Abstract
Nd-doped TiO2 nanoparticles of different concentration are synthesized using a wet chemical route, followed by structural, optical and magnetic property examination. X-ray diffraction of the Nd-doped samples exhibits a tetragonal type anatase crystal structure similar to that of the pure TiO2. Crystallite size and strain of the nanoparticles are calculated by Scherrer’s and Williamson–Hall equation. FTIR shows the existence of functional groups at the conjunction of TiO2 nanoparticles and thereby confirming strong chemical bonding. Microstructural investigation of the prepared samples by FESEM and HRTEM shows the presence of nano-sized irregular spherical-shaped particles. The presence of defects and vacancies is observed by multicolour PL emission spectra. Further, availability of unpaired electrons and paramagnetic centres of Nd-doped samples is been identified by EPR spectra. The magnetic analysis reveals that pure TiO2 show weak ferromagnetic behaviour, whereas the Nd-doped TiO2 samples exhibit unsaturated ferromagnetic behaviour at high field, which delivers the interesting interaction between dopant and host lattice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanoscale materials are of immense interest owing to their exceptional physical properties providing oppurtunities to delve into their applications. In recent years, dilute magnetic semiconductors (DMS) utilised both charge and spin in single device. Therefore, extensive attention is given to obtain DMS materials which exhibit ferromagnetism at and above room temperature, due to their prime application of spin-oriented electronic devices [1]. To envisage aforesaid spintronic devices, it’s important to establish semiconductor compounds displaying room temperature ferromagnetism. Significance of this field was catalysed by the theoretical prediction of RTFM in ZnO doped with Mn by Dietl and his co-workers [2]. Thereby, experimental and theoretical examinations of other transition metals doped oxide-based semiconductors such as SnO2, TiO2, In2O3 and HfO2 commenced [3]. The occurrence of high Tc ferromagnetism in TiO2 thin films doped with Co [4] paves the way for continuous research on this material [5,6,7,8]. However, TiO2 is a n-type semiconductor with energy bandgap value of 3.2 eV, which is tuned and utilised for various applications like dye-sensitized solar cells, protective coatings, LEDS, gas sensors, photoelectrochemical cells, photocatalyst for water splitting, etc. [9]. TiO2 is been focus of interest for its distinctive properties namely higher refractive index, dielectric constant with good optical transmittance and high dissolving capability of magnetic dopant ions which makes it suitable for magneto-optical devices as well [10].
Even with many years of research on understanding ferromagnetism in oxide-based diluted magnetic semiconductors, the origin of it is not clear upto now. Doping methods are extensively used for altering the electronic structure of TiO2 to enhance the optical, magnetic and catalytic properties, where the dopants can segregate on surface of TiO2 nanostructures or incorporate into their lattice or both [11]. Thus, doping with a suitable dopant makes it more or less efficient. Transition metal doping of such semiconductors as DMS materials is intensively investigated in past decade [12]. The drawbacks of TM doping such as weak magnetism, occurrence of secondary or impure metallic phases, half filled 3d outer shell or reproducibility could be minimised with rare earth doping. Rare-earth doped DMS have caught up great interests in recent years because of their unique properties such as luminescence, high emission quantum yields and high magnetic moment per atom due to high orbital momentum. The emission in the visible region enables the surface state to form oxygen vacancies, trapped state and surface defect state which act as emission centres. Rezlescu’s claims, the large ionic radius and stable valence state of 3+ of rare earth atoms makes it to modify the properties of ferrite structure [13]. Room temperature ferromagnetism and paramagnetism at low temperature is observed in molten salt synthesised Sm doped and pure TiO2 nanorods [14]. Susmita Paul et al. [15] observed paramagnetism of Gd-doped TiO2 samples being reduced due to antiferromagnetic interaction caused by exchange interaction of Gd ions. Further, rare earth-doped ZnO, SnO2 are intensively investigated for dilute magnetic semi-conducting (DMS) applications [12, 16].
Among the rare earth elements, neodymium (Nd) finds numerous applications as permament magnets, high power lasers, luminescent materials, catalysts and data storage devices. In comparison to other rare earth, Nd-doped TiO2 system is less considered for its ferromagnetic properties inspite of its advanced applications in modern technology. However, the method of preparation, chemical composition, sintering temperature and dopant level greatly affects the magnetic properties of TiO2 materials as different synthesis method results in diverse defect concentrations, structures and morphologies. Hence, here in present work, sol–gel method is adopted which permits manipulation of dopant concentrations and high purity. Even though TiO2 doped with rare earth elements is extensively studied for photocatalytic properties, conversely the induced ferromagnetic behaviour in Nd-doped TiO2 nanoparticles are hardly reported in literature. Hence, motivation of this work is to study the influence of neodymium doping on some physical properties of TiO2 nanoparticles.
