Abstract
Herein, we demonstrate a simple one-step method for synthesizing Ag NPs embedded free-standing PVA/chitosan (PVA/Cs) films. The influence of gamma radiation on the dielectric constants, dispersion, and optical characteristics of PVA/Cs/Ag nanocomposite films was studied. The results confirmed the successful formation of silver NPs in the matrix through the appearance of the surface plasmon absorption at 420 nm. The indirect energy gap decreases from 2.11 to 2.01 eV, while the direct energy gap decreases from 2.6 to 2.5 eV by increasing the irradiation doses. Besides, the oscillator energy and the dispersion energy are varied by increasing the irradiation doses. Furthermore, the optical conductivity increases by the increase in γ-doses but decreases at 15 kGy due to the degradation process. Overall, the optical characteristics of PVA/Cs/Ag nanocomposite films make them promising materials for flexible optoelectronic devices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Recently, the integration of metal nanoparticles into polymeric materials has introduced a new class of materials that are useful for many applications. The interest in polymer/metal nanocomposites rose because of their biological, optical, electronic, and magnetic applications. Polymers are now known to be excellent host material for metal nanoparticles, where the polymers can protect nanoparticles from aggregation and acting as reducing agents participating in NPs’ synthesis. The polymer matrix gives additional important properties such as processability, solubility, and thermal stability of the systems [1].
Polyvinyl alcohol (PVA) is a characteristic water-soluble and biocompatible polymer with many technological, pharmaceutical, and biomedical applications. PVA acts as a good host material due to its thermal stability, chemical resistance, and mechanical strength. PVA has gained attention due to its nontoxicity and its reduction ability [2, 3]. Chitosan (CS) is a linear polysaccharide derived from partial deacetylation of chitin. Chitosan is a copolymer comprising of N-acetyl-2-amino-2-deoxy-d-glucopyranose and 2-amino-2-deoxy-d-glucopyranose, where the two types of repeating units are linked together by (1 → 4)-glycosidic bonds. The chitosan has reactive functional groups, i.e., amino and hydroxyl groups; thus, it can be used as a reactive polymer template for stabilizing and size controlling metal nanoparticles. The chitosan has been extensively used due to its desirable properties and benefits that include low cost, nontoxicity, biodegradability, biocompatibility, and being environmentally friendly [4,5,6,7].
Polymers blending are an important method to have new materials with some desired properties varying from each component and is an alternative to the high cost of developing new materials [8]. Many studies have been carried out blending PVA with chitosan [9,10,11], where the blending is predictable to provide a good film with good chemical and mechanical assets as a result of the intermolecular interactions between PVA and chitosan. Also, the blending has much attention for medical and pharmaceutical applications related to their nontoxic, non-carcinogenic, and biocompatibility characterizations. The PVA–chitosan blend is easy to prepare related to its physical and chemical characterizations [12, 13].
Radiation modification of polymer is an attractive process for the creation of specific physical and optical properties. When polymers are exposed to ionizing radiation, ionization of the atoms and formation of a highly excited state have occurred. After that, the dissipation of excitation energy occurs in a number of ways, forming a very reactive intermediate, free radicals, ions, and excited states that causing cross-linking and/or degradation and in turn modifies the structure as well as properties of the polymer. The two processes (cross-linking and degradation) happen simultaneously, and one predominates depending upon the absorbed irradiation doses [14,15,16,17]. Moreover, for metal nanoparticles modification, gamma radiation is capable of regulating the particle size through changing the available the radiation parameters like applied irradiation doses or dose rate. This technique permits the effective loading of controllable metal nanoparticles inside polymeric films [18,19,20,21].
Silver nanoparticles have gained increasing attention due to their unique properties such as good chemical stability, electrical conductivity, antimicrobial activity as well as their extraordinary optical properties, which lead to wide applications in the fields of electronic and optical materials [22,23,24,25,26].
In the present study, we have been exploring the effects of gamma radiation on the dielectric constants, dispersion, optical characteristics of free-standing PVA/Cs/Ag nanocomposite films. The PVA/Cs/Ag nanocomposite films were characterized using XRD, FTIR, and UV/Vis spectroscopy.
2 Materials and methods
2.1 Materials
Silver nitrate was purchased from Nice chemicals PVT.LTD, India. Polyvinyl alcohol with a degree of polymerization ≈1700–1800 and M.wt = 18,000 gm/mol was provided from laboratory Rasayan, Cario, Egypt. Chitosan with deacetylation degree > 90%, viscosity = 50–300 and purity 90% was provided from Bio Basic Canada Inc.
