Abstract
This work demonstrated a dry electrode for electrocardiogram (ECG) monitoring based on graphene-coated cotton fabric by screen printing process. The cotton fabric was fully covered by the graphene flakes, which makes the sheet resistance of cotton fabric reaches as low as 42.2 Ω/sq. The ECG signals obtained from the graphene-coated textile electrode show comparable performance with the Ag/AgCl electrode under both sitting, standing, and rotating conditions. The Pearson correlation coefficient between the conventional commercial electrode and the graphene-coated textile electrode is 99.47%. The graphene-coated textile electrode exhibited an excellent flexibility with an increase resistance of 4.5% after 1000 bending cycles. The graphene-coated textile electrode after bending showed good performance for ECG signal acquisition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Healthcare monitoring electronics has attracted great research interest due to their ability to provide real time monitoring to prevent people suffer from chronic diseases, such as cardiovascular diseases [1, 2]. In general, the heart-related diseases, such as arrhythmias and related deaths, can be prevented by the diagnosis of electrocardiogram (ECG) signals [3]. The ECG signals can reflect the electrical activity of heart muscles [4], which have been widely used to observe the activity of human heart and evaluate the cardiac and cardiovascular functions [5]. In a conventional ECG system, the ECG signals are recorded by attaching the silver/silver chloride (Ag/AgCl) electrodes on the skin to capture the bio-potential signals and then convert the signals to electrical signals with digital filtering system [6]. The Ag/AgCl electrodes are normally used together with a conductive hydrogel and an adhesive layer to reduce the impedance between the electrode and skin [7]. However, the hydrogel and adhesive layer can gather hair and cause skin inflammation, and the drying of hydrogel over time might lead to low quality of signals and is not suitable for long-term monitoring [8].
A promising ECG electrode should fill the requirements of low contact impedance and motion artifacts, with expected long-time stability, as well as good flexibility to withstand some extensional strain [6]. Over the years, a range of dry electrodes has been investigated to avoid the problems associated with the hydrogel in wearable ECG monitoring systems [5, 9, 10]. To maintain intimate electrode/skin contact, a common strategy is to develop a flexible skin-like conductive film by mixing stretchable polymers with conduct additives, such as conductive polymer [11], silver nanowires [12], carbon nanotubes [13], and carbon black powder [14]. These dry electrodes solve the disadvantages of Ag/AgCl electrodes with skin irritation and long-term wear, but the fabrication processes of such electrodes are complicated, not systematic, and unsuitable for mass-production. Another strategy to fabricate dry electrode is based on textiles. Conductive textile electrode can be fabricated by depositing conductive materials, including PEDOT:PSS [15], silver paste [16], and graphene [17], on fabrics by printing technique such as screen printing [18], or inkjet printing [17, 19]. The printing technique, which has been widely used in wearable and flexible electronics [20, 21], offers a promising versatility for ECG monitoring.
Graphene, a two-dimensional carbon nanomaterial with a hexagonal honeycomb lattice, has excellent conductivity, good flexibility, and less affected with temperature changes [22], which makes it attract extensive attentions in materials science and biomedicine, and has great potential in healthcare monitoring [23]. Graphene has been widely used in electron and quantum dots [24], MEMS and microfluidics [25, 26], epidermal electronics [27], supercapacitors [28, 29], batteries [30-35] and flexible electronics [36, 37]. Graphene dry electrode produced by chemical vapor deposition (CVD) method and transfer process has been used for ECG monitoring [38, 39]. Celik et al. [38] transferred CVD synthesized graphene on the surface of Ag/AgCl electrodes to prepare electrodes. The prepared graphene is deposited on rigid substrates, and the long-term monitoring comfort needs to be improved. Lou et al. [39] prepared graphene electrodes on the PET substrate, which has good toughness. However, The PET substrate is not conducive to skin breathing. Moreover, the CVD and transfer processes are complex, which are not suitable for large scale fabrication. Graphene flakes can be exfoliated from nature graphite in large scale through liquid phase [40, 41], which can be further dispersed in solvents to form graphene-based conductive inks and deposited on textile for wearable ECG monitoring by printing technique [42]. The graphene–textile electrodes are normally prepared by coating textile with graphene oxide, following with a reduction process to convert the graphene oxide to graphene [8, 17]. However, the effective reduction of graphene oxide is normally need harmful chemicals [8], which limits its practical application for ECG monitoring. Moreover, there is a certain degree of graphene shedding during the reduction process, which affects the electrode quality.
