Abstract
A simple solvothermal route was successfully implemented for fabrication of Li-rich Mn-based layered cathode Li1.15Ni0.15Co0.15Mn0.7M0.01O2 (LMLC) materials through incorporation of different trivalent cations (M3+: Al3+, Cr3+ or Fe3+). The structural properties of mixed solid solution of rhombohedral LiNiO2 phase and monoclinic Li2MnO3 phases were checked by X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS). LMLC-Fe sample shows the highest coercivity Hc value of about 198.1 Oe, and the lowest dielectric constant εr about 74. LMLC-Cr sample exhibits the lowest activation energy (Ea = 0.163 eV) for electric conduction as a result of the smallest cell volume and particle size. LMLC-Al electrode material delivers the highest initial charge and discharge capacity of about 140 and 104 mAh/g at 0.1 C, respectively maintaining around 70% of the initial capacity at 0.1C. Both LMLC-Al and LMLC-Cr samples show better high rate capabilities than that of LMLC-Fe sample.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The accelerated development of electric vehicles (EVs) and hybrid electric vehicles (HEVs) as well as the installation of huge renewable energy power plants has enforced the need to high energy density batteries mainly lithium-ion batteries [1, 2]. Considering that, the Li-rich layered cathode materials have been extensively investigated and attracted higher attention than the conventional cathodes made of LiCoO2, LiMn2O4 and LiFePO4 owing to their high storage capacities [3,4,5]. During the last few years, the electrochemical performance of Li-rich Mn-based layered cathode (LMLC) materials xLi2MnO3-(1-x)LiNiO2 has clearly surpassed that of commercial Ni-rich layered cathodes materials (NMC) [6,7,8]. The reason for their preference for next generation of EVs can be attributed to the enhanced structural and thermal stability as a result of the presence of inactive Li2MnO3, delivering large charge-discharge capacities and its lower cost and toxicity [9,10,11]. However, the solid solution system of LMLC materials still suffers from some operational problems such as low initial coulombic efficiency or low electric conductivity leading to a poor rate capability [12,13,14]. Huge efforts have been made to overcome all the aforementioned practical difficulties through several strategies such as surface modifications [15, 16], doping with transition metals ions [17, 18] and controlling the morphology of particles [19, 20]. Apparently, previous research studies demonstrated that the bulk doping with cations like Mg2+ [21], Cr3+ [22], Zr4+ [23], Al3+ [24], Mo4+/6+ [19], V4+/5+ [25], Ti4+ [26], Fe3+ [27] or Sn4+ [28] would stabilize the layered material superstructure and significantly hinder the spinel phase transformation upon cycling [29,30,31]. In addition, various preparation wet chemical methods, e.g. co-precipitation [32], sol-gel [33] and solvothermal [34], have been employed to prepare different morphologies of LMLC materials in the nanosized scale to boost the lithium ions diffusion rate and hence improving the storage capacity and coulombic efficiency as well. In this view, the effect of incorporation of a small proportion of trivalent metal ions (~ 1%) on the structural, morphological and electrochemical performance of LMLC materials was explored as principal objective of this work. Thus, three samples of Li1.15Ni0.15Co0.15Mn0.7M0.01O2 materials doped with 1wt% of Al3+, Cr3+ and Fe3+ trivalent cations were synthesized via solvothermal technology. The fabricated electrodes from these materials were investigated by cyclic voltammetry and electrochemical impedance spectroscopy to clarify the reaction mechanism. The charge/discharge experiments were also conducted by applying different C-rates as well as long-term stability tests.
2 Experimental
2.1 Synthesis of layered metal oxides
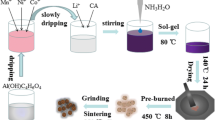
A single phase of Li-rich and Mn-based layered and subsequently doped by trivalent metal-ion Li1.15Ni0.15Co0.15Mn0.7M0.01O2 nanoparticles, where M: Al3+, Cr3+ or Fe3+ ions were prepared via simple solvothermal route. A mixture of ethylene glycol (density = 1.11 g/cm3) and absolute ethanol (1:1, by volume) serves as organic solvent. The three M-doped samples Li1.15Ni0.15Co0.15Mn0.7Al0.01O2 (LMLC-Al), Li1.15Ni0.15Co0.15Mn0.7Cr0.01O2 (LMLC-Cr) and Li1.15Ni0.15Co0.15Mn0.7Fe0.01O2 (LMLC-Fe) were prepared by adding stoichiometric amounts of lithium nitrate trihydrate LiNO3.3H2O (99.9%, 1.4 g), nickel (II) acetate tetrahydrate Ni(CH3CO2)2·4H2O, (99.9%, 0.75 g), manganese acetate tetrahydrate Mn(CH3CO2)2·4 H2O (99.9%, 3.43 g), cobalt acetate tetrahydrate Co(CH3CO2)2·4H2O (99%, 0.75 g) and either Al(NO3)3·9H2O (99.9%, 0.078 g) or chromium (III) nitrate nonahydrate Cr(NO3)3·9H2O (99.9%, 0.08 g) or iron (III) nitrate nonahydrate Fe(NO3)3·9H2O (99.9%, 0.08 g) to 120 ml of the solvent mixture. Afterwards the salts were completely dissolved and the molar ratio of Li:Ni:Co:Mn:M3+ was 1.15:0.15:0.15:0.7: 0.01. The pH of the three mixtures was adjusted to 9.7 using an ammonium hydroxide solution (33%) to form the corresponding hydroxides. The formed suspensions were stirred for 15 min to achieve higher homogeneity. This new homogeneous mixture was then transferred into a closed Teflon container. The container was heated up to 180 °C (4.5 bar), and afterwards the temperature was hold for 12 h. Subsequently, the obtained solvothermal precursor solutions/suspensions were boiled at 105 °C under ambient pressure to evaporate the organic solvents. Each dried precursor was then pre-calcined at 500 °C (4 h) in air. The pre-calcined powders were finally calcined at 800 °C (3 h) in air and subsequently quenched at open air conditions. Finally, the as-prepared powders were intensely ground and subjected to further characterizations.