2.2 Preparation of PVA/Cs/Ag nanocomposite film
PVA/Cs/Ag nanocomposite film was fabricated by a casting technique. First, 5 g PVA was dissolved in 100 mL of deionized water using a magnetic stirrer at 85 °C for 2 h until the complete uniformity. Second, 1 g chitosan was solubilized in 100 mL of 1% (v/v) aqueous acetic acid at 60 °C using a magnetic stirrer for 2 h until the polymer becomes completely soluble. Then, (70:30) ml of (PVA:Cs) was mixed and stirred for 1 h to ensure complete homogeneity. After that, 6 mM AgNO3 was dissolved in the blend solution and kept in stirring at room temperature for 1 h. This blend solution was then poured in glass Petri dish plate and left to dry in darkness for free-standing film formation of PVA/Cs/Ag nanocomposite. Finally, the PVA/Cs/Ag composite films were gamma-irradiated (0, 15, 25, 50 kGy) using Co-60 γ-cell-220 sources, at a dose rate equal 1.14 kGy/h, at National Center for Radiation Research and Technology, Egyptian Atomic Energy Authority (see Schematic 1).
2.3 Characterization techniques
The crystal structure the untreated and treated PVA/Cs/Ag nanocomposite films with γ-rays was obtained via (XRD 6000, Shimadzu) with power voltage 40 kV and current 30 mA [27,28,29]. UV–Vis spectrophotometry was utilized as an analytical tool to track the silver NPs formation inside the polymeric matrix. UV–Vis measurements were taken by a Unicam double beam UV–Vis spectrophotometer. The structural analysis by Fourier transform infrared spectroscopy in attenuated total reflection (ATR) mode was performed using Bruker Vertex 70 FTIR spectrophotometer.
3 Result and discussion
3.1 Structural analyses
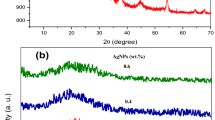
Figure 1 displays the XRD patterns for the untreated and treated PVA/Cs/Ag nanocomposite films with γ-rays. Also, the figure of PVA/Cs/Ag nanocomposite films indicates a notable peak of almost 2θ = 19.68° that interpreting its semicrystalline characteristics of PVA [30]. XRD patterns also reveal that no peaks of Ag NPs are evident; this proved the uniform distribution of Ag NPs within the PVA/Cs blend without aggregation emerging [31]. Further, we remarked that the peak’ intensity of irradiated PVA/Cs/Ag film with 15 kGy is higher than that for the pristine PVA/Cs/Ag nanocomposite film and then reduces with increasing the doses up to 50 kGy. These notes match the PVA/Cs/Ag film degradation and PVA/Cs/Ag film cross-linking processes [31]. The increase in intensity at 15 kGy indicates an increase in ordering due to degradation (chain scission) that arising due to the ionizing effect of gamma which reduces the intermolecular stress in the amorphous portions. Consequently increases the mobility of the chains that allowing the reordering of particular molecules. On the other hand, the reduction in the peak intensity of PVA/Cs/Ag nanocomposite films means a decreasing in the degree of crystallinity of films through crosslinking. That can be correlated to the decrease in the particle size in the films due to enhancement the bonding between Ag NPs and the polymer blend with increasing these doses up to 50 kGy, as examined earlier in [32]. On other words, the marked degradation for PVA/Cs/Ag film at 15 kGy induces the repulsion occurring within the Ag NPs molecules, increments the degree of the arrangement characteristics of the PVA/Cs chains (i.e., decline in the amorphous appearance of PVA/Cs) of the PVA/Cs/Ag nanocomposite films. The irradiation with a gamma dose of 15 kGy promotes the hydrogen bonding between Ag NPs and the PVA/Cs blend’ chains. Also, it supports the Ag NPs, which possess a crystalline characteristic, to be embedded into the PVA/Cs matrix, hence decreasing the intermolecular stress and intensifying the order arrangement [31,32,33], while at doses (25 kGy and 50 kGy), the intensity declines. This indicates that the free radicals were created in the PVA/Cs/Ag films. Subsequently, these radicals are chemically active and prompted the PVA/Cs/Agcross-linking that leads an emerging extraordinary intermolecular interaction between the chains of PVA/Cs blend and Ag NPs, and this behavior was noticed in numerous investigations [31, 33, 34], Alhazime et al. [33] have reported occurring the degradation for Pani-Ag/PVA films after exposed to at \(\gamma\)-rays dosage between 20 and 40 kGy, while the cross-linking for the chain of the irradiated Pani-Ag/PVA films was observed at \(\gamma\)-rays dosage between 80 kGy and 150 kGy.