In this work, we describe the fabrication of a dry electrode for ECG monitoring by screen printing the water-based graphene ink on a commercial cotton textile. Graphene nanoplatelets were chosen as the conductive materials due to its good conductivity and flexibility. Carboxymethyl Cellulose (CMC) was chosen as the surfactant for dispersing graphene because of its bio-compatibility and good stabilizability in water. The graphene ink can be successfully deposited on the textile substrate by screen printing process, following with a low drying temperature to remove water and form conductive graphene pattern. The graphene electrodes printed on textile present good contact with skin surface and comparable performance against conventional Ag/AgCl pre-gelled electrodes in ECG measurements. Moreover, the graphene-based textile electrodes showed excellent performance in recording of ECG signals even after 1000 bending cycles. The proposed approach paves the way for the development of bio-friendly, economical, and scalable dry electrodes for ECG monitoring.
2 Material and methods
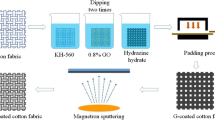
The fabrication processes of graphene-coated textile electrode for ECG monitoring are illustrated in Fig. 1a. The graphene ink was prepared from graphene and Carboxymethyl Cellulose (CMC, Sigma-Aldrich) with a ratio of 5:1 by weight. Firstly, 0.9 g of CMC was dissolved in 50 ml of deionized (DI) water and magnetically stirred on a magnetic stirrer for 30 min at 550 r/min. Then 4.5 g of graphene nanoplatelets (M15, XG Sciences) was added into the CMC aqueous solution, followed by putting into Vertical planetary ball mill (MITR-YXQM-1L) and stirring for 15 min at the rate of 350 r/min to obtain the final graphene ink. A commercial cotton fabric with yarn count 40 × 40, density 133 × 100 and weighting 130 g/m2 was chosen as the substrate.
Screen printing was performed using an aluminum alloy screen printer with polyester screen printing mesh (150 mesh count) and designed pattern, as shown in Fig. 1b. The graphene ink was transferred to the screen printing template with a spoon and scraped at a 45° using a neutral blade, following by annealing in an oven at 80 °C for 30 min. For patterns with more than one printing layer, the printed patterns were dried at 80 °C for 30 min before the next printing cycle. Figure 1c shows the electrode sensing effective area with 20 mm × 20 mm, and the strip size is 5 mm × 10 mm. To obtain a stable signal, copper tape and conductive silver paste is applied around the contact surface of the narrow strip. Then, a layer of medical tape is applied on the copper tape. The sheet resistance of printed graphene patterns was measured using a handheld four-probe tester (M3, Suzhou Jingge Electronic). Each sample was measured 6 times, the average value and standard deviation of each data point were calculated. All tests were performed at room temperature. Impedance measurements were carried out using a LCR meter (HTOKI-IM3536) with scan frequency from 4 to 1000 Hz. The bending test was carried out by a home-build bending test machine. X-ray photoelectron spectroscopy (XPS) was measured on a Kratos Axis Ultra DLD XPS spectrometer (Kratos Analytical Ltd, Manchester, UK).
2.1 Results and discussion
To identify the quality of graphene, we measured scan electron microscopy (SEM) and XPS data of the graphene nanoplatelets, as shown in Fig. 2. It can be seen that the size distribution of graphene nanoplatelets is range from ~ 1 to 8 µm, which had an average size of 3.6 ± 1.3 µm (Fig. 2a, b). Figure 2c shows the XPS spectrum of graphene sample, which has a strong C 1 s peak, a small O 1 s peak, and almost invisible N 1s and S 2p peaks. Using the elements analysis, the atom weight of C, O, N, and S are 96.63%, 2.68%, 0.30%, and 0.39%, respectively. Moreover, the AFM data of typical graphene nanoplatelets show the thickness of graphene nanoplatelets is ~ 10 nm, which is similar to the value (8–10 nm) in the data sheet supported by the supplier.
