makhlouf, K.S. Shaaban, Novel borosilicate glass system: Na2B4O7-SiO2-MnO2: synthesis, average electronics polarizability, optical basicity, and gamma-ray shielding features. J. Non-Cryst. Solids 553, 120509 (2021). https://doi.org/10.1016/j.jnoncrysol.2020.120509
A. Dahshan, Y.B. Saddeek, K.A. Aly, K.H.S. Shaaban, M.F. Hussein, A.O. Abo El Naga, S.A. Shaban, S.O. Mahmoud, Preparation and characterization of Li2B4O7 – TiO2 – SiO2 glasses doped with metal-organic framework derived nano-porous Cr2O3. J. Non-Cryst. Solids 508, 51–61 (2019). https://doi.org/10.1016/j.jnoncrysol.2019.01.002
K.S. Shaaban, A.M. Al-Baradi, Z.A. Alrowaili, A.M. Ali, M.S. Al-Buriahi, E.A.A. Wahab, Structural, thermal, and mechanical characteristics of yttrium lithium borate glasses and glass–ceramics. J. Mater. Sci.: Mater. Electron. 32, 28065–28075 (2021). https://doi.org/10.1007/s10854-021-07158-w
A.F.A. El-Rehim, H.Y. Zahran, I.S. Yahia, S.A. Makhlouf, K.S. Shaaban, Radiation, crystallization, and physical properties of cadmium borate glasses. SILICON 13, 2289–2307 (2021). https://doi.org/10.1007/s12633-020-00798-3
S. Alomairy, A.M. Aboraia, E.R. Shaaban, K.S. Shaaban, Comparative studies on spectroscopic and crystallization properties of Al2O3 -Li2O- B2O3-TiO2 glasses. Braz. J. Phys. 51, 1237–1248 (2021). https://doi.org/10.1007/s13538-021-00928-1
E.A. Abdel Wahab, K.S. Shaaban, E.S. Yousef, Enhancement of optical and mechanical properties of sodium silicate glasses using zirconia. Opt. Quant. Electron. (2020). https://doi.org/10.1007/s11082-020-02575-3
A.F.A. El-Rehim, H.Y. Zahran, I.S. Yahia, A.M. Ali, K.S. Shaaban, Physical, radiation shielding and crystallization properties of Na2O-Bi2O3- MoO3-B2O3- SiO2-Fe2O3 glasses. SILICON (2020). https://doi.org/10.1007/s12633-020-00827-1
T. Alharbi, H.F.M. Mohamed, Y.B. Saddeek, A.Y. El-Haseib, K.S. Shaaban, Study of the TiO2 effect on the heavy metals oxides borosilicate glasses structure using gamma-ray spectroscopy and positron annihilation technique. Radiat. Phys. Chem. 164, 108345 (2019). https://doi.org/10.1016/j.radphyschem.2019.108345
K.S. Shaaban, Y.B. Saddeek, Effect of MoO3 content on structural, thermal, mechanical and optical properties of (B2O3-SiO2-Bi2O3-Na2O-Fe2O3) glass system. SILICON 9, 785–793 (2017). https://doi.org/10.1007/s12633-017-9558-5
K.H. Mahmoud, A.S. Alsubaie, E.A.A. Wahab, F.M. Abdel-Rahim, K.S. Shaaban, Research on the effects of yttrium on bismuth titanate borosilicate glass system. SILICON (2021). https://doi.org/10.1007/s12633-021-01125-0
K.S. Shaaban, I. Boukhris, I. Kebaili, M.S. Al-Buriahi, Spectroscopic and attenuation shielding studies on B2O3-SiO2-LiF-ZnO-TiO2 glasses. SILICON (2021). https://doi.org/10.1007/s12633-021-01080-w
K.S. Shaaban, A.M. Al-Baradi, E.A.A. Wahab, The Impact of Y2O3 on physical and optical characteristics, polarizability, optical basicity, and dispersion PARAMETERS of B2O3 – SiO2 – Bi2O3 – TiO2 Glasses. SILICON (2021). https://doi.org/10.1007/s12633-021-01309-8
A.M. Al-Baradi, A.F.A. El-Rehim, Z.A. Alrowaili, M.S. Al-Buriahi, K.S. Shaaban, FT-IR and gamma shielding characteristics of 22SiO2- 23Bi2O3-37B2O3-13TiO2-(5–x) LiF- x BaO glasses. SILICON (2021). https://doi.org/10.1007/s12633-021-01481-x
A.M. Ali, Z.A. Alrowaili, A.M. Al-Baradi, M.S. Al-Buriahi, E.A.A. Wahab, K.S. Shaaban, A study of thermal, and optical properties of 22SiO2- 23Bi2O3-37B2O3-13TiO2-(5–x) LiF- x BaO glasses. SILICON (2021). https://doi.org/10.1007/s12633-021-01440-6