2 Experimental
2.1 Synthesis of Nd–TiO2 nanoparticles
The chemical used as starting materials are of analytical grade and do not require any additional treatment. Pure and Nd-doped TiO2 samples are synthesized using sol–gel method. To start with, titanium tetraisopropoxide (C12H28O4Ti) is dissolved in ethanol and acetic acid to form a transparent solution. To the above solution, neodymium nitrate hexahydrate (Nd(NO3)3·6H2O) dispersed in ethanol and water is added dropwise under vigorous stirring. The mixture is stirred continuously for 3 h and aging of the solution results in gel formation. The obtained transparent gel is dried at temperature of 100 °C, ground into powders using a mortar and calcined at 400 °C to obtain Nd-doped TiO2 nanoparticles. Pure TiO2 is prepared in the absence of neodymium. The amounts of neodymium ions are in order of 1%, 3% and 5% and are labelled as NT1, NT3 and NT5 respectively.
2.2 Materials characterisation
XRD patterns of powdered samples are recorded using Bruker D8 PXRD system with radiation source CuKα (λ = 0.15406 nm) and diffraction angle ranging from 20° to 80°. Fourier transform infrared spectra (FT-IR) are recorded in the frequency range of 400–4000 cm−1 by Spectra-RX spectrophotometer to study the presence of vibrational bands. Laser Raman spectra are recorded using spectrometer (Horiba Jobin, LabRAM HR) with the excitation source of 514 nm at room temperature. Surface morphology of the prepared samples is being studied with FEG 200 FESEM and JEOL JEM-2010 HRTEM. Nitrogen adsorption–desorption isotherms is recorded at liquid nitrogen temperature (− 196 °C) on a Micromeritics Model ASAP 2020 and specific surface areas are determined at relative pressure (P/P0 = 0.99) using BET (Brunauer–Emmett–Teller) method. All the samples are degassed at 150 °C prior to measurements. The UV–Vis spectra in the diffuse reflectance mode are registered using a spectroscopy instrument Jasco V-700. PL measurements are performed with the excitation of 315 nm using a Spectrofluorometer. EPR spectra are recorded with Bruker TMX spectrometer (X band). Room temperature magnetic behaviour of TiO2 nanoparticles is studied using Lakeshore, 7410 Model vibrating sample magnetometer.
3 Results and discussion
3.1 PXRD analysis
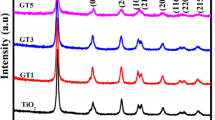
Crystal structure of Nd-doped and pure TiO2 samples are studied by PXRD and presented in Fig. 1a. All visible XRD peaks are listed to tetragonal structure of anatase TiO2 with standard JCPDS data (no: 075–1537). The thermal treatment at 400 °C aids in the conversion of amorphous solid to crystalline anatase structure. It is distinct that XRD peaks of doped samples are found shifting towards the lower 2θ values (Δθ = 0.17° for NT1). The diffraction peak shift observed for the maximum intense peak (101) is depicted in Fig. 1b. From earlier reports, the substitution of Nd ions in Ti–O lattice has their maximum intensity plane (101) shifted to lower angle side indicating that dopant ions perturbs the lattice of host appreciably. As the content of Nd increases, there is a peak broadening and decrease in intensity which shows that the dopant interact with Ti–O lattice and reduce the crystallinity and crystallite size. In addition, Nd ions involve in growth mechanism and reduce the grain size of TiO2 nanoparticles [17].
a Powder X-ray diffraction pattern of Nd-doped and pure TiO2 nanoparticles calcined at 400° C for 2 h in air. All samples indicate anatase TiO2 as dominating phase. b Peak shift of (101) plane of Nd-doped and pureTiO2 nanoparticles showing lower angle shift indicating lattice distortion. c Williamson–Hall plot with its experimental data and linear fit of Nd-doped and pure TiO2 nanoparticles. d Crystallite size calculated from Debye–Scherrer plotted against Nd content. Crystallite size decreases with concentration of Nd-doped TiO2 nanoparticles
The crystallite size, D of the samples is found by Scherer’s equation:
where λ is X-ray wavelength used, β is full width at half maximum and θ is angle of diffraction. Crystallite size from Scherer’s equation is calculated for prepared samples and presented in Table 1.
Lattice strain and crystallite size are distinguished and correlated by Williamson and Hall plot (W–H) method. This is done by plotting βcosθ in dependence of 4sinθ and the parameters are calculated using equation:
where ε is lattice strain. The above equation is that of a straight line and its slope presents strain of the lattice and intercept gives crystallite size. Figure 1c shows the W–H plots of pure and Nd-doped TiO2 nanoparticles. The strain present in samples indicates the presence of imperfections which may arise due to Nd dopant ions, growth conditions and thermal energy relaxations.
The equation for calculating lattice parameters of the nanoparticle is:
where a and c are lattice parameters, h, k, l are Miller indices and d, the interplanar distance [18]. Unit cell parameters ‘a’ increases and ‘c’ decreases with increase in Nd content and this indicates the possible incorporation of Nd ions into lattice of anatase TiO2 [19].