Figure 3a demonstrates a typical scanning electron microscope (SEM, TESCAN MIR3) images of untreated cotton fabric, in which the microstructure of the cotton fibers is clearly visible and the fiber surface is relatively smooth. After the deposition process, the morphology of cotton fabric surface is clearly covered with graphene flakes, as shown in Fig. 3c. It can be seen that graphene flakes are successfully attached onto the surface of cotton fibers after screen printing deposition. Figure 3b, d exhibits the cross-sectional SEM images of cotton fabric before and after graphene deposition. The untreated cotton fiber is looser, while the cotton fibric become relatively tight and flat after deposition. This can be attributed to the binding ability of the binder we used in the ink.
Figure 4a shows the variation of the sheet resistance of the graphene coated cotton fabrics with the number of printing repetitions. It can be seen that the sheet resistance of coated fabric was ~ 200 Ω/sq with one printing passes. There was a sharp decrease in sheet resistance by ~ 65% for the second printing pass. The sheet resistance of the coated fabric with three and four printing passes shows a small change from 46.6 to 42.2 Ω/sq. This could possibly be explained by coverage ratio of graphene flakes over fabric with increasing printing passes and form a continuous and uniform conductive film after three printed passes. The intrinsic impedance of graphene textile electrode with different printed passes is shown in Fig. 4b. It can be seen that the intrinsic impedance is almost constant for all frequencies for the various printing passes of graphene-coated fabric electrodes. The electrode with four printing passes shows an intrinsic internal resistance around 30 Ω, indicating the excellent conductivity of graphene film.
a Variation of sheet resistance with increasing number of printing passes. b The intrinsic impedance of graphene fabric with increasing number of printing passes. Variation with frequency in the electrode–skin impedance of Ag/AgCl and graphene electrodes with different printing passes: c dry condition and d wet condition
Figure 4c, d shows the contact impedance of the electrode–skin with the frequency range from 4 to 1000 Hz at dry and wet conditions. The graphene–fabric electrodes were placed on a left wrist that is 2 cm apart and have not been pretreated by skin. For comparison, pre-gelled Ag/AgCl electrodes were attached on the same position as textile electrodes. Figure 4c exhibits the electrode–skin impedance of the Ag/AgCl and graphene-coated textile electrodes under dry condition. The contact resistance values of the Ag/AgCl and the graphene electrodes (one layer) at 4 Hz are 183 KΩ, and 1.25 MΩ, respectively. Figure 4d shows the electrode–skin contact impedance of the Ag/AgCl and graphene electrodes under wet condition (~ 1 ml of a natural emulsion, purchased at the CHANDO flagship store, milky white, used for ordinary skin care hydration, water retention, was applied to the contact interface of the skin and electrode). It can be seen that the skin-contact resistance of graphene-based fabric electrodes with different layers shows little difference with using emulsion, which indicates good coupling of the electrodes to the skin. Thus, the experiments mentioned below are carried out on the graphene electrodes with printing one layer.
The schematic of ECG signal acquisition, processing, and transmission is shown in Fig. 5a. The ECG signal obtained by electrodes is amplified by a preamplifier (IN129, TI) and then passed through a filtering module, including a high-pass filter with cut-off frequency at 0.05 Hz, a 50 Hz notch filter and a low-pass filter with cut-off frequency at 100 Hz. The obtained ECG signal is further amplified by the post amplifier with adjustable gain and sampled at 333 Hz with a microprocessor (STM32F407, ST) to digitalize. Finally, the signal is transmitted to the terminal device via the Bluetooth module (CC2541, TI). Figure 5b illustrates the experimental setup of ECG signal acquisition.
Figure 6a shows the ECG signals acquired from the Ag/AgCl (black line) and the graphene-coated textile (red and blue lines) electrodes during sitting, standing, and rotating conditions. It can be seen that the amplitude of ECG signal obtained from the graphene-coated textile electrodes is similar to that obtained from the Ag/AgCl electrode at sitting and stand conditions, which indicates the good skin-contact performance of the graphene textile electrodes. In contrast, both the Ag/AgCl and dry graphene textile electrodes show obviously baseline drift under rotating condition, while the wet graphene-coated textile electrode exhibits a relatively stable, low noise signal. Figure 6b shows the morphology of P-QRS-T waves obtained from Ag/AgCl and graphene electrodes. The P wave, QRS complex wave and T wave which are the characteristic waves of the ECG signal can be clearly distinguished. The signals are almost coincident except for a slightly larger in amplitude from Ag/AgCl electrode. The Pearson correlation coefficient of Ag/AgCl and graphene electrode is as high as 99.47%. These results indicate that the graphene-coated textile electrodes, even in dry condition, have comparable performance with the Ag/AgCl electrode for ECG signal acquisition.