2.2 Characterizations
Bruker AXS diffractometer (D8-ADVANCE) with Cu Kα radiation (λ = 1.5406 Ǻ) operating at 40 kV and 10 mA was utilized to record X-ray powder diffraction (XRD) pattern. The diffraction scan was done between 2θ range 10°–80° with a rate of about 0.5° min−1. The XPS spectra for the LMLC-Al sample were measured using X-ray photoelectron spectrometer (Thermo Scientific, Model: K-Alpha surface analysis) equipped with monochromatic small-spot XPS system to scan spectra in the range of 0–1000 eV using X-ray source–Al Kα source (λ = 8.339 Ǻ) and calibrated by C 1 s line. The surface morphology and particle size of the as-prepared nanopowders were examined by field emission scanning electron microscope (FE-SEM) using the model QUANTA FEG250 from Genesis, Holland. The elemental analysis was performed with built-in unit for energy-dispersive X-ray spectroscopy analysis (EDS). The morphology of the cathode materials was identified by high-resolution transmission electron microscopy (HR-TEM) with a TECNAI G2 S-Twin microscope operating at 200 kV. The magnetization characteristics of all samples were measured at room temperature using a vibrating sample magnetometer (7400–1 VSM, U.S. Lake Shore Co., Ltd., USA) at maximum applied field 20 kOe. A thin-film layer of silver suspension ink was coated on the sintered pellet sides, in order to serve as electrodes for the dielectric measurements. Dielectric and ac conductivity measurements were determined in the temperature range of 300–525 K at 10 kHz using a Stanford Research RCL Bridge (Model: SR-720). The voltage bias: 2 VDC (internal) and 0–40 VDC (external).
2.3 Battery assembly and electrochemical testing
All processing steps for the as-synthesized positive electrode materials have been carried out in Argon filled glove box. The electrode slurry was prepared by mixing each active material (LMLC-Al, LMLC-Cr or LMLC-Fe) with carbon black (Super P C 65, Imerys) as conducting additive and polyvinylidene fluoride (PVDF, Solef 5130, Solvay Solexis) as binder in mass ratio NCM/CB/PVDF 83/10/7 in N-methyl pyrrolidone (NMP). The formed homogeneous slurry was coated on Al-foil as current collector and the as-prepared electrodes were dried under vacuum at 90 °C for (8 h). The cathode material loading is 4 mg/cm2. Finally, circular electrodes with a diameter of 12 mm were laser-cut and dried at 90 °C for 24 h under vacuum. At least three-electrode cells (Swagelok®) with Li as reference and counter electrode were assembled with each of the as-prepared electrodes. Freudenberg FS 2190 served as separator. A commercial carbonate mixture EC/DMC (1:1) (Sigma-Aldrich) with 1 M LiPF6 conducting salt was utilized as electrolyte. The rate capability tests and the long-term cycling were carried out between 3.0 and 4.8 V versus Li/Li+ with the charge/discharge procedure showed in Table S1 “Supplementary file” by means of a Maccor 4200 battery tester. Cyclic voltammetry measurements were performed in three-electrode cells as well in the voltage range of 3.0–4.8 V (vs. Li+/Li) at scan rate of 10 µVs−1 by means of a BioLogic VSP Potentiostat.
3 Results and discussion
3.1 Structural properties
The as-prepared layered samples (LMLC-Al, LMLC-Cr or LMLC-Fe) exhibit the same diffraction patterns as shown in Fig. 1. The XRD results of each composition indicate the presence of superstructure composed of two different phases, Rhombohedral phase is appeared as the main phase, and its peak signatures are matching to the common LiNiO2 layered structure with R-3 m space group (JCPDS card no. 96-0063) [35]. The other minor peaks correspond to the monoclinic Li2MnO3 phase with C2/m space group (JCPDS card no. 84-1634) [36]. The crystallographic parameters are calculated using the integrated software analysis (Topas 4.1) and listed in Table 1. The crystallite size (D) was calculated using the common Scherrer equation D = kλ/βcosθ, where k is the Scherrer constant (k = 0.89), λ is the X-ray wavelength, β is the peak width at half maximum, and θ is the Braggs diffraction angle. The higher the intensity ratio between the two reflections (003) and (104) than 1.2, the higher is the cation mixing in the layered LMO2 material. Meanwhile, if the I(003)/I(104) ratio is below 1.2, a week bonding of transition elements with Li atoms in the crystal structure is caused [37, 38]. Obviously, the three layered samples exhibited higher values of I(003)/I(104) ratio above 1.2 and LMLC-Al electrode material showed the highest value of about 2.15, which suggest high degree of transition elements occupation inside the Li–O layers. Moreover, the appearance of well separated peaks corresponding to (101) and (006) planes indicates the formation of the well crystalline layered Li2MnO3 structure of (Li[Li1/3Mn2/3]O2) type. Basically, the combination of these two different crystal structures could be possible at the atomic level as the close packed layers have similar interlayer spacing; thus their integration on molecular level is deeply observed in the present case [39, 40]. Accordingly, a successful formation of complex structure was achieved by the proposed solvothermal technique. The calculated average crystallite sizes are about 120, 74 and 102 nm for Al-, Cr- and Fe-doped LMLC layered structures, respectively. Thus, the significant decrease in the crystallinity of LMLC-Cr and LMLC-Fe could be attributed to the decrease in the crystallinity and crystallite size. It is indicated that LMLC-Al has the maximum cell volume, while the Cr-doped LMLC samples possessed the smallest unit cell volume. The reason for increasing the cell volume of LMLC-Al > LMLC-Cr > LMLC-Fe because atomic mass of Al (27 u) is lower than Cr (52 u) and Fe (56 u), thus the number of doped atoms of Al is higher than Cr and Fe leading to more octahedral centers which creates more inter spaces in the structure resulting in the lattice expansion along a and c axes for LMLC-Al and shrinkages in the lattice parameters a and c of LML-Cr. The influence of the trivalent doping cation on the oxidation state of Mn, Ni and Co can be investigated by applying the X-ray photoelectron spectroscopy (XPS) analysis for LMLC-Al sample as an example. Figure 1b reveals the two principle peaks of Mn2p3/2 and Mn2p1/2 at binding energies 642.2 and 653.7 eV, respectively. The separation between these two peaks is about 11.5 eV, which indicates the presence of Mn4+ [41, 42]. In addition, the depth XPS scan of Ni 2p in Fig. 1c displays two strong peaks of Ni2p3/2 at 854.3 eV and Ni2p1/2 at 872.5 eV as a result of Ni2+ cation existence. The other mild peaks observed at 856.5, 861.5 and 879 eV provide an additional confirmation for the presence of Ni2+ cations [43, 44]. The observation of the weak peak at 866 eV signifies the presence of Ni3+ traces (about 0.5%), which results from the valence degeneracy between Mn4+ and Ni2+ cations through the reversible reaction: Mn4+ + Ni2+ ↔ Ni3+ + Mn3+ [45, 46] or due to the effect of Al3+ doping. Figure 1d illustrates the Co2p XPS spectra, which shows two main peaks of Co2p3/2 and Co2p1/2 at binding energies about 780 and 795.2 eV, respectively. The observed splitting distance between these two peaks of about 15.2 eV confirms the existence of Co3+ cations [47, 48]. Moreover, the detection of two satellite peaks around 790 and 802 eV gives strong evidence also for Co3+ cations formation [48, 49].