Lattice specifications, cell volume, crystallite size (Scherrer’s and W–H equation) of the prepared compounds are tabulated (Table 1). Decreasing crystallite size with increase in Nd dopant concentration confirmed by the peak broadening is depicted in Fig. 1d. The variation in lattice parameters, crystallite size and strain may be related to difference in their ionic radius (Ti = 0.068 nm and Nd = 0.098 nm) leading to microstructural heterogeneity. Hence, Nd ions are mainly distributed on the surface in Nd-doped samples leading to a larger surface area (Table 2), which is also reported [20].
3.2 FT-IR analysis
Fourier transform infrared spectroscopy (FT-IR) provides complete revelation of the structure and positions of ions in the samples, where the presence of absorption peaks and their positions are depending on crystalline structure, chemical composition of the materials. IR absorption spectra of Nd-doped and pure TiO2 nanoparticles at room temperature are presented in Fig. 2. The absorption in the domain of 400–800 cm−1 associated with stretching modes of metal oxide. This region presents sub bands at 460, 602, 656, and 754 cm−1 which are not distinct as the Nd dopant concentration increases. The peaks at 602, 656, and 754 cm−1 are due to symmetric O–Ti–O stretch, while peak at 460 cm−1 are due to the vibrations of Ti–O or Nd–O bond [21]. These peaks refer to vibration modes of anatase structure which is also supported by PXRD data. The intense absorption at 1124 cm−1 is virtue of C–O stretching modes. The absorption peaks by 1404, 1630 cm−1 is marked to H–O–H bending of adsorbed water. The wide absorption region of 3100–3400 cm−1 is related to stretching modes of hydroxyl groups [22]. The weak absorption peak by 2335 cm−1 is due to CO2 present in the samples trapped during sample characterisation measurements [23]. Hence, the observed metal oxide vibrations in frequency range of 400–4000 cm−1 reveal the phase and chemical purity of all the pure and Nd-doped TiO2 compounds.
3.3 Raman analysis
Crystallographic structure and variation in local symmetry of prepared samples are studied by Raman spectroscopic technique and the observed spectra are shown in Fig. 3. According to the factor group analysis, anatase TiO2 belongs to the symmetry space group D4h19: I41 /amd which is tetragonal with two formula units (six atoms) per primitive cell has six Raman modes (A1g + 2 B1g + 3Eg) [24]. From earlier reports the allowed vibrational modes appear by 144 cm−1, 197 cm−1 and 639 cm−1 (Eg), 513 cm−1 (A1g), 399 cm−1 and 519 cm−1 (B1g) for anatase TiO2. All doped samples exhibit active Raman modes that are as same as the pure phase. The sharp, narrow peak at 144 cm−1 corresponds to the Eg mode of anatase phase TiO2. Other than these peaks, two low intense peaks are seen at 197 and 639 cm−1, respectively. The B1g mode occurs at 399 cm−1, overlapped A1g and B1g mode appears at 516 cm−1 [15]. The nonappearance of the other characteristic vibrations in Raman spectra suggests the absence of Nd2O3 segregation in Ti–O lattice. It is known, A1g peak is the result of antisymmetric bending, the B1g peak results due to symmetric bending and Eg peak is characteristic of the symmetric stretching vibrations in TiO2 lattice [25]. The mural inset shows there was a notable shift to high wave number after Nd doping, which affirms that Nd may be introduced into the lattice of titania causing a small extent of structural distortion. Further, it is seen that intensity of the most intense peak at 144 cm−1 decreases, indicating that particle size of the doped TiO2 decreases with increasing Nd doping [26]. This also elucidates the increase in oxygen vacancy with increase in Nd dopant concentration. It is also observed that broadening of the peaks and position of peak by 144 cm−1 shows blue shift while the low frequency mode Eg peak by 639 cm−1 shows red shift with increase in Nd doping, indicating the increase in oxygen vacancies or defects induced via incorporation of Nd into TiO2 lattice [27]. Thus, Raman spectra show a consistent result of the XRD patterns that TiO2 has well crystallized anatase structure.
3.4 Surface analysis
3.4.1 FESEM analysis
The morphological analysis of Nd-doped and pure TiO2 nanoparticles is performed with the help of field emission scanning electron microscopy. Figure 4a shows the SEM micrographs of the pure and neodymium-doped TiO2 powders calcined at 400 ºC. All the synthesised samples show irregular spherical-shaped particles, regardless of Nd ratio. It is seen the undoped sample has a nearly invariable particle size in the dimension of 17–23 nm with average particle size, 21 nm. With Nd doping, the NT1 sample has particle size in the range of 23–27 nm with mean size of 26 nm whereas NT5 has a mean size of 20 nm. The image also depicts the decrease in grainsize with increase in Nd doping, which is also evident from increased surface area summarised in Table 2. The trend of increased surface area to volume ratio causes the agglomeration of the Nd-doped TiO2 nanoparticles [28].
Elemental compositions of the prepared Nd-doped and pure TiO2 samples are estimated by EDAX studies. Figure 4b shows EDAX spectra of NT3 and NT5 with elemental composition. The spectra projects the main components are Ti and O with low contents of Nd. The non-appearance of other elements confirms purity of Nd-doped and pure TiO2 nanoparticles.