To investigate the mechanical flexibility of the graphene-coated textile electrodes, cyclic bending test was carried out using a stepper motor bending test machine, as shown in Fig. 7a. The change of the resistance of graphene textile electrode is less than 2% over 100 bending cycles, as shown in Fig. 7b. Even at an increase cycle number of 1000, only a ~ 4.5% increase of the resistance is observed, which indicates the good flexbility of graphene-coated texitle electrode. Due to the excellent bending tolerance of graphene-coated textile electrode, it can successfully acquire ECG signals even after 1000 times bending cycles, as shown in Fig. 8. The measured ECG signals from graphene-coated textile electrodes after bending show the characteristic waves of the ECG signal at sitting, standing, and rotating conditions. These results prove that the graphene-coated textile electrode exhibits good flexible tolerance, which is important for its application in wearable ECG signal monitoring.
To further investigate the long-term performance of graphene electrode and commercial gel electrode, we used both electrodes to monitor the ECG signal for 24 h, as shown in Fig. 9a. It can be seen that the ECG signal obtained from the pre-gelled Ag/AgCl electrode and the graphene electrode showed typical ECG waves at both sitting and rotating state. After wearing 24 h, the ECG signal obtained from the pre-gelled Ag/AgCl electrode showed a decreasing in amplitude at sitting state and a large baseline drift under rotating state. This is due to the poor contact between the Ag/AgCl electrode and the skin with gel drying after 24 h. However, the ECG signal obtained from the graphene electrode showed changeless amplitude at sitting state and small baseline drift under rotating state. Moreover, after 24 h of wearing, the skin attached with graphene electrode was not significantly abnormal, while the skin attached with the Ag/AgCl electrode appeared red rash, as shown in Fig. 9b. These results further prove the suitability of the graphene-coated textile electrode for long-time ECG monitoring.
3 Conclusions
In conclusion, we fabricated a graphene-based dry electrode on textile for its applications in ECG monitoring through screen printing technique. The ECG signals acquired from the graphene-coated textile electrode exhibited good quality, which were comparable or even better in wet condition with commercial pre-gelled Ag/AgCl electrodes. Moreover, the graphene-coated textile electrode showed excellent bending tolerance. ECG recordings from the graphene electrode displayed stable characteristics even after a thousand bending cycles. These results have laid an important foundation for the further development of textile electrodes for ECG monitoring. Considering this electrode can be fabricated with low-cost and mass production, it would be a promising tool for widely application in wearable bio-potential monitoring systems for the prevention of cardiovascular diseases.
References
M. Ha, S. Lim, H. Ko, Wearable and flexible sensors for user-interactive health-monitoring devices. J. Mater. Chem. B 6, 4043–4064 (2018)
Y.R. Yang, W. Gao, Wearable and flexible electronics for continuous molecular monitoring. Chem. Soc. Rev. 48, 1465–1491 (2019)
J.J. Oresko, Z.P. Jin, J. Cheng, S.M. Huang, Y.W. Sun, H. Duschl, A.C. Cheng, A wearable smartphone-based platform for real-time cardiovascular disease detection via electrocardiogram processing. IEEE Trans. Inf. Technol. Biomed. 14, 734–740 (2010)
B.Y. Liu, Z.Y. Luo, W.Z. Zhang, Q. Tu, X. Jin, Carbon nanotube-based self-adhesive polymer electrodes for wireless long-term recording of electrocardiogram signals. J. Biomat. Sci. Polym. E 27, 1899–1908 (2016)
J.S. Lee, J. Heo, W.K. Lee, Y.G. Lim, Y.H. Kim, K.S. Park, Flexible capacitive electrodes for minimizing motion artifacts in ambulatory electrocardiograms. Sensors 14, 14732–14743 (2014)
G. Anna, H. Stefan, M. Jörg, Novel dry electrodes for ECG monitoring. Physiol. Meas. 28, 1375 (2007)
V. Marozas, A. Petrenas, S. Daukantas, A. Lukosevicius, A comparison of conductive textile-based and silver/silver chloride gel electrodes in exercise electrocardiogram recordings. J. Electrocardiol. 44, 189–194 (2011)
M.K. Yapici, T. Alkhidir, Y