For investigating the gamma radiation effect on PVA/Cs/Ag nanocomposite film, the FTIR spectra of pristine and γ-irradiated PVA/Cs/Ag nanocomposite films are illustrated in Fig. 2. In the spectrum of pristine PVA/Cs/Ag nanocomposite film, a strong, broadband around 3299 cm−1 is due to N–H and O–H stretching vibrations. The absorption peaks in the 2800–2900 cm−1 region are due to the stretching vibrations of the CH bonds. The peaks at 1729 and 1569 cm−1 are attributed to the stretching C=O and bending N–H vibration, respectively [35, 36]. The band at around 1089 cm−1 corresponds to C–O stretching vibration [10, 37, 38].
Upon exposure to gamma rays, the spectra of PVA/Cs/Ag nanocomposites exhibit the same feature of the pristine film, but the intensity of the interaction peaks with silver NPs [39] was changed: First, a slight shifting and decrease in intensity of the peak at 3299 cm−1 of hydroxyl (–OH) and amino (–NH) groups. Second, the C=O stretching vibration band at 1629 cm−1 is shifted to 1621 cm−1. Third, NH band at 1569 cm−1 was shifted to 1561 cm−1 with a decrease in its intensity. These observations suggest the particle size of silver nanoparticles was affected due to gamma radiation.
3.2 Optical properties of PVA/Cs/Ag nanocomposite films
3.2.1 UV/Vis absorption spectra
Figure 3 shows the UV/Vis absorption spectra for the untreated and treated PVA/Cs/Ag nanocomposite films with γ-rays. Apparently, the free-standing PVA/Cs/Ag nanocomposite film displays strong absorption in the visible region at 420 nm which is attributed to the surface plasmon resonance (SPR) of silver NPs [19]. When PVA/Cs/Ag nanocomposite film were irradiated by gamma rays, the SPR band undergoes a significant blue shift with increasing the irradiation dose, which meaning the formation of a smaller nano-sized particles (i.e., quantum confinement effect) [40]. The synthetic mechanism of Ag NPs inside polymeric matrix obtained by reduction of AgNO3 by PVA/Cs polymer blend, which behaves as a reducing and stabilizing agent as observed by UV–visible spectroscopy. The Ag NPs formation mechanism is based on the coordination of Ag+ by NH2, OH and C=O function groups of Cs and PVA [39], followed by the complete reduction by γ-irradiation. It is important to note that the polymeric matrix acts as a capping and/or stabilizing agent for silver NPs synthesis and growth. Upon exposure to gamma rays, the individual macromolecules of polymer are cross-linked with each other forming a three-dimensional network [41,42,43]. The percent of polymer chains surrounding the nanoparticle increased by cross-linking of polymer molecules, and this prevents the aggregation of nanoparticles; as a result, smaller-sized particles are generated [43].
3.2.2 The reflectance and transmittance spectra of PVA/Cs/Ag nanocomposite films
Figure 4A presents the T(λ) spectra for the untreated and treated PVA/Cs/Ag nanocomposite films with γ-rays. We notice the absorption edge for the untreated PVA/Cs/Ag nanocomposite film with γ-rays near 420 nm, and for different doses, the absorption edge shifted to lower wavelengths. Also, it is seen that the value of T(λ) for the untreated and treated PVA/Cs/Ag nanocomposite films with γ-rays was growth for the irradiated film with 15 kGy and then reduced sharply as gamma doses increased from 25 to 50 kGy. This result is perfectly consistent with the obtained results from XRD and UV/Vis absorbance spectra. This trend is attributed to a decrease in the energy bandgap. Also, the R(λ) spectra behave the same trend with the variation in the γ-doses (Fig. 4B), but R(λ) decreases for the irradiated film with 15 kGy and then increases as the gamma doses increase up to 50 kGy [32].
3.2.3 Estimating of optical bandgap energy
The optical bandgap energy (Eg) for the untreated and treated PVA/Cs/Ag nanocomposite films with γ-rays can be evaluated via Tauc’ equation [28, 44]
where B is represented a constant and t designates to the transitions’ type, the optical energy gap is achieved by scheming (αhν)1/r against (hν) as shown in Fig. 5A, B. We examined that the energy gap of PVA/Cs/Ag nanocomposite films reduces with boosting the irradiation doses (Table 1). The decline in the value of Eg of PVA/Cs/Ag nanocomposite films may be attributable to the formation of chemical bonding that initiated between PVA/Cs blend chains and Ag NPs. This configuration is satisfying the creation of localized states within the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) band edges [45,46,47,48]. This enhances the micro-strain and the values of dislocation density, leading to creating crystal deficits inside the Ag NPs’ lattice, consequently reducing the value of Eg [33, 49, 50].
3.2.4 Refractive index and dispersions constants
The extinction coefficient (k) and the refractive index (n) for the untreated and treated PVA/Cs/Ag nanocomposite films with γ-rays can be estimated via [51,52,53]:
Figure 6A, B displays the relationship between (n and k) versus λ (nm) for the untreated and treated PVA/Cs/Ag nanocomposite films with γ-rays.