3.2 Surface morphology
3.2.1 Scanning electron microscope
The microstructure of the three powders is shown in Fig. 2a–e at two different magnifications: 10000X and 30000X. The LMLC samples are homogenous and highly dispersed and exhibit both rhombohedral (or trigonal bipyramids) and monoclinic morphologies as detected by the XRD measurements. The average particle sizes are (500–1000 nm), (300–500 nm) and (300–700 nm) for Al-, Cr- and Fe-doped samples, respectively. The observed sharp edges in each regular particle geometry confirm the successful incorporation of doping metal ions without crystal lattice destruction and reflects the crystallinity improvement as well. It is worth to mention that the pores and path depths created between both types of crystal morphologies in each sample demonstrate good electronic paths for Li ions during charge/discharge processes.
The EDS analysis of LMLC electrode materials confirmed the incorporation of trivalent cations (Al3+, Cr3+, Fe3+). It is noticed that Fe3+ cation is found to be actually doped in the LMLC with higher stoichiometric amount (1.66%) than the intended percentage (1%). Meanwhile, both Al3+ and Cr3+ cations are found to be existed in the LMLC structure with lower percentages 0.42% and 0.68% than the intended one. The elemental analysis of other elements Mn, Ni, Co and O in all the three LMLC samples are quite different. The reason for this divergence could be attributed to the difference in the area exposed to the electron beam and the thickness of each powder LMLC sample mounted on the carbon tape could affect on the accuracy of EDS results (Fig. 3).
3.2.2 Transmission electron microscope
TEM/HR-TEM images with different magnifications for LMLC crystals, lattice pattern and selected area electron diffraction patterns (SAED) can be observed in Fig. 4a–i. The normal TEM images, Fig. 4a, b and c, of both LMLC-Al (a) and LMLC-Cr (b, c) materials exhibit well crystalline nanoplates of rectangular-like shape with different side dimensions, about 350–400 nm length, 250–280 nm width and 15–30 nm thickness. Alongside to this, the LMLC-Al particles have larger size than that of LMLC-Cr particles suggesting a good agreement with XRD and SEM results. Actually, the rhombohedral LiMO2 and monoclinic Li2MnO3 phases of the LMLC-Fe sample can be easily distinguished from HRTEM images and SAED patterns, as depicted in Fig. 4d, e, f, h.
TEM images of Li and Mn rich layered samples calcined at 800 °C for 3 h: a LMLC-Al, b, c LMLC-Cr, HR-TEM images and d-spacings of R-3 m phase (d) and C2/m phase in LMLC-Fe nanocrystals (e). Fast Fourier Transformation (FFT) of lattice fringes of C2/m phase in LMLC-Fe nanocrystals (f). The corresponding Miller indices of the FFT of LMLC-Fe composite structure. Observed and simulated electron diffraction pattern for the corresponding crystallographic orientations (Miller indices) of LMLC-Fe nanoparticles (h, i)
Figure 4d reveals the HR-TEM image for the LMLC-Fe nanocrystals which indicates the existence of two d-spacing distances 0.2 and 0.47 nm corresponding to the indexed planes (104) and (0 0 3) planes, respectively, of LiMO2 lattice pattern [50, 51]. Figure 4e f shows the HR-TEM analysis for another spot in LMLC-Fe nanoparticles with lattice fringes of Li2MnO3 (d020 = 0.43 nm) calculated by the built-in Fast Fourier Transformation (FFT) software. Thus, the corresponding miller indices of the FFT of LMLC-Fe composite structure are illustrated in Fig. 4g. Moreover, the observed and simulated electron diffraction pattern for the corresponding crystallographic orientations (Miller indices) of LMLC-Fe nanoparticles is shown in Fig. 4h, i. The ring pattern is a common type of the diffraction pattern that explains the existed phases according to the d-spacing (the radius of each ring) and hkl. It is clear that the bright and intense rings in SAED are related to rhombohedral LiMO2 phase, while the small and less bright rings correspond to monoclinic Li2MnO3 phase.
3.3 Magnetic and dielectric properties
As the movement of lithium ions and the transfer of electrons can take place simultaneously. Thus, applying the localized electrons model is highly preferred due to the fact that many cathode materials are poor conductors. Therefore, magnetic properties of the three LMLC samples were tested in order to investigate the change in the magnetic interactions within transition metal layers by doping with different trivalent cations which can lead to a change in the orbital overlapping (d- and –p orbitals) causing a change in the ions mobility and electronic properties. Hence, prediction of electron transfer pathways and characteristics gives an indication about the electrochemical behavior of multivalent electrode materials.