Z.A. Alrowaili, A.M. Al-Baradi, M.A. Sayed, A. Mossad Ali, E.A. Abdel Wahab, M.S. Al-Buriahi, K.S. Shaaban, The impact of Fe2O3 on the dispersion parameters and gamma / fast neutron shielding characteristics of lithium borosilicate glasses. Optik (2021). https://doi.org/10.1016/j.ijleo.2021.168259
Y.B. Saddeek, K.A. Aly, K.S. Shaaban, A.M. Ali, M.A. Sayed, The Effect of TiO2 on the optical and mechanical properties of heavy metal oxide borosilicate glasses. SILICON 11, 1253–1260 (2019). https://doi.org/10.1007/s12633-018-9912-2
A.A. El-Maaref, E.A.A. Wahab, K.S. Shaaban, R.M. El-Agmy, Enhancement of spectroscopic parameters of Er3+-doped cadmium lithium gadolinium silicate glasses as an active medium for lasers and optical amplifiers in the NIR-region. Solid State Sci. 113, 106539 (2021). https://doi.org/10.1016/j.solidstatesciences.2021.106539
A.F.A. El-Rehim, K.S. Shaaban, Influence of La2O3 content on the structural, mechanical, and radiation-shielding properties of sodium fluoro lead barium borate glasses. J. Mater. Sci.: Mater. Electron. 32, 4651–4671 (2021). https://doi.org/10.1007/s10854-020-05204-7
K.S. Shaaban, S. Alomairy, M.S. Al-Buriahi, Optical, thermal and radiation shielding properties of B2O3–NaF–PbO–BaO–La2O3 glasses. J. Mater. Sci.: Mater. Electron. (2021). https://doi.org/10.1007/s10854-021-05885-8
A.F.A. El-Rehim, A.M. Ali, H.Y. Zahran, I.S. Yahia, K.S. Shaaban, Spectroscopic, structural, thermal, and mechanical properties of B2O3-CeO2-PbO2 glasses. J. Inorg. Organomet. Polym Mater. 31, 1774–1786 (2021). https://doi.org/10.1007/s10904-020-01799-w
K.S. Shaaban, E.S. Yousef, Optical properties of Bi2O3 doped boro tellurite glasses and glass ceramics. Optik 203, 163976 (2020). https://doi.org/10.1016/j.ijleo.2019.163976
M.A. Sayed, A.M. Ali, A.F. Abd El-Rehim et al., Dispersion parameters, polarizability, and basicity of lithium phosphate glasses. J. Elec. Mater. 50, 3116–3128 (2021). https://doi.org/10.1007/s11664-021-08921-9
A.M. Fayad, K.S. Shaaban, W.M. Abd-Allah, M. Ouis, Structural and optical study of CoO doping in borophosphate host glass and effect of gamma irradiation. J. Inorg. Organomet. Polym Mater. 30, 5042–5052 (2020). https://doi.org/10.1007/s10904-020-01641-3
K.S. Shaaban, E.S. Yousef, S.A. Mahmoud, E.A.A. Wahab, E.R. Shaaban, Mechanical, structural and crystallization properties in titanate doped phosphate glasses. J. Inorg. Organomet. Polym Mater. 30, 4655–4663 (2020). https://doi.org/10.1007/s10904-020-01574-x
A.A. El-Maaref, S. Badr, K.S. Shaaban, E.A. Abdel Wahab, M.M. Elokr, Optical properties and radiative rates of Nd3+ doped zinc-sodium phosphate glasses. J. Rare Earths 37, 253–259 (2019). https://doi.org/10.1016/j.jre.2018.06.006
E.A. Abdel Wahab, A.A. El-Maaref, K.S. Shaaban, J. Börcsök, M. Abdelawwad, Lithium cadmium phosphate glasses doped Sm3+ as a host material for near-IR laser applications. Opt. Mater. 111, 110638 (2021). https://doi.org/10.1016/j.optmat.2020.110638
A. El-Taher, A.M. Ali, Y.B. Saddeek, R. Elsaman, H. Algarni, K. Shaaban, T.Z. Amer, Gamma ray shielding and structural properties of iron alkali alumino-phosphate glasses modified by PbO. Radiat. Phys. Chem. 165, 108403 (2019). https://doi.org/10.1016/j.radphyschem.2019.108403
A. Yadav, M.S. Dahiya, A. Hooda, P. Chand, S. Khasa, Structural influence of mixed transition metal ions on lithium bismuth borate glasses. Solid State Sci. 70, 54–65 (2017). https://doi.org/10.1016/j.solidstatesciences.2017.06.011