The refractive index decreases for the irradiated PVA/Cs/Ag nanocomposite film with 15 kGy of γ-rays and then increasing as the γ-ray doses increased up to 50 kGy as illustrated in Fig. 6A. The irradiated the PVA/Cs/Ag nanocomposite film with 15 kGy of γ-rays, the refractive index declines attributable to the degradation mechanism occurring in PVA/Cs/Ag nanocomposite film. While the increase in γ-rays doses from 25 to 50 kGy, the γ-rays dose promotes the production of free radicals that are chemically active, supporting the generation of covalent bonds between diverse chains (cross-linking) and leading to an increase in refractive index [54]. Also, Fig. 6B illustrates that the k spectra for the untreated and treated PVA/Cs/Ag nanocomposite films with γ-rays containing band around 420 nm are attributed to the surface plasmon resonance (SPR) of silver NPs [19], this band shifted to lower wavelengths as γ-rays doses increases [49, 55].
3.2.5 Dispersion and the dielectric parameters
The oscillator energy Eo, the dispersion energy Ed for the untreated and treated PVA/Cs/Ag nanocomposite films with γ-rays are estimated (Table 1) by the single oscillator model and the Wemple and DiDomenico relationship [56].
Figure 7 exhibits the (n2 − 1)−1 versus (hν)2 plots for the untreated and treated PVA/Cs/Ag nanocomposite films with γ-rays. The remarkable influence of γ-irradiation on the (Eo and Ed) energies of PVA/Cs/Ag nanocomposite films was detected. We see that the oscillator and the dispersion energies besides the oscillator strength decrease in the case of 15 kGy then increase in the case of 25 kGy and 50 kGy.
The static refractive index n0 (at hυ = 0), the static dielectric constant, εs, and optical oscillator strengths (f) for the untreated and treated PVA/Cs/Ag nanocomposite films with γ-rays were estimated via the relations [57, 58]:
The values of n0 and εs for the untreated and treated PVA/Cs/Ag nanocomposite films with γ-rays are calculated, where it is clear that the n0 and εs values were increased at the gamma dose of 15 kGy and then decreased at the γ-doses 25 kGy and 50 kGy (see Table 1) [58]. While the optical oscillator strengths was decreased at 15 kGy and then increased at the γ-doses 25 kGy and 50 kGy.
The real and imaginary parts (εr and εi) of the dielectric constant for the untreated and treated PVA/Cs/Ag nanocomposite films with γ-rays were estimated via the following relations [59, 60].
The electronic band structure of PVA/Cs/Ag nanocomposite films is closely dependent on dielectric constants. Figure 8A, B exhibits the dielectric constants (εr and εi) or the untreated and treated PVA/Cs/Ag nanocomposite films with γ-rays. We noted that improved both dielectric constants with the rising in the photon energy, while these constants decline at the gamma dose of 15 kGy and then increased for the γ-doses 25 kGy and 50 kGy.
The ratio of free carrier concentration to the effective mass (N/m*) and the lattice dielectric constant, εL, for the untreated and treated PVA/Cs/Ag nanocomposite films with γ-rays which were estimated via Eq. [61]:
Figure 9 presents the (n2 − k2) versus λ2 plots for the untreated and treated PVA/Cs/Ag nanocomposite films with γ-rays. These parameters decreased at the gamma dose of 15 kGy and then increased for the γ-doses 25 kGy and 50 kGy (see Table 1).
3.2.6 Volume and surface energy loss functions
The energy loss functions through the volume and surface (VELF and SELF) display the expected loss in energy for the fast electrons that passing in bulk and at the surface of the material. The VELF and SELF functions were evaluated via equations [62]:
Figure 10A, B presents the VELF and SELF versus (hυ) parts for the untreated and treated PVA/Cs/Ag nanocomposite films with γ-rays. Both energy loss through bulk (VELF) and surface (SELF) behave roughly the same as previously observed behavior for the other optical parameters with radiation doses. Further, the VELF function values were more in magnitude than SELF values. This proved that the transition energy for the electrons is changeable as an effect of the ionizing dose alteration [63].
3.2.7 The optical conductivity
The optical conductivity (σopt) initiated as a result of charge carriers that transported under an alternating electric field of the incident electromagnetic waves. The real and imaginary (σr and σi) parts of the optical conductivity can be assessed utilizing the subsequent relations [64].
Figure 11A, B presents both (σr and σi) parts for the untreated and treated PVA/Cs/Ag nanocomposite films with γ-rays. It is discerned that the increase in the γ-doses enhances the optical conductivity. The notable decrease in optical conductivity for the PVA/Cs/Ag nanocomposite film at 15 kGy is due to degradation process for PVA/Cs blend.