The field-dependent magnetization measurements are conducted at room temperature to study the magnetic behavior of LMLC materials as shown in Fig. 5a and the corresponding data are represented in Table 2. Weak ferromagnetic behavior was clearly demonstrated for the LMLC material. In particular, the magnitudes of saturation magnetization (Ms) and remanence magnetization (Mr) were decreased with increasing ionic radius of dopant ions Fe3+ > Cr3+ > Al3+. However, the coercivity (Hc) was significantly increased with increasing ionic radius of metal ions as well as decreasing average particle sizes and magnetocrystalline anisotropy. The obtained results showed good consistency with the previous reports of layered cathode materials [52, 53]. Furthermore, the ground samples of layered phases were pressed and sintered in air at 830 °C to study the electrical properties of sintered LMLC materials. The electronic conductivity measurements are conducted at 10 kHz by using ac impedance spectroscopy technique to unveil the mobility of Li cations and electrons in the tunnels of layered framework structures as depicted in Fig. 5b, c. It is found that the dielectric constant of all samples strongly increases with increasing temperature from 40 to 50 °C. This behavior directly changed to be decreased with further increase in the temperature reaching the steady state at 80 °C. Likely, the dielectric loss of all samples decreased with increasing the temperature from 50 to 90 °C and then start to increase up to 140 °C. It is obvious that the LMLC-Fe sample showed the lowest values of both dielectric loss (tan δ = 1.75) and dielectric constant (εr = 74) at room temperature and the same dielectric behavior achieved with rising the temperature. In addition, the LMLC-Al sample possessed the maximum value of (εr) and (tan δ) in the temperature range 30–70 °C, and shows moderate values above 70 °C. On the other hand, the common Arrhenius relation is implemented to calculate the required activation energy for electronic conduction in the bulk structure [54], as plotted in Fig. 5d. Generally, the electrical conductivity linearly increases with rising the temperature from 50 to 150 °C. Accordingly, the LMLC-Cr sample exhibited the lowest value of activation energy (Ea = 0.163 eV) of conduction as a result of smallest cell volume and particle size as well. Meanwhile, LMLC-Fe sample revealed the highest value of activation energy of conduction (Ea = 0.207 eV) over the temperature range (40–160 °C) which might be correlated to the less homogeneity in the layered structure and large cell volume. Table 3 summarizes the electrical and dielectric properties of the sintered pellets of layered LMLC samples at 10 kHz and 298 K. The measured LMCL samples showed similar electrical and dielectric behavior to that of other layered cathode materials [55,56,57].
3.4 Electrochemical examination
3.4.1 Galvanostatic cycling and cyclic voltammetry
The charge–discharge profiles at 0.2C rates for the first four cycles of LMLC-Al, LMLC-Cr and LMLC-Fe electrode materials are depicted in Fig. 6a, c and e while the corresponding cyclic voltammograms (CV) of LMLC-Al, LMLC-Cr and LMLC-Fe are illustrated in Fig. 6b, d and f. It is found that the LMLC-Fe achieves the maximum initial charge capacity about 165 mAh/g at 1 C which correspond to 60% of the theoretical capacity. In contrast, LMLC-Cr achieves the maximum coulombic efficiency about 92.5% during the subsequent 4th cycle. The reason for this change can be significantly explained by the cyclic voltammetry and electrochemical impedance spectroscopy studies. As visible in Fig. 6b, d and f at the beginning of the first cycle, from open circuit potential up to 3.7 V, the Mn ions remain electrochemically inactive, however in the following cycles between 3.0 V and ca. 3.7 V, the oxidation of Mn3+ to Mn4+ takes place. This is visible by the broad current peaks from the second cycle and further in the CV of each material under study [58]. The Mn4+ to Mn3+ reduction occurs around 3.4 V.
For all three test samples, it can be noted that oxidation reactions of Ni2+ to Ni3+ and then to Ni4+ as well as Co3+ and Co4+ during charge are responsible for the first anodic peaks around 4.0 V [18]. On the cathodic side of the cyclic voltammogram, this is the reduction of Ni4+ to Ni2+ and Co4+ to Co3+. The sharp peak during the first cycle around 4.6 V is only seen in the samples LMLC-Cr and LMLC-Fe due to irreversible electrochemical material activation reaction that decomposes Li2MnO3 into Li2O and MnO2 and yet oxygen is released. This irreversible reaction causes a crystal structure transformation of the cathode material according to the literature also. However, this occurrence has no negative influence on the electrochemical performance of the cathode, and it is very necessary for the further stable function of Li-rich cathode materials [59]. The peak is not present in the 2-nd, 3-rd and 4-th cycle for both materials. Generally, the subsequent cycles overlap each other, which is an evidence for a very good electrochemical reversibility of the as-presented layered cathode materials.
3.4.2 Electrochemical impedance spectroscopy (EIS)
Figure 7a, b illustrates the Nyquist plots for the assembled LMLC batteries before and after galvanostatic cycling at 0.2 C. In addition, the measured EIS parameters after fitting of the obtained Nyquist plots for all cells are listed in Table 4. It is revealed that LMLC-Al cell demonstrates the lowest values for ohmic resistance (Rs), while the LMLC-Cr cell possesses the lowest resistance values for transport of charges (Rct) and lithium ions diffusion (Zw) before and after cycling as depicted in Table 4. In contrast to that, the LMLC-Fe cell reveals the maximum values for Rs, Rct and Zw among the three cells before and after cycling that may lead to poor electrochemical performance upon cycling at different current rates or for large number of cycles. The reason for such behavior because LMLC-Fe sample has the lowest degree of transition elements occupation inside the Li–O layers and lowest amount of layered Li2MnO3 structure of (Li[Li1/3Mn2/3]O2) type as previously discussed in the XRD results.
3.4.3 Rate performance and cycle life
The electrode geometrical area was 1.1 cm2; the active mass loadings of LMLC-Al, LMLC-Cr or LMLC-Fe were 4.01, 3.67 and 4.93 mg, respectively, and the calculated theoretical capacities were as follows: 278.4, 277.46 and 277.63 mAh/g. The practical discharge capacities from selected test cells of the three cathode materials as a function of the C-rate are presented in Fig. 8. The coulombic efficiencies during the formation cycle were about 69, 60 and 50% for LMLC-Cr, LMLC-Al and LMLC-Fe, respectively. The low coulombic efficiency of the layered NCM electrodes is well described in the literature [60,61,62,63]. However, a low initial coulombic efficiency after formation is not a necessary prerequisite for bad electrode performance, since it usually rises in the subsequent cycles, reaching 90–99%, as it was also in the case at hand.
In the initial cycle after formation, the mean discharge capacities at 0.1C were 104, 93 and 95 mAh/g for LMLC-Al, LMLC-Cr and LMLC-Fe cells, respectively. At 1 C the LMLC-Al and LMLC-Cr materials maintained around 70% of their initial capacity at 0.1C while the LMLC-Fe exhibited capacity fade down to ca. 60%. Generally, during the galvanostatic tests all the layered M-doped LMLC cathode materials showed around 65% lower capacities than the theoretically calculated and a relatively poor rate capability, particularly after 2C discharge rate (Fig. 8).