K.S. Shaaban, Z.A. Alrowaili, A.M. Al-Baradi, A.M. Ali, E.A.A. Wahab, M.S. Al-Buriahi, Mechanical and thermodynamic characteristics of 22SiO2- 23Bi2O3-37B2O3-13TiO2-(5–x) LiF- x BaO glasses. SILICON (2021). https://doi.org/10.1007/s12633-021-01441-5
E. Şakar, Ö.F. Özpolat, B. Alım, M.I. Sayyed, M. Kurudirek, PhyX / PSD: Development of a user-friendly online software for calculation of parameters relevant to radiation shielding and dosimetry. Radiation Phys. Chem. 166, 108496 (2020). https://doi.org/10.1016/j.radphyschem
K.S. Shaaban, E.A. Abdel Wahab, A.A. El-Maaref, M. Abdelawwad, E.R. Shaaban, E.S. Yousef, H. Wilke, H. Hillmer, J. Börcsök, Judd-Ofelt analysis and physical properties of erbium modified cadmium lithium gadolinium silicate glasses. J. Mater. Sci.: Mater. Electron. 31, 4986–4996 (2020). https://doi.org/10.1007/s10854-020-03065-8
K.S. Shaaban, M.S.I. Koubisy, H.Y. Zahran, I.S. Yahia, Spectroscopic properties, electronic polarizability, and optical basicity of titanium-cadmium tellurite glasses doped with different amounts of lanthanum. J. Inorg. Organomet. Polym Mater. 30, 4999–5008 (2020). https://doi.org/10.1007/s10904-020-01640-4
A.A. El-Maaref, R.M. El-Agmy, K.S. Shaaban, E.A. Abdel Wahab, Optical and spectroscopic study of Nd2O3-doped SBN glass in the near-infrared, visible and UV regions under pumping up-conversion emissions. Eur. Phys. J. Plus (2021). https://doi.org/10.1140/epjp/s13360-021-01798-x
E.A.A. Wahab, A.M. Aboraia, A.M.E. Shafey, K.S. Shaaban, A.V. Soldatov, The effect of ZrO2 on the linear and non-linear optical properties of sodium silicate glass. Opt. Quant. Electron. (2021). https://doi.org/10.1007/s11082-021-03164-8
E.A. Abdel Wahab, K.S. Shaaban, S. Alomairy, M.S. Al-Buriahi, Electronegativity and optical basicity of glasses containing Na/Pb/B and their high performance for radiation applications: role of ZrO2 nanoparticles. Eur. Phys. J. Plus (2021). https://doi.org/10.1140/epjp/s13360-021-01572-z
A.F.A. El-Rehim, K.S. Shaaban, H.Y. Zahran, I.S. Yahia, A.M. Ali, M.M.A. Halaka, S.A. Makhlouf, E.A.A. Wahab, E.R. Shaaban, Structural and mechanical properties of lithium bismuth borate glasses containing molybdenum (lbbm) together with their glass-ceramics. J. Inorg. Organomet. Polym Mater. 31, 1057–1065 (2021). https://doi.org/10.1007/s10904-020-01708-1
A.F.A. El-Rehim, H.Y. Zahran, I.S. Yahia, E.A.A. Wahab, K.S. Shaaban, Structural, elastic moduli, and radiation shielding of SiO2-TiO2-La2O3-Na2O glasses containing Y2O3. J. Mater. Eng. Perform. 30, 1872–1884 (2021). https://doi.org/10.1007/s11665-021-05513-w
B. Albarzan, A.H. Almuqrin, M.S. Koubisy, E.A. Abdel Wahab, K.A. Mahmoud, Kh.S. Shaaban, M.I. Sayyed, Effect of Fe2O3 doping on structural, FTIR and radiation shielding characteristics of aluminium-lead-borate glasses. Prog. Nucl. Energy 141, 103931 (2021). https://doi.org/10.1016/j.pnucene.2021.103931
S. Alomairy, M.S. Al-Buriahi, E.A. Abdel Wahab, C. Sriwunkum, K. Shaaban, Synthesis, FTIR, and neutron/charged particle transmission properties of Pb3O4–SiO2–ZnO–WO3 glass system. Ceram. Int. 47, 17322–17330 (2021). https://doi.org/10.1016/j.ceramint.2021.03.045
M. Bala, S. Agrohiya, S. Dahiya, A. Ohlan, R. Punia, A.S. Maan, Effect of replacement of Bi2O3 by Li2O on structural, thermal, optical and other physical properties of zinc borate glasses. J. Mol. Struct. 1219, 128589 (2020). https://doi.org/10.1016/j.molstruc.2020.128589