4 Conclusion
A simple one-step method for synthesizing Ag nanoparticles embedded free-standing PVA/Cs films and then exposed to gamma irradiation is presented. The nanocomposite films were confirmed by XRD, FTIR, and UV–VIS absorption spectroscopy. The indirect energy gap decreases from 2.11 to 2.01 eV, while the indirect energy gap declines from 2.60 to 2.50 eV by increasing the irradiation doses. The refractive index decreases for the irradiated PVA/Cs/Ag nanocomposite film with 15 kGy of γ-rays and then increasing as the γ-ray doses increased up to 50 kGy. These notes match the PVA/Cs/Ag film degradation and PVA/Cs/Ag film cross-linking processes. Also, the dielectric constants of PVA/Cs/Ag thin films display an increase as both the photon energy and γ-doses were boosting. Besides, a remarkable influence of γ-irradiation on the oscillator and the dispersion energies of PVA/Cs/Ag nanocomposite films was detected. Further, static refractive index and the static dielectric constant values were increased at the gamma dose of 15 kGy and then decreased for the γ-doses 25 kGy and 50 kGy. Furthermore, it is discerned that the increase in the γ-doses enhances the optical conductivity. The notable decrease in optical conductivity for γ-irradiated PVA/Cs/Ag nanocomposite film with 15 kGy is due to the film degradation. Based on the preceding, the dielectric, dispersion, optical characteristics of irradiated PVA/chitosan/Ag nanocomposite films qualify them as proper materials for flexible optoelectronic devices.
References
N. Rouabah, B. Boudine, R. Nazir, M. Zaabat, M. Sebais, O. Halimi, M.T. Soltani, A. Chala, Structural, optical and photocatalytic properties of PVC/CdS nanocomposites prepared by soft chemistry method. J. Inorg. Organomet. Polym. Mater. 31(3), 1102–1110 (2021)
P.V. Gaikwad, S.K. Sharma, K. Sudarshan, V. Kumar, A. Kshirsagar, P.K. Pujari, Molecular packing of polyvinyl alcohol in PVA-gold nanoparticles composites and its role on thermo-mechanical properties. Polym. Compos. 39(4), 1137–1143 (2018)
A.A. Menazea, A.M. Mostafa, E.A. Al-Ashkar, Effect of nanostructured metal oxides (CdO, Al2O3, Cu2O) embedded in PVA via Nd:YAG pulsed laser ablation on their optical and structural properties. J. Mol. Struct. 1203, 127374 (2020)
S.K. Shukla, A.K. Mishra, O.A. Arotiba, B.B. Mamba, Chitosan-based nanomaterials: a state-of-the-art review. Int. J. Biol. Macromol. 59, 46–58 (2013)
S. El-Sayed, A.M. El Sayed, Synthesis, characterisation, dielectric, and optical properties of the chitosan/poly(ethylene glycol)/magnesia biopolymer nanocomposites. Mater. Technol. 34(10), 602–614 (2019)
K. Kalantari, E. Mostafavi, B. Saleh, P. Soltantabar, T.J. Webster, Chitosan/PVA hydrogels incorporated with green synthesized cerium oxide nanoparticles for wound healing applications. Eur. Polym. J. 134, 109853 (2020)
R.P. Sharma, S.D. Raut, A.S. Kadam, R.M. Mulani, R.S. Mane, In-vitro antibacterial and anti-biofilm efficiencies of chitosan-encapsulated zinc ferrite nanoparticles. Appl. Phys. A 126(10), 824 (2020)
A.Y. Yassin, Dielectric spectroscopy characterization of relaxation in composite based on (PVA–PVP) blend for nickel–cadmium batteries. J. Mater. Sci. Mater. Electron. 31(21), 19447–19463 (2020)
A.M. Pandele, M. Ionita, L. Crica, E. Vasile, H. Iovu, Novel Chitosan-poly(vinyl alcohol)/graphene oxide biocomposites 3D porous scaffolds. Compos. B Eng. 126, 81–87 (2017)
K.E. Bourakadi, N. Merghoub, M. Fardioui, M.E.M. Mekhzoum, I.M. Kadmiri, E.M. Essassi, A.E.K. Qaiss, R. Bouhfid, Chitosan/polyvinyl alcohol/thiabendazoluim-montmorillonite bio-nanocomposite films: mechanical, morphological and antimicrobial properties. Compos. Part B Eng. 172, 103–110 (2019)