3.4.2 HRTEM analysis
The HRTEM studies give additional details on microstructure of nanoparticles such as crystalline nature, lattice imperfections, defects and other structural information. HRTEM images of Nd-doped and pure TiO2 nanoparticles are displayed, Fig. 5a. From the images, it can be observed that Nd-doped and pure TiO2 nanoparticles show spherical shaped particles with irregular framework. Further with increase of Nd dopant concentration, decreasing of particle size with decrease in crystalliity is apparently seen in TEM images which is also consistent with XRD and other rare earth metal dopants like Gd-doped TiO2 [15]. The nanoparticles shows good surface covering with high surface area leading to agglomeration of the nanoparticles. The added Nd dopant reduces the grain boundaries hindering the grain growth thereby reducing the particle size [28]. High magnification images (Fig. 5b) show equally spaced and well-aligned distinguishable planes. Lattice fringes of pure TiO2 are clearly seen with interplanar distances (dh k l = 0.346 nm), which matches well with (1 0 1) plane at 2θ = 25.6° from diffraction pattern of anatase TiO2. From Fig. 5b, interplanar spacing of the doped and pure TiO2 nanoparticles shows trivial variation. This further affirms the embodiment of only few neodymium ions into the TiO2 lattice [15]. SAED pattern (Fig. 5c) of samples displays specific circular rings which reveal polycrystallinity of nanoparticles. The well-defined rings are catalogued to tetragonal anatase structure of TiO2. As shown in Fig. 5c, the concentric rings in SAED pattern of pure TiO2 indicates the polycrystalline type of particles whereas for maximum dopant concentration the discrete spots in the circular rings are not intense indicating the occurrence of random orientation of grains [16]. This also commends the decrease of grainsize with an increase in Nd dopant [29].
a High-resolution transmission electron microscopy images of Nd-doped and pure TiO2 nanoparticles. b High magnification (5 nm) TEM images showing lattice spacing of Nd-doped and pure TiO2 nanoparticles. c SAED pattern of Nd-doped and pure TiO2 nanoparticles showing polycrystalline nature. d EDS images of Nd-doped TiO2 nanoparticles confirms absence of other chemical elements
An energy dispersive X-ray spectrum (EDS) brought out the elements present in the sample. Figure 5d evidently justifies the presence of Ti, Nd and O elements with no impurities. The existence of copper ions is from the copper grid of sample holder in HRTEM analysis.
3.4.3 BET analysis
Morphology of the material is important in determining textural parameters Fig. 6a presents N2 adsorption–desorption isotherms of Nd-doped and pure TiO2 nanoparticles. Referring to IUPAC classification, adsorption isotherms of samples are representing type IV with hysteresis loop of type H1, suggesting the occurance of mesopores [30]. A hysteresis loop of this type is interpreted as indicating the materials with cylindrical pore geometry with high pore size consistency due to the agglomerates or spherical particles placed in an adequately even way [31]. This is in agreement with FESEM and HRTEM studies. The adsorption and desorption isotherm for Nd-doped TiO2 are similar to that of pure TiO2 suggesting uniform distribution of Nd in the prepared samples. The intensity of the hysteresis curve increases with Nd doping but decreases for increase in Nd concentration. This results from the capillary condensation occurring in the mesopores at higher pressure range [32]. It also indicates the smaller particle size and higher density of particles for Nd-doped TiO2 nanoparticles. Pore size distributions of Nd doped and pure samples exhibited in Fig. 6b presents a vast distribution of pores which clearly shows that the particles are of mesoporous nature [22]. Though the poresizes are beyond mesopore scale (> 50 nm), it does not affect the hysteresis curve which depicts narrow pore size distribution. Mean particle size of the nanoparticles is calculated using the equation [20]:
where S is specific surface area (m2 g−1), ρ is powder density (g cm−3) and D denotes mean particle size (nm) measured via the BET method and listed in Table 2. The calculated values satisfy with the obtained XRD results. Specific surface area (SBET), pore radius and pore volume of the samples calculated using BET (Brunauer–Emmett–Teller) technique and BJH (Barrett–Joyner–Halenda) method are compiled in Table 2. It is ascertained that the doped samples have comparatively higher surface area as that of pure TiO2. The exceeding surface area for Nd-doped TiO2, in comparison with pure, is also reported [17]. This efficacy is related to the decreasing crystallite size of doped TiO2 nanoparticles discussed in PXRD analysis and the larger ionic radii of Nd ions than Ti ions making them distributed mainly on the surface. But the pore volume and pore radius decrease with increase in Nd concentration though it is higher when compared to pure TiO2. Thus, the introduction of Nd increases the surface area with high porosity, ceasing the congregation process and influencing the textural properties greatly.