Figure 9 shows the subsequent long-term cycling (500 cycles) performances at 1C/2C of charge–discharge rate. During the long-term cycling the LMLC-Fe cathode showed the highest capacity decline down to zero, already after 360 cycles. This result is also in accordance with the measured EIS, where it was found that the cell with Fe-doped LMLC showed the maximum charge transfer resistance and a relatively poor Li+ ion diffusion between the electrolyte and electrode surface. The capacity of LMLC-Al after 500 cycles faded to 7% of the initial one, before the galvanostatic C-rate tests. Meanwhile, the LMLC-Cr maintained approximately 10 mAh/g after 500 cycles, which corresponds to ca. 10% of the initial capacity before the C-rate testing.
According to the galvanostatic tests, the Al-doped LMLC cell exhibits the highest capacity after formation, until 0.1C. Thereafter, its capacity output mostly overlaps with those of LMLC-Cr up to 4 C and both materials perform generally better as the LMLC-Fe. The higher the C-rate, the less the capacity difference between the samples as displayed in Fig. 10. The compared results of mean discharge capacities at different C-rates are in a good agreement with CV and EIS measurements. The excellent storage capabilities of LMLC-Al at initial cycles and lower C-rates correspond to the highest occupation of Li–O layers in the Li2MnO3 structure resulting in large extent of lithium ion extraction. The improved electrochemical behavior for LMLC-Cr cathode at higher C-rates could be assigned to the smallest crystallite/particle size and high electronic conductivity as well as the enhanced diffusion of lithium ions [64, 65].
4 Conclusions
The present study successfully employed the facile processing of three kinds of LMLC materials via solvothermal method. The as-prepared electrode materials were comparatively inspected for their crystal structure, chemical composition and microstructure through the advanced characterization techniques e.g. XRD, XPS, FE-SEM and HR-TEM. The results indicated that doping of LMLC with very small percentage of Al3+ causes significant increase in the crystallite size and cell volume which enable hosting larger extent of Li–O layers in the layered framework structure. Moreover, as Cr-doped LMLC material exhibited the smallest particle size, it also demonstrated the best electronic and ionic conductivity. Although LMLC-Fe recorded the highest Hc value, it also possessed the lowest Ms and Mr values. The Al-doped LMLC layered cathode indicated an improved electrochemical storage behavior during initial formation cycles due to the improved structure stability with good Li extraction giving rise in the electrolyte conductivity. The incorporation of 1% Cr3+ to the LMLC layered structure relatively improves the rate capability performance over 50 cycles and promotes the cyclability over subsequent 500 cycles. Nevertheless, Fe-doped layered LMLC showed the maximum charge transfer resistance and poor Li+ ion diffusion between the electrolyte and electrode surface.
References
A.M. Gaikwad, B.V. Khau, G. Davies, B. Hertzberg, D.A. Steingart, A.C. Arias, A high areal capacity flexible lithium-ion battery with a strain-compliant design. Adv. Energy Mater. 5, 1401389 (2015). https://doi.org/10.1002/aenm.201401389
M.S. Whittingham, Introduction: batteries. Chem. Rev. 114, 11413–11413 (2014). https://doi.org/10.1021/cr500639y
Z. Lu, D.D. Macneil, J.R. Dahn, Layered cathode materials Li[Nix(2/3–x/3)]O2 for lithium-ion batteries. Electrochem. Solid-State Lett. 34, 191 (2001). https://doi.org/10.1149/1.1407994
M.M. Thackeray, S.H. Kang, C.S. Johnson, J.T. Vaughey, S.A. Hackney, Comments on the structural complexity of lithium-rich Li1+ xM1− xO2 electrodes (M= Mn, Ni, Co) for lithium batteries. Electrochem. Commun. 8, 1531–1538 (2006). https://doi.org/10.1016/j.elecom.2006.06.030
X.J. Guo, Y.X. Li, M. Zheng, J.M. Zheng, J. Li, Z.L. Gong, Y. Yang, Structural and electrochemical characterization of xLi[Li]1/3Mn2/3O2-(1-x)Li[Ni1/3Mn1/3Co1/3]O2 (0<x<0.9) as cathode materials for Li ion batteries. J Power Sources 184, 414–419 (2008). https://doi.org/10.1016/j.jpowsour.2008.04.013
H. Yu, H. Kim, Y. Wang, P. He, D. Asakura, Y. Nakamura, H. Zhou, High-energy composite layered manganese-rich cathode materials via controlling Li2MnO3 phase activation for lithium-ion batteries. Phys. Chem. Chem. Phys. 14, 6584–6595 (2012). https://doi.org/10.1039/C2CP40745K
H. Pan, T. Zhu, S. Zhang, Y. Jiang, J. Chen, M. Gao, Y. Liu, Li- and Mn-rich layered oxide cathode materials for lithium-ion batteries: a review from fundamentals to research progress and applications. Mol. Syst. Des. Eng. 3, 748–803 (2018). https://doi.org/10.1039/c8me00025e
A. Manthiram, J.C. Knight, S.-T. Myung, S.-M. Oh, Y.-K. Sun, M. Gu, I. Belharouak, Nickel-rich and lithium-rich layered oxide cathodes: progress and perspectives. Adv. Energy Mater. 6, 1501010 (2016). https://doi.org/10.1002/aenm.201501010