S.K. Lakkaboyana, K. Soontarapa, Vinaykumar, R.K. Marella, K. Kannan, Preparation of novel chitosan polymeric nanocomposite as an efficient material for the removal of Acid Blue 25 from aqueous environment. Int. J. Biol. Macromol. 168, 760–768 (2021)
J.H. Kim, J.Y. Kim, Y.M. Lee, K.Y. Kim, Properties and swelling characteristics of cross-linked poly(vinyl alcohol)/chitosan blend membrane. J. Appl. Polym. Sci. 45(10), 1711–1717 (1992)
M.J. Tommalieh, H.A. Ibrahium, N.S. Awwad, A.A. Menazea, Gold nanoparticles doped polyvinyl alcohol/chitosan blend via laser ablation for electrical conductivity enhancement. J. Mol. Struct. 1221, 128814 (2020)
V. Rao, 14 - Radiation processing of polymers. in Advances in Polymer Processing, ed. by S. Thomas, Y. Weimin (Woodhead Publishing, 2009), pp. 402–437
A.G. Chmielewski, M. Haji-Saeid, S. Ahmed, Progress in radiation processing of polymers. Nucl. Instrum. Methods Phys. Res., Sect. B 236(1), 44–54 (2005)
V. Ravindrachary, Ismayil, S.P. Nayak, D. Dutta, P.K. Pujari, Free volume related fluorescent behavior in electron beam irradiated chalcone doped PVA. Polym.er Degrad. Stab. 96(9), 1676–1686 (2011)
Ismayil, R. Vasachar, R.F. Bhajantri, P.S. Dhola, G. Sanjeev, Impact of electron-beam irradiation on free-volume related microstructural properties of PVA:NaBr polymer composites. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 342, 29–38 (2015)
Z.I. Ali, O.A. Ghazy, G. Meligi, H.H. Saleh, M. Bekhit, Radiation-induced synthesis of copper/poly(vinyl alcohol) nanocomposites and their catalytic activity. Adv. Polym. Technol. 37(2), 365–375 (2018)
M. Bekhit, M.N. Abu el-naga, R. Sokary, R.A. Fahim, N.M. El-Sawy, Radiation-induced synthesis of tween 80 stabilized silver nanoparticles for antibacterial applications. J. Environ. Sci. Health Part A 55(10), 1210–1217 (2020)
R. Sokary, M.N. Abu el-naga, M. Bekhit, S. Atta, A potential antibiofilm, antimicrobial and anticancer activities of chitosan capped gold nanoparticles prepared by γ-irradiation. Mater. Technol. 1–10 (2021)
K. Čubová, V. Čuba, Synthesis of inorganic nanoparticles by ionizing radiation—a review. Radiat. Phys. Chem. 158, 153–164 (2019)
W.H. Eisa, M.F. Zayed, B. Anis, L.M. Abbas, S.S.M. Ali, A.M. Mostafa, Clean production of powdery silver nanoparticles using Zingiber officinale: the structural and catalytic properties. J. Clean. Prod. 241, 118398 (2019)
S. Jiang, C. Tang, Z. Gong, Z. Zhang, D. Wang, M. Fan, Facile preparation of chitosan coated silver nanoparticles embedded cotton fabric for point-of-use water disinfection. Mater. Lett. 277, 128256 (2020).
K. Ryu, Y.-J. Moon, K. Park, J.-Y. Hwang, S.-J. Moon, Electrical property and surface morphology of silver nanoparticles after thermal sintering. J. Electron. Mater. 45(1), 312–321 (2016)
T.T.H. Pham, N.D. Dien, X.H. Vu, T.T. Tran, N.X. Ca, N. Van Truong, P.M. Tan, H.T. Van, P. Van Do, Synthesis and in-depth study of the mechanism of silver nanoplate and nanodecahedra growth by LED irradiation for SERS application. J. Electron. Mater. 49(8), 5009–5027 (2020)
S. Ahmad, K. Subhani, A. Rasheed, M. Ashraf, A. Afzal, B. Ramzan, Z. Sarwar, Development of conductive fabrics by using silver nanoparticles for electronic applications. J. Electron. Mater. 49(2), 1330–1337 (2020)
M.I.A. Abdel Maksoud, A. El-Ghandour, G.S. El-Sayyad, R.A. Fahim, A.H. El-Hanbaly, M. Bekhit, E.K. Abdel-Khalek, H.H. El-Bahnasawy, M. Abd Elkodous, A.H. Ashour, A.S. Awed, Unveiling the effect of Zn2+ substitution in enrichment of structural, magnetic, and dielectric properties of cobalt ferrite. J. Inorg. Organomet. Polym. Mater. 30(9), 3709–3721 (2020)
E.M. Abou Hussein, M.I.A. Abdel Maksoud, R.A. Fahim, A.S. Awed, Unveiling the gamma irradiation effects on linear and nonlinear optical properties of CeO2–Na2O–SrO–B2O3 glass. Opt. Mater. 114, 111007 (2021)