3.5 Optical studies
3.5.1 UV-DRS analysis
Diffused reflectance spectra of Nd-doped and pure TiO2 are measured in the domain of 200–900 nm at room temperature, to study the optical response of doped TiO2 nanoparticles set out in Fig. 7a and Fig. 7b. From the figure, it is perceptible that no visible light (> 400 nm) absorption for pure TiO2, whereas Nd-doped TiO2 nanoparticles displayed a wide absorption band in the range 400–900 nm as seen from inset of Fig. 7a. It is seen that there are five typical absorption peaks at 527 nm, 586 nm, 746 nm, 804 nm and 876 nm. Moreover with increasing Nd content, intensity of absorption peaks increases with appearance of additional weak absorption peaks at 432 nm, 476 nm and 684 nm, elemental characteristic peaks of Nd doping [33]. For pure TiO2, it is generally understood that the absorption in the ultraviolet range (λ = 318 nm) is associated with the excitation of the O 2p electron to the Ti 3d level [34]. However absorption in visible light range of 400–900 nm is attributed to 4f electron transition of Nd ions. The positions of these absorption peaks at 527 nm, 586 nm, 746 nm, 804 nm and 876 nm are attributable to the internal transitions from the 4I9/2 ground state to higher excited states 2P1/2 and 2D5/2 (432 nm), 2D3/2 and 4G11/2 (460 nm), 2G9/2 and 2K15/2 (476 nm), 2K13/2 and 4G7/2 (527 nm), 2G7/2 and 4G5/2 (586 nm), 4F9/2 (684 nm), 4F7/2 and 4S3/2 (762 nm) and 4F5/2 and 2H9/2 (802 nm) [35]. In general, Nd doping of TiO2 enhances the optical absorption capability which is attributed to morphology, strain, defects and incorporation of impurities during synthesis in the nanomaterials [20].The bandgap values are being extrapolated from the manipulation of Tauc equation:
where A is a constant characteristic of the material, ν is frequency, Eg is optical bandgap, α is absorption coefficient, n corresponds to electronic transitions (n = ½ and 2, the transitions are indirect and direct, respectively) and h stands for Planck’s constant. The calculated bandgap values presented in Fig. 7c evidences less significant change in the bandgap values with increasing dopant concentration.
3.5.2 Photoluminescence studies
Room temperature PL spectra of Nd loaded and pure TiO2 upon ultra bandgap excitation (λ = 340 nm) are shown in Fig. 8. It is seen that Nd-doped and pure TiO2 nanoparticles exhibit distinct excitonic PL signals alongwith homogeneous curve shape except the band edge emission peak typically observed in pure and NT5 with increased intensity. Furthermore, the presence of neodymium does not arise new PL signals yet the indicative contribution of Nd is in the intensity of PL emission peaks where the intensity increases for NT1 and NT3, despite it decreases for NT5 doping. The enhancement in intensity of PL emission peaks is attributed to increase in defects like oxygen vacancies. These results elucidate surface oxygen vacancies reaches maximum when Nd content was 3% [36]. All of the samples are found to exhibit expanded emission band in visible wavelength region. A pronounced UV emission at 348 nm and several relatively weak visible emissions in the domain of 400–500 nm attributed to the doubly ionized oxygen vacancy formation for pure TiO2 [37], as seen in inset of Fig. 8. The visible luminescence peaks results from binding of electrons with oxygen vacancies present on surface of TiO2 nanoparticles [38]. The visible emission at 466 nm is generally ascribed to oxygen vacancies in addition to other defect states in the host material during synthesis [39].
3.6 Electron paramagnetic analysis
EPR spectroscopy is well-known spectroscopic technique to probe the co-ordination environment and provide details of elemental composition. It also investigates paramagnetic ions on oxide surfaces which may include surface defects, inorganic or organic radicals, metal cations or clusters [40]. EPR spectra of Nd-doped and pure TiO2 nanoparticles at room temperature are presented in Fig. 9. Pure TiO2 and Nd-doped samples display an isotropic signal related to ferromagnetic behaviour accompanied with a distinct sharp derivative signal related to paramagnetic centres containing unpaired electrons. This is further confirmed by the ‘g’ value which is sensitive to micromagnetic domain structures and changes in the local symmetry of spins. The ‘g’ factor is calculated using the relation
where H, magnetic field and B, Bohr magneton [41]. The pronounced EPR signal at g = 2.0002 nearly equals the signal of free electron value, indicating the existence of unpaired electrons in the host lattice which mainly occurs due to the presence of singly charged oxygen vacancies [5, 8]. Presence of signal at g = 1.98 indicates the presence of paramagnetic Ti3+ centres in both pristine and Nd-doped samples, which is commonly explained by the generation of oxygen vacancies. The surface Ti3+ tends to absorb O2 forming superoxide (O2−) ions or the free electrons occupy interior Ti position, thereby generating Vo and Ti3+ defects in the lattice [31]. In addition, Tseng et al. attributes signal at g value of 2.8 to ferromagnetic ordering in undoped TiO2 [42]. The subsurface oxygen vacancies trapping photogenerated electrons and holes at defect centres are responsible for this signal [43], which is more intense for maximum Nd dopant concentration. The signal with g value of 3.1 is related to disordering or surface-related defects. Thus Nd doping of TiO2 enhances free ions of oxygen vacancies, Ti3+ defects, interstitials and surface defects resulting in ferromagnetic ordering in the samples.