X. Yang, X. Wang, Q. Wei, H. Shu, L. Liu, S. Yang, B. Hu, Y. Song, G. Zou, L. Hu, L. Yi, Synthesis and characterization of a Li-rich layered cathode material Li1.15[(Mn1/3Ni1/3Co1/3)0.5(Ni1/4Mn3/4)0.5]0.85O2 with spherical core–shell structure. J. Mater. Chem. 22, 19666–19672 (2012). https://doi.org/10.1039/C2JM34259F
H.J. Yu, H.S. Zhou, High-energy cathode materials (Li2MnO3–LiMO2) for lithium-ion batteries. J. Phys. Chem. Lett. 4, 1268–1280 (2013). https://doi.org/10.1021/jz400032v
X. Dong, Y.L. Xu, S. Yan, S.C. Mao, L.L. Xiong, X.F. Sun, Towards low-cost, high energy density Li2MnO3 cathode materials. J. Mater. Chem. A 3, 670–679 (2015). https://doi.org/10.1039/C4TA02924K
J.R. Croy, D. Kim, M. Balasubramanian, K. Gallagher, S.H. Kang, M.M. Thackeray, Countering the voltage decay in high capacity xLi2MnO3-(1–x)LiMO2 electrodes (M: Mn Ni, Co) for Li+-ion batteries. J. Electrochem. Soc. 159, A781–A790 (2012). https://doi.org/10.1149/2.080206jes
S. Yang, G. Huang, S. Hu, X. Hou, Y. Huang, M. Yue, G. Lei, Improved electrochemical performance of the Li1.2Ni0.13Co0.13Mn0.54O2 wired by CNT networks for Li-ion batteries. Mater. Lett. 118, 8–11 (2014). https://doi.org/10.1016/j.matlet.2013.11.071
J.R. Croy, M. Balasubramanian, K.G. Gallagher, A.K. Burrell, Review of the U.S. Department of Energy’s “deep dive” to understand voltage fade in Li- and Mn-rich cathodes. Acc. Chem. Res. 48, 2813–2821 (2015). https://doi.org/10.1021/acs.accounts.5b00277
A. Kumar, R. Nazzario, L. Torres-Castro, A. Pena-Duarte, M.S. Tomar, Electrochemical properties of MgO-coated 0.5 Li2MnO3–0.5 LiNi0.5Mn0.5O2 composite cathode material for lithium ion battery. Int. J. Hydrog. Energy 40, 4931–4935 (2015)
L. Zhong, H. Jian-He, H. Gang, L. Lu, Effect of FePO4 coating on performance of Li1.2Mn0.54Ni0.13Co0.13O2 as cathode material for Li-ion battery. J. Inorg. Mater. 30, 129–134 (2015)
S. Yamamoto, H. Noguchi, W.W. Zhao, Improvement of cycling performance in Ti substituted 0.5Li2MnO3–0.5LiNi0.5Mn0.5O2 through suppressing metal dissolution. J. Power Sources 278, 76–86 (2015). https://doi.org/10.1016/j.jpowsour.2014.12.038
H. Chen, Q. Hu, Z. Huang, Z. He, Z. Wang, H. Guo, X. Li, Synthesis and electrochemical study of Zr-doped Li[Li0.2Mn0.54Ni0.13Co0.13]O2 as cathode material for Li-ion battery. Ceram. Inter. 42, 263–269 (2016). https://doi.org/10.1016/j.ceramint.2015.08.104
Y. Zang, C. Ding, X. Wang, Z. Wen, C. Chen, Molybdenum-doped lithium-rich layered-structured cathode material Li1.2Ni0.2Mn0.6O2 with high specific capacity and improved rate performance. Electrochem. Acta 168, 234–239 (2015). https://doi.org/10.1016/j.electacta.2015.03.223
Z. He, Z. Wang, H. Guo, X. Li, P. Yue, J. Wang, X. Xiong, Synthesis and electrochemical performance of xLi2MnO3·(1–x)LiMn0.5Ni0.4Co0.1O2 for lithium ion battery. Powder Technol. 235, 158–162 (2013). https://doi.org/10.1016/j.powtec.2012.09.020
H. Xu, S. Deng, G. Chen, Improved electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 by Mg doping for lithium ion battery cathode material. J. Mater. Chem. 2, 15015–15021 (2014). https://doi.org/10.1039/C4TA01790K
H. Li, H. Guo, Z. Wang, J. Wang, X. Li, N. Chen, W. Gui, Improving rate capability and decelerating voltage decay of Li-rich layered oxide cathodes by chromium doping. Inter. Journal Hydr. Ener. 43, 11109–11119 (2018). https://doi.org/10.1016/j.ijhydene.2018.04.203
F. Nobili, F. Croce, R. Tossici, I. Meschini, P. Reale, R. Marassi, Sol–gel synthesis and electrochemical characterization of Mg-/Zr-doped LiCoO2 cathodes for Li-ion batteries. J. Power Sources 197, 276–284 (2012). https://doi.org/10.1016/j.jpowsour.2011.09.053
L. Croguennec, J. Bains, J. Bréger, C. Tessier, P. Biensan, S. Levasseur, C. Delmas, Effect of aluminum substitution on the structure, electrochemical performance and thermal stability of Li1+x(Ni0.40Mn0.40Co0.20−zAlz)1−xO2. J. Electrochem. Soc. 158, A664–A670 (2011). https://doi.org/10.1149/1.3571479
C. Lu, S. Yang, H. Wu, Y. Zhang, X. Yang, T. Liang, Enhanced electrochemical performance of Li-rich Li1.2Mn0.52Co0.08Ni0.2O2 cathode materials for Li-ion batteries by vanadium doping. Electrochem. Acta 209, 448–455 (2016). https://doi.org/10.1016/j.electacta.2016.05.119
X. Feng, Y. Gao, L. Ben, Z. Yang, Z. Wang, L. Chen, Enhanced electrochemical performance of Ti-doped Li1.2Mn0.54Co0.13Ni0.13O2 for lithium-ion batteries. J. Power Sources 317, 74–80 (2016). https://doi.org/10.1016/j.jpowsour.2016.03.101
Y. Zhao, G. Sun, R. Wu, Synthesis of nanosized Fe-Mn based Li-rich cathode materials for lithium-ion battery via a simple method. Electroch. Acta 96, 291–297 (2013). https://doi.org/10.1016/j.electacta.2013.01.059
Q.-Q. Qiao, L. Qin, G.-R. Li, Y.-L. Wang, X.-P. Gao, Sn-stabilized Li-rich layered Li(Li0.17Ni0.25Mn0.58)O2 oxide as a cathode for advanced lithium-ion batteries. J. Mater. Chem. A 3, 17627–17634 (2015). https://doi.org/10.1039/C5TA03415A
C.-C. Wang, Y.-C. Lin, P.-H. Chou, Mitigation of layer to spinel conversion of a lithium-rich layered oxide cathode by substitution of Al in a lithium ion battery. RSC Adv. 5, 68919–68928 (2015). https://doi.org/10.1039/C5RA11665A