A.S. Awed, M.I.A.A. Maksoud, M.M. Atta, R.A. Fahim, Nonlinear optical properties of irradiated 1,2-dihydroxyanthraquinone thin films: merged experimental and TD-DFT insights. J. Mater. Sci. Mater. Electron. 30(8), 7858–7865 (2019)
R.P. Chahal, S. Mahendia, A.K. Tomar, S. Kumar, γ-Irradiated PVA/Ag nanocomposite films: materials for optical applications. J. Alloy. Compd. 538, 212–219 (2012)
A.A. Alhazime, M.M.M. Barakat, K. Benthami, S.A. Nouh, Gamma irradiation‐induced modifications in the structural, thermal, and optical properties of polyvinyl alcohol‐polyethylene glycol/cobalt oxide nanocomposite films. J. Vinyl Addit. Technol. (2020)
B. Alshahrani, H.I. ElSaeedy, S. Fares, A.H. Korna, H.A. Yakout, M.I.A.A. Maksoud, R.A. Fahim, A.H. Ashour, A.S. Awed, The effect of gamma irradiation on structural, optical, and dispersion properties of PVA/Zn0.5Co0.4Ag0.2Fe2O4 nanocomposite films. J. Mater. Sci. Mater. Electron. (2021)
A.A. Alhazime, K.A. Benthami, B.O. Alsobhi, G.W. Ali, S.A. Nouh, Pani-Ag/PVA nanocomposite: gamma induced changes in the thermal and optical characteristics. J. Vinyl Addit. Technol. 27(1), 47–53 (2021)
S.A. Nouh, K. Benthami, Gamma induced changes in the structure and optical properties of ZnS/PVA nanocomposite. J. Vinyl Addit. Technol. 25(3), 271–277 (2019)
W.H. Eisa, T. Abdelnaby, S. Mostafa, M.Y. Elzayat, In situ preparation of chitosan/gold nanocomposite: structural and catalytic properties. Adv. Polym. Technol. 37(6), 2095–2101 (2018)
Z.I. Ali, F.M. Mosallam, R. Sokary, T.A. Afify, M. Bekhit, Radiation synthesis of ZnS/chitosan nanocomposites and its anti-bacterial activity. Int. J. Environ. Anal. Chem. 101(3), 379–390 (2021)
S. Tripathi, G.K. Mehrotra, P.K. Dutta, Physicochemical and bioactivity of cross-linked chitosan–PVA film for food packaging applications. Int. J. Biol. Macromol. 45(4), 372–376 (2009)
M.T. Khorasani, A. Joorabloo, H. Adeli, Z. Mansoori-Moghadam, A. Moghaddam, Design and optimization of process parameters of polyvinyl (alcohol)/chitosan/nano zinc oxide hydrogels as wound healing materials. Carbohydr. Polym. 207, 542–554 (2019)
R. Kaur, D. Goyal, S. Agnihotri, Chitosan/PVA silver nanocomposite for butachlor removal: fabrication, characterization, adsorption mechanism and isotherms. Carbohydr. Polym. 262, 117906 (2021)
S. Hajji, R.B.S.-B. Salem, M. Hamdi, K. Jellouli, W. Ayadi, M. Nasri, S. Boufi, Nanocomposite films based on chitosan–poly(vinyl alcohol) and silver nanoparticles with high antibacterial and antioxidant activities. Process Saf. Environ. Prot. 111, 112–121 (2017)
N.M. El-Sawy, M.B. El-Arnaouty, A.M.A. Ghaffar, γ-Irradiation effect on the non-cross-linked and cross-linked polyvinyl alcohol films. Polym.-Plast. Technol. Eng. 49(2), 169–177 (2010)
W.H. Eisa, Y.K. Abdel-Moneam, Y. Shaaban, A.A. Abdel-Fattah, A.M. Abou Zeid, Gamma-irradiation assisted seeded growth of Ag nanoparticles within PVA matrix. Mater. Chem. Phys. 128(1), 109–113 (2011)
Z.I. Ali, M. Bekhit, R. Sokary, T.A. Afify, Radiation synthesis of copper sulphide/poly(vinyl alcohol) nanocomposites films: an efficient and reusable catalyst for p-nitrophenol reduction. Int. J. Environ. Anal. Chem. 99(13), 1313–1324 (2019)
J. Krumhansl, The solid state. Annu. Rev. Phys. Chem. 8(1), 77–104 (1957)
C.U. Devi, A. Sharma, V.N. Rao, Electrical and optical properties of pure and silver nitrate-doped polyvinyl alcohol films. Mater. Lett. 56(3), 167–174 (2002)
H. Zidan, Electron spin resonance and ultraviolet spectral analysis of UV-irradiated PVA films filled with MnCl2 and CrF3. J. Appl. Polym. Sci. 88(1), 104–111 (2003)