3.7 Magnetization analysis
The effects of Nd doping on coercivity, saturation and remanent magnetization are studied by recording M–H hysteresis loop using VSM in the magnetic field range of -15,000 to 15,000 Gauss at room temperature. Figure 10 shows the hysteresis curves for all samples under investigation. From the figure, pure TiO2 exhibits weak ferromagnetic behaviour, though bulk TiO2 exhibits diamagnetism. From XRD analysis, undoped TiO2 has the smallest unit cell volume which may also enhance ferromagnetic coupling between Ti ions [44]. Yahya Alivov et al. [45] studied the provenance of magnetism in pristine TiO2 nanotubes. All anatase, amorphous and rutile TiO2 nanotubes exhibited paramagnetic behaviour in a wide temperature range of 1.8–300 K. Qian et al. [46] reported room temperature ferromagnetism in undoped amorphous HfO2 nano-helix arrays and justifies it by the presence of singly charged oxygen vacancies. Hacchimi et al. [47] reported Nd-doped ZnO exhibiting paramagnetism at room temperature and ferromagnetism at low temperature. Theoretical calculations predicted an antiferromagnetic phase for low concentration of Nd atoms [47]. Zheng et al. [48] reports dilute ferromagnetic property at room temperature for Nd-doped ZnO and ascribes to oxygen vacancy and the s–f coupling between rare earth ions ‘f’ and ZnO host ‘s’ states. Paramagnetism in Gd-doped TiO2 and pure TiO2 exhibiting weak ferromagnetic behaviour at room temperature is reported earlier [25]. Ferromagnetic signals resulted from enhanced oxygen vacancies and Gd doping induces paramagnetic transition.
The pure TiO2 samples exhibits weak ferromagnetic behaviour, whereas the Nd-doped samples shows ferromagnetic behaviour at lower magnetic field and unsaturated ferromagnetic behaviour at higher field. The unsaturated ferromagnetism of the Nd-doped TiO2 samples is a characteristic of weak ferromagnetic behaviour [49]. This not saturated magnetic behaviour may also result from surface spin effects resulting from surface Nd ions or size effects resulting from nanosized particles wherein BET studies also confirms the accumulation of Nd ions on the surface [50]. However applying the laws of saturation, saturation magnetisation values are found and depicted in Table 3. Dimri et al. [51]. reports non saturation of M–H loops of Nd-doped CeO2 results from a paramagnetic component. In a similar case, the hysteresis curve of NT1 and NT3 shows paramagnetic contributions arising from inhibited carrier mediations. But a strong S curve with a coercivity of 61 Oe indicates ferromagnetic ordering. This type of magnetic behaviour of Nd-doped TiO2 nanoparticles illustrates a frustrated magnetic system.
The variation in magnetic behaviour is explained on basis of oxygen vacancies and strong exchange interactions in s–f coupling between the Nd ions ‘f’ and TiO2 host ‘s’ states [1]. The trivalent (Nd3+) doping in Ti–O lattice induces oxygen vacancies and interstitials, which contributes the ferromagnetic behaviour in host lattice [52]. From EPR analysis, it is evident that oxygen vacancy related defect increases for Nd-doped samples. In addition, surface morphology may play an indispensable role for the induced ferromagnetic behaviour in TiO2 nanoparticles. Thus, pure TiO2 exhibiting weak ferromagnetism is further enhanced with addition of Nd. The electrons are trapped in defects like Ti interstitials, Ti vacancies and oxygen vacancies. These trapped electrons enriche the magnetic behaviour of the Nd-doped TiO2 nanoparticles. It is further identified from high Mr value of NT5 sample retaining the ferromagnetic alignment because of the intrinsic magnetic moment of Nd ions. Mojtaba et al. [53] reported Nd-doped TiO2 films exhibiting weak ferromagnetism and attributed to the subsistence of ‘f’orbital of Nd ions.
Table 3 displays coercivity (Hc), saturation and remanent magnetization (Ms and Mr) for all the samples. From the table, coercivity of NT1 and NT3 samples increase whereas it decreases for NT5 nanoparticles. The saturation magnetisation (Ms) value is found to increase with Nd addition depicting the ferromagnetic alignment of the magnetic domains. But the remanent magnetisation (Mr) values decreases for NT1 and NT3 and increases for NT5 resulting from increased magnetic moment and magnetic anisotropy of Nd ions. The changes in coercivity are attributed to differences in oxygen vacancies and carrier mediation present in each sample [1]. PL curves demonstrate the increase in intensity for NT1 and NT3 sample whereas it decreases for NT5 with its maximum dopant concentration reaching saturation. The single electrons trapped in F centres mediate the exchange coupling generating ferromagnetism.
Thus from the magnetic studies, as the Nd concentration increases, the unsaturated ferromagnetic behaviour at higher field is increased, owing to Nd–Nd interaction. The unpaired electron pairs from rare earth ions interact with host lattice and enhance the magnetic interaction. Thus, the magnetic behaviour of TiO2 depends on surface morphology, charge carriers, defects and oxygen vacancies.