P.K. Nayak, J. Grinblat, M. Levi, E. Levi, S. Kim, J.W. Choi, D. Aurbach, Al doping for mitigating the capacity fading and voltage decay of layered Li and Mn-rich cathodes for Li-ion batteries. Adv. Energy Mater. (2016). https://doi.org/10.1002/aenm.201502398
P.K. Nayak, J. Grinblat, E. Levi, M. Levi, B. Markovsky, D. Aurbach, Understanding the influence of Mg doping for the stabilization of capacity and higher discharge voltage of Li- and Mn-rich cathodes for Li- ion batteries. Phys. Chem. Chem. Phys. 19, 6142–6152 (2017). https://doi.org/10.1039/C6CP07383B
Y.-J. Kang, J.-H. Kim, S.-W. Lee, Y.-K. Sun, The effect of Al(OH)3 coating on the Li[Li0.2Ni0.2Mn0.6]O2 cathode material for lithium secondary battery. Electroch. Acta 50, 4784–4791 (2005). https://doi.org/10.1016/j.electacta.2005.02.032
T. Zhao, S. Chen, L. Li, X. Zhang, R. Chen, I. Belharouak, F. Wu, K, , Amine, Synthesis, characterization, and electrochemistry of cathode material Li[Li0.2Co0.13Ni0.13Mn0.54]O2 using organic chelating agents for lithium-ion batteries. J. Power Sources 228, 206–213 (2013). https://doi.org/10.1016/j.jpowsour.2012.11.099
F. Fu, Y.Y. Huang, P. Wu, Y.K. Bu, Y.B. Wang, J.N. Yao, Controlled synthesis of lithium-rich layered Li1.2Mn0.56Ni0.12Co0.12O2 oxide with tunable morphology and structure as cathode material for lithium-ion batteries by solvo/hydrothermal methods. J. Alloys Compd. 618, 673–678 (2015). https://doi.org/10.1016/j.jallcom.2014.08.191
E. Markevich, G. Salitra, Y. Talyosef, U.-H. Kim, H.-H. Ryu, Y.-K. Sun, D. Aurbach, High performance LiNiO2 cathodes with practical loading cycled with Li metal anodes in fluoroethylene carbonate based electrolyte solution. ACS Appl. Energy Mater. 1, 2600–2607 (2018). https://doi.org/10.1021/acsaem.8b00304
A. Boulineau, L. Croguennec, C. Delmas, F. Weill, Structure of Li2MnO3 with different degrees of defects. Solid State Ionics 180, 1652–1659 (2010). https://doi.org/10.1016/j.ssi.2009.10.020
S. Alagar, C.-C. Yang, C. Karuppiah, R. Madhuvilakku, S. Piraman, Temperature-controlled synthesis of Li- and Mn-Rich Li1.2Mn0.54Ni0.13Co0.13O2 hollow nano/sub-microsphere electrodes for high-performance lithium-ion battery. ACS Omega 4(23), 20285–20296 (2019). https://doi.org/10.1021/acsomega.9b02766
B. Seteni, N. Rapulenyane, J.C. Ngila, H. Luo, Structural and electrochemical behavior of Li1.2Mn0.54Ni0.13Co0.13-xAlxO2 (x = 0.05) positive electrode material for lithium ion battery. Mater Today Proc 5, 10479–10487 (2018). https://doi.org/10.1016/j.matpr.2017.12.379
S. Rajarathinam, S. Mitra, R.K. Petla, Li2MnO3 rich-LiMn0.33Co0.33Ni0.33O2 integrated nano-composites as high energy density lithium-ion battery cathode materials. Electrochem. Acta 108, 135–144 (2013). https://doi.org/10.1016/j.electacta.2013.06.102
D. Mohanty, A.S. Sefat, S. Kalnaus, J. Li, R.A. Meisner, E.A. Payzant, D.P. Abraham, D.L. Wood, C. Daniel, Investigating phase transformation in the Li1.2Co0.1Mn0.55Ni0.15O2 lithium-ion battery cathode during high-voltage hold (4.5 V) via magnetic, X-ray diffraction and electron microscopy studies. J. Mater. Chem. A 1, 6249–6261 (2013). https://doi.org/10.1039/C3TA10304H
Q. Xia, D.G. Ivey, X. Zhao, M. Xu, W. Wei, Z. Ding, J. Liu, L. Chen, A Li-rich layered@spinel@carbon heterostructured cathode material for high capacity and high rate lithium-ion batteries fabricated via an in situ synchronous carbonization-reduction method. J. Mater. Chem. A 3, 3995–4003 (2015). https://doi.org/10.1039/C4TA05848H
Y. Tian, M. Chen, S. Xue, Y. Cai, Q. Huang, X. Liu, W. Li, Template-determined microstructure and electrochemical performances of li-rich layered metal oxide cathode. J. Power Sources 401, 343–353 (2018). https://doi.org/10.1016/j.jpowsour.2018.09.010
K. Amine, H. Tukamoto, H. Yasuda, Y. Fujita, A new three-volt spinel Li1+xMn1.5Ni0.5O4 for secondary lithium batteries. J. Electrochem. Soc. 143, 1607–1613 (1996). https://doi.org/10.1149/1.1836686
K.M. Shaju, R.G.V. Subbu, B.V.R. Chowdari, Performance of layered LiNi1/3Co1/3Mn1/3O2 as cathode for Li-ion batteries. Electrochim. Acta 48, 145–151 (2002). https://doi.org/10.1016/S0013-4686(02)00593-5
J. Huang, X. Fang, Y. Wu, L. Zhou, Y. Wang, Y. Jin, W. Dang, L. Wu, Z. Rong, X. Chen, X. Tang, Enhanced electrochemical performance of LiNi0.8Co0.1Mn0.1O2 by surface modification with lithium-active MoO3. J. Electroanaly. Chem. 823, 359–367 (2018)
Y. Zhao, M. Xia, X. Hu, Z. Zhao, Y. Wang, Z. Lv, Effects of Sn doping on the structural and electrochemical properties of Li1.2Ni0.2Mn0.8O2 Li-rich cathode materials. Electrochim. Acta 174, 1167–1174 (2015). https://doi.org/10.1016/j.electacta.2015.05.068
Z. Chen, J. Wang, D.L. Chao, T. Baikie, L. Bai, S. Chen, Y. Zhao, T.C. Sum, J. Lin, Z. Shen, Hierarchical porous LiNi1/3Co1/3Mn1/3O2 nano-/micro spherical cathode material: minimized cation mixing and improved Li+ mobility for enhanced electrochemical Performance. Sci. Rep. 6, 25771 (2016). https://doi.org/10.1038/srep25771
J. Hong, K. Kang, H. Gwon, S.K. Jung, K. Ku, Review—Lithium- excesslayered cathodes for lithium rechargeable batteries. J. Electrochem. Soc. 162, A2447–A2467 (2015). https://doi.org/10.1149/2.0071514jes