W.H. Eisa, Y.K. Abdel-Moneam, Y. Shaaban, A.A. Abdel-Fattah, A.M. Abou Zeid, Gamma-irradiation assisted seeded growth of Ag nanoparticles within PVA matrix. Mater. Chem. Phys. 128(1–2), 109–113 (2011)
N. Bhat, M. Nate, M. Kurup, V. Bambole, S. Sabharwal, Effect of γ-radiation on the structure and morphology of polyvinyl alcohol films. Nucl. Instrum. Methods Phys. Res. Sect. B 237(3–4), 585–592 (2005)
S. Nouh, K. Benthami, A. Massoud, N. El-Shamy, Effect of gamma irradiation on the structural and optical properties of PVA/CdS nanocomposite films prepared by ex-situ technique. Radiat. Eff. Defects Solids 173(11–12), 956–969 (2018)
A. El-ghandour, A.S. Awed, M.I.A. Abdel Maksoud, M.A. Nasher, 1,2-Dihydroxyanthraquinone: synthesis, and induced changes in the structural and optical properties of the nanostructured thin films due to γ-irradiation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 206, 466–473 (2019)
H. Zeyada, M. El-Nahass, I. Elashmawi, A. Habashi, Annealing temperatures induced optical constant variations of methyl violet 2B thin films manufactured by the spin coating technique. J. Non-Cryst. Solids 358(3), 625–636 (2012)
D. Sell, H. Casey Jr, K. Wecht, Concentration dependence of the refractive index for n‐ and p‐type GaAs between 1.2 and 1.8 eV. J. Appl. Phys. 45(6), 2650–2657 (1974)
N. El-Ghamaz, A. El-Sonbati, M. El-Mogazy, Effect of γ-radiation on the structural and optical properties of poly (3-allyl-5-[(4-nitrophenyl) diazenyl]-2-thioxothiazolidine-4-one) thin films. J. Mol. Liq. 248, 556–563 (2017)
S. Nouh, M. Abdel-Salam, A.A. Morsy, Electrical, optical and structural behaviour of fast neutron-irradiation-induced CR-39 SSNTD. Radiat. Meas. 37(1), 25–29 (2003)
A. El-Ghandour, A. Awed, M.A. Maksoud, M. Nasher, 1, 2-Dihydroxyanthraquinone: synthesis, and induced changes in the structural and optical properties of the nanostructured thin films due to γ-irradiation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 206, 466–473 (2019)
S. Wemple, M. DiDomenico Jr., Behavior of the electronic dielectric constant in covalent and ionic materials. Phys. Rev. B 3(4), 1338 (1971)
G. Sakr, I. Yahia, M. Fadel, S. Fouad, N. Romčević, Optical spectroscopy, optical conductivity, dielectric properties and new methods for determining the gap states of CuSe thin films. J. Alloy. Compd. 507(2), 557–562 (2010)
T.A. Taha, Optical properties of PVC/Al2O3 nanocomposite films. Polym. Bull. 76(2), 903–918 (2019)
A. Reyes-Coronado, C.G. Ortíz-Solano, N. Zabala, A. Rivacoba, R. Esquivel-Sirvent, Analysis of electromagnetic forces and causality in electron microscopy. Ultramicroscopy 192, 80–84 (2018)
K.-S. Lee, T.-M. Lu, X.-C. Zhang, The measurement of the dielectric and optical properties of nano thin films by THz differential time-domain spectroscopy. Microelectron. J. 34(1), 63–69 (2003)
E.D. Palik, Handbook of Optical Constants of Solids (Academic, Orlando, 1985). Google Scholar, pp. 286–297
K.L. Chopra, Thin film phenomena (1969)
A. Ali, J. Son, A. Ammar, A.A. Moez, Y. Kim, Optical and dielectric results of Y0. 225Sr0. 775CoO3±δ thin films studied by spectroscopic ellipsometry technique. Results Phys. 3, 167–172 (2013)
A. Abu El‐Haija, A. Rousan, L. Abuhassan, The basic optical properties, optical constants and optical conductivity of bismuth single thin films and bismuth–copper bilayer systems. Physica Status Solidi (a) 168(2), 505–517 (1998)
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdel Maksoud, M.I.A., Awed, A.S., Sokary, R. et al. Effect of gamma irradiation on the free-standing polyvinyl alcohol/chitosan/Ag nanocomposite films: insights on the structure, optical, and dispersion properties. Appl. Phys. A 127, 619 (2021). https://doi.org/10.1007/s00339-021-04776-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-021-04776-3