4 Conclusion
Nd-doped and pure TiO2 nanoparticles are prepared by sol–gel route. XRD studies revealed the tetragonal anatase structure with polycrystalline nature of the samples. The observed decrease in intensity of diffraction peak and its broadening with lower angle shift in diffraction pattern, changes in d value, cell volume and stress confirms the lattice perturbation of Nd ions in TiO2 lattice. FTIR analysis confirms the presence of Nd–O, Ti–O and O–Ti–O–Nd metal oxide vibrations. Raman spectra reveal that Nd doping influences the structure of TiO2 to certain extent. Surface morphology studies elucidate the decrease of average grain size and decrease in crystallinity with the addition of Nd, in confirmation with PXRD analysis. The EDS result confirms the successful substitution of Nd dopant in TiO2 matrix. BET studies reveal the mesoporosity of nanoparticles with Nd-doped samples having higher surface area than the pure TiO2. Diffuse reflectance spectra shows absorption in visible region of Nd-doped TiO2 nanoparticles. PL spectra and EPR investigations shows Nd doping of TiO2 enhances occurrence of oxygen vacancies, Ti3+ defects and surface defects which plays a key role in establishing room temperature ferromagnetism for both Nd-doped and undoped TiO2 nanoparticles. The magnetic study demonstrate that pure TiO2 exhibits ferromagnetic behaviour, whereas Nd-doped samples shows unsaturated ferromagnetic behaviour at higher applied field, this reveals the Nd ion exchange interaction with Ti–O lattice. Thus, the present work may provide an insight on the magnetic property of Nd-doped and pure TiO2 samples for dilute magnetic semiconductors and its applications.
References
G. Vijayaprasath, R. Murugan, T. Mahalingam, Y. Hayakawa, G. Ravi, Ceram. Int. 41, 10607 (2015)
T. Dietl, H. Ohno, F. Matsukura, J. Cibert, D. Ferrand, Science 287, 1019 (2000)
J.M.D. Coey, M. Venkatesan, C.B. Fitzgerald, Nat. Mater. 4, 173 (2005)
Y. Matsumoto, M. Murakami, S. Tomoji, T. Hasegawa, T. Fukumra, M. Kawasaki, P. Ahmet, C. Toyohiro, S.Y. Koshihara, H. Koinuma, Science 291, 854 (2001)
S. Batakrushna, P.K. Giri, D. Soumen, I. Kenji, F. Minoru, J. Phys. D 47, 235304 (2014)
S. Naseem, W. Khan, S. Khan, S. Husain, A. Ahmad, J. Magn. Magn. Mater. 447, 155 (2018)
S. Sudesh, C. Sujeet, K.C. Subhash, K.S. Shiv, J. Appl. Phys. 109, 0839057 (2011)
B. Choudhury, M. Dey, A. Choudhury, Int. Nano. Lett. 25, 2 (2013)
X. Lu, X. Mou, J. Wu, D. Zhang, L. Zhang, F. Huang, Adv. Funct. Mater. 20, 509 (2010)
L. Kernazhitsky, V. Shymanovska, T. Gavrilko, V. Naumov, L. Fedorenko, V. Kshnyakin, J. Lumin. 166, 253 (2015)
M. Manzoor, A. Rafiq, M. Ikram, M. Nafees, S. Ali, Int. Nano. Lett. 8, 1 (2018)
G. Vijayaprasath, R. Murugan, Y. Hayakawa, G. Ravi, J. Lumin. 178, 375 (2016)
N. Rezlesu, E. Rezlescu, C. Pasnicu, M.L. Craus, J. Phys, Condens. Matter 6(29), 5707 (1994)
L.-T. Tseng, Xi. Luo, S. Li, J. Yi, J. Alloys Compd. 687, 294 (2016)
S. Paul, B. Choudhury, A. Choudhury, J. Alloys Compd. 601, 201 (2014)
R. Adhikari, A.K. Das, D. Karmakar, J. Ghatak, J. Magn. Magn. Mater. 322, 3631 (2010)
T.L.R. Hewer, E.C.C. Souza, T.S. Martins, E.N.S. Muccillo, R.S. Freire, J. Mol. Catal A 336, 58 (2011)
P. Bushra, H. Mahmood-ul, K. Zeeshan, R. Saira, N. Shahzad, J. Appl. Res. Technol. 15, 132 (2017)
V. Stengl, S. Bakardjieva, N. Murafa, Mater. Chem. Phys. 114, 217 (2009)
R. Joanna, G. Tomasz, W.S. Janusz, L. Wojciech, G. Maria, O. Bunsho, Z. Adriana, Appl. Surf. Sci. 307, 333 (2014)