H. Pan, S. Zhang, J. Chen, M. Gao, Y. Liu, T. Zhu, Y. Jiang, Li- and Mn-rich layered oxide cathode materials for lithium-ion batteries: a review from fundamentals to research progress and applications. Mol. Syst. Des. Eng. 3, 748–803 (2018). https://doi.org/10.1039/C8ME00025E
N.A. Chernova, M. Ma, J. Xiao, M.S. Whittingham, J.C. Jiménez, C.P. Grey, Magnetic studies of layered cathode materials for lithium ion batteries. Mater. Res. Soc. Symp. Proc. 972, 610 (2007). https://doi.org/10.1557/PROC-0972-AA06-10
N.A. Chernova, M. Ma, M. Jie Xiao, Stanley Whittingham, Julien Breger, Clare P. Grey, Layered LixNiyMnyCo1–2yO2 cathodes for lithium ion batteries: understanding local structure via magnetic properties. Chem. Mater. 19, 482–4693 (2007). https://doi.org/10.1021/cm0708867
M.N. Ates, S. Mukerjee, K.M. Abraham, A high rate Li-rich layered MNC cathode material for lithium-ion batteries. RSC Adv. 5, 27375–27386 (2015). https://doi.org/10.1039/C4RA17235C
Y. Errami, M. Ouassaid, M. Cherkaoui, M. Maaroufi, Maximum power point tracking control based on a nonlinear back stepping approach for a permanent magnet synchronous generator wind energy conversion system connected to a utility grid. Ener. Technol. 3, 743–757 (2015). https://doi.org/10.1002/ente.201500026
D. Harbaoui, M.M.S. Sanad, C. Rossignol, E. Hlil, N. Amdouni, S. Obbade, Synthesis and structural, electrical, and magnetic properties of new iron–aluminum alluaudite phases β-Na2Ni2M(PO4)3 (M = Fe and Al). Inorg. Chem. 56, 13051–13061 (2017). https://doi.org/10.1021/acs.inorgchem.7b01880
N. Murali, K.V. Babu, K.E. Babu, V. Veeraiah, Structural and conductivity studies of LiNi0.5Mn0.5O2 cathode materials for lithium-ion batteries. Mater. Sci-Pol. 34, 404–411 (2016)
N. Murali, V. Veeraiah, Preparation, dielectric and conductivity studies of LiNi1-xMgxO2 cathode materials for lithium-ion batteries. Process. Appl. Ceram. 11, 258–264 (2017). https://doi.org/10.2298/PAC1704258M
N. Murali, S.J. Margarette, V. Veeraiah, Synthesis, dielectric, conductivity and magnetic studies of LiNi1/3Co1/3Mn(1/3)−xAlxO2 (x=0.0, 0.02, 0.04 and 0.06) for cathode materials of lithium-ion batteries. Results Phys. 7, 1379–1388 (2017). https://doi.org/10.1016/j.rinp.2017.02.037
H. Arai, S. Okada, Y. Sakurai, J.-I. Yamaki, Reversibility of LiNiO2 cathode. Solid State Ionics 95, 275–282 (1997). https://doi.org/10.1016/S0167-2738(96)00598-X
S.S. Zhang, K. Xu, T.R. Jow, Formation of solid electrolyte interface in lithium nickel mixed oxide electrodes during the first cycling. Electrochem. Solid-State Lett. 5, A92–A94 (2002). https://doi.org/10.1149/1.1464506
J.P. Peres, C. Delmas, A. Rougier, M. Broussely, F. Perton, P. Biensan, P. Willmann, The relationship between the composition of lithium nickel oxide and the loss of reversibility during the first cycle. J. Phys. Chem. Solids 57, 1057–1060 (1996). https://doi.org/10.1016/0022-3697(95)00395-9
C. Delmas, J.P. Peres, A. Rougier, A. Demourgues, F. Weill, A. Chadwick, M. Broussely, F. Perton, P. Biensan, P. Willmann, On the behavior of LixNiO2 system: an electrochemical and structural overview. J. Power Sources 68, 120–125 (1997). https://doi.org/10.1016/S0378-7753(97)02664-5
J.R. Mueller-Neuhaus, R.A. Dunlap, J.R. Dahn, Understanding irreversible capacity in Lix Ni1 − y Fey O 2 cathode materials. J. Electrochem. Soc. 147, 3598–3605 (2000)
J. Choi, A. Manthiram, Investigation of the irreversible capacity loss in the layered LiCo1/3Ni1/3Mn1/3O2 cathodes. Electrochem. Solid-State Lett. 8, C102–C105 (2005). https://doi.org/10.1149/1.1943567
A.Y. Shenouda, M.M.S. , Sanad, Synthesis, characterization and electrochemical performance of Li2NixFe1−xSiO4 cathode materials for lithium ion batteries. Bull. Mater. Sci. 40, 1055–1060 (2017). https://doi.org/10.1007/s12034-017-1449-2
A. Abdel-Aziz, A.Y. Shenouda, M.M.S. Sanad, B. Kishore, H.F.Y. Khalil, M.M.B. El-Sabbah, Effect of ionic substitutions on the physicochemical, morphological, and electrochemical properties of lithium-rich vanadium phosphate and pyrophosphate compounds. Ionics 25, 969–980 (2019). https://doi.org/10.1007/s11581-019-02843-7
Acknowledgements
The author is grateful and thankful to Science and Technology Development Fund (STDF) for the financial support of the work through German-Egyptian Research Fund (GERF) grant ID: 23038. Great thanks are extended to the The Hydrogen and Fuel Cell Center–Duisburg (Germany) for performing the rate capability and long-term cycling test of batteries under the assistance and supervision of Dipl.-Ing. Bernd Oberschachtsiek and Dr. George Topalov at the department of electrolysis and batteries. Deep acknowledgments are presented to Dr. Sebastian Wennig (Hydrogen and Fuel Cell Center-Duisburg-Germany) for providing language and scientific help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sanad, M.M.S., Toghan, A. Unveiling the role of trivalent cation incorporation in Li-rich Mn-based layered cathode materials for low-cost lithium-ion batteries. Appl. Phys. A 127, 733 (2021). https://doi.org/10.1007/s00339-021-04884-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-021-04884-0