S. Bale, N.S. Rao, S. Rahman, Spectroscopic studies of Bi2O3–Li2O–ZnO–B2O3 glasses. Solid State Sci. 10, 326–331 (2008). https://doi.org/10.1016/j.solidstatesciences.2007.09.017
M. Hubert, A.J. Faber, On the structural role of boron in borosilicate glasses. Eur. J. Glass Sci. Technol. Part B Phys. Chem. Glasses 55(3), 136–158 (2014)
M.S. Gaafar, S.Y. Marzouk, Mechanical and structural studies on sodium borosilicate glasses doped with Er2O3 using ultrasonic velocity and FTIR spectroscopy. Physica B 388(1–2), 294–302 (2007). https://doi.org/10.1016/j.physb.2006.06.132
T. Walia, K. Singh, Mixed alkaline earth modifiers effect on thermal, optical and structural properties of SrO-BaO-SiO2-B2O3-ZrO2 glass sealants. J. Non-Cryst. Solids 564, 120812 (2021). https://doi.org/10.1016/j.jnoncrysol.2021.120812
E.A. Abdel Wahab, K.S. Shaaban, Structural and optical features of aluminum lead borate glass doped with Fe2O3. Appl. Phys. A (2021). https://doi.org/10.1007/s00339-021-05062-y
K.S. Shaaban, A.M. Al-Baradi, A.M. Ali, Investigation of BaO reinforced TiO2–P2O5–li2O glasses for optical and neutron shielding applications. RSC Adv. 12, 3036–3043 (2022). https://doi.org/10.1039/d2ra00171c
E.A.A. Wahab, K.S. Shaaban, A.M. Al-Baradi, Enhancement of optical and physical parameters of lead zinc silicate glasses by doping W+3 ions. SILICON (2021). https://doi.org/10.1007/s12633-021-01236-8
M.A. Alothman, Z.A. Alrowaili, J.S. Alzahrani, E.A.A. Wahab, I.O. Olarinoye, C. Sriwunkum, K.S. Shaaban, M.S. Al-Buriahi, Significant influence of MoO3 content on synthesis, mechanical, and radiation shielding properties of B2O3-Pb3O4-Al2O3 glasses. J. Alloy. Compd. 882, 160625 (2021). https://doi.org/10.1016/j.jallcom.2021.160625
S. Alomairy, Z.A. Alrowaili, I. Kebaili, E.A.A. Wahab, C. Mutuwong, M.S. Al-Buriahi, K.S. Shaaban, Synthesis of Pb3O4-SiO2-ZnO-WO3 glasses and their fundamental properties for gamma shielding applications. SILICON (2021). https://doi.org/10.1007/s12633-021-01347-2
M.H.A. Mhareb, M.I. Sayyed, Y.S.M. Alajerami, M. Alqahtani, N. Dwaikat, A.M. Alsagry, M. Al-Yatimi, M. Zakariah, Structural and radiation shielding features for a new series of borate glass samples: part I. Eur. Phys. J. Plus (2021). https://doi.org/10.1140/epjp/s13360-020-00984-7
Y.B. Saddeek, K.H.S. Shaaban, R. Elsaman, A. El-Taher, T.Z. Amer, Attenuation-density anomalous relationship of lead alkali borosilicate glasses. Radiat. Phys. Chem. 150, 182–188 (2018). https://doi.org/10.1016/j.radphyschem.2018.04.028
Acknowledgements
We would like to thank Taif University Research Supporting Project number (TURSP-2020/24), Taif University, Taif, Saudi Arabia. Moreover, the authors express their gratitude to the Deanship of Scientific Research at King Khalid University for funding this work through the research groups program under Grant No. R.G.P. 1/298/42.
Author information
Authors and Affiliations
Contributions
All the authors have given their approval for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
The manuscript has not been published elsewhere.
Consent to participate and publication
The author’s consent to participate and publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shaaban, K.S., Al-Baradi, A.M. & Ali, A.M. Cr2O3 effect on the structure, optical, and radiation shielding properties of Na2B4O7–SiO2–CaO–Cr2O3 glasses. Appl. Phys. A 128, 208 (2022). https://doi.org/10.1007/s00339-022-05348-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-022-05348-9