A.H. Mohammed, N.S. Fawaz, S.A. Adnan, Int. J. Appl. Innov. Eng. Manag. 2, 432 (2013)
J. Yang, J. Dai, J. Li, Appl. Surf. Sci. 257, 8965 (2011)
Wu. Du Jun, Z.S. Qi, Gu. Xin, L. Jiao, G. Haizhi, Z. Wenlong, P. Hailong, Z. Jianguo, J. Rare Earths 33, 148 (2015)
S.H. Hoda, M. Saif, J.T. McLeskey, M.S.A. Abdel-Mottaleb, I.S. Yahia, T. Story, W. Knoff, Int. J. Photoenergy (2009). https://doi.org/10.1155/2009/240402
N. Nithyaa, N.V. Jaya, J. Supercond. Nov. Magn. (2018). https://doi.org/10.1007/s10948-018-4693-9
G. Yang, Z. Jiang, H. Shi, T. Xiao, Z. Yan, J. Mater. Chem. 20, 5301 (2010)
K. Sandeep, S. Patel, S.G. Namdeo, J. Magn. Magn. Mater. 330, 21 (2013)
B. Parveen, M. Hassan, S. Atiq, S. Riaz, S. Naseem, M.A. Toseef, Prog. Nat. Sci. Mater (2017). https://doi.org/10.1016/j.pnsc.2017.04.007
S. Kumar, P.D. Sahare, Mater. Res. Bull. 51, 217 (2014)
A. Zeid, A.L. Othman, Materials 5, 2874 (2012)
M. Rajendran, K.K. Mallick, A.K. Bhattacharya, Mater. Lett. 37, 10 (1998)
V. Hiremath, R. Shavi, J.G. Seo, Chem. Eng. J 308, 177 (2017)
J. Xuefeng, Y. Lu, L. Peng, L. Xi, S. Jian, Colloids Surf. B. 79, 69 (2010)
Xu. Yue-Hua, C. Chen, X.-L. Yang, X. Li, B.-F. Wang, Appl. Surf. Sci. 255, 8624 (2009)
R. Kralchevska, M. Milanova, D. Hristov, A. Pintar, D. Todorovsky, Mater. Res. Bull. 47, 2165 (2012)
Qi. Xiao, Z. Si, Yu. Zhiming, G. Qiu, Mater. Sci. Eng. B 137, 189 (2007)
G. Singh, C.S. Ravi, Ceram. Int. 43, 2350 (2017)
P. Susmita, C. Pawan, C. Biswajit, A.A. Gazi, C. Amarjyoti, J. Colloid Interface Sci. 439, 54 (2015)
K.S. Babu, A.R. Reddy, C. Sujatha, K.V. Reddy, A.N. Mallika, J. Adv. Ceram. 2, 260 (2013)
D.M. Murphy, Metal Oxide Catal. (2008). https://doi.org/10.1002/9783527626113.ch1
G. Yang, D. Gao, J. Zhang, J. Zhang, Z. Shi, D. Xue, J. Phys. Chem. C 115, 16814 (2011)
T. Li-Ting, L. Xi, T.T. Thiam, L. Sean, Y. Jiabao, Nanoscale Res. Lett. 9, 1 (2014)
P.K. Chinthala, O.G. Neeruganti, C.W. Ting, W. Ming-Show, C.K. Shyue, J. Phys. Chem. B 110, 5223 (2006)
A.A. Dakhel, M. El-Hilo, J. Appl. Phys. 107, 123905 (2010)
A. Yahya, G. Ted, C. Cigdem, W. Iwamoto, P.G. Pagliuso, M. Sabee, Nanotechnology 24, 275704 (2013)
X. Qian, W. Wei-Peng, X. Zheng, Z. Peng, Li. Zheng-Cao, Z. Zheng-Jun, Chin. Phys. B. 24, 057503 (2015)
A.G. El Hachimi, H. Zaari, M. Hamedoun, A. Benyoussef, A. El Kenz, O. Mounkachi, J. Magn. Magn. Mater. 444, 416 (2017)
J.H. Zheng, J.L. Song, Z. Zhao, Q. Jiang, J.S. Lian, Cryst. Res. Technol. 47, 713 (2012)
G. Vijayaprasath, R. Murugan, S. Asaithambi, G.B. Anandha, P. Sakthivel, T. Mahalingam, Y. Hayakawa, G. Ravi, Appl. Phys. A (2016). https://doi.org/10.1007/s00339-016-9655-0
B. Poornaprakash, U. Chalapathi, S. Babu, S.-H. Park, Physica E (2017). https://doi.org/10.1016/j.physe.2017.06.007
M.D. Chandra, H. Khanduri, H. Kooskora, J. Subbi, I. Heinmaa, A. Mere, J. Krustok, R. Stern, Phys. Status Solidi A 209, 353–358 (2012)
S. Kumar, P.D. Sahare, J. Rare Earths 30, 761 (2012)
M. Mohammadi, N. Shahtahmasebi, M. Karimipour, R. Sarhaddi, Indian J. Sci. Technol. 5, 2912 (2012)
Acknowledgement
The authors gratefully acknowledge DST, New Delhi for providing financial support to carry out this research work under PURSE II scheme. One of the authors Mrs. Nithyaa. N is thankful to DST, New Delhi for the award of DST-PURSE fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nithyaa, N., Victor Jaya, N. Effect of Nd on structural, optical and magnetic behaviour of TiO2 nanoparticles. Appl. Phys. A 127, 69 (2021). https://doi.org/10.1007/s00339-020-04140-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-020-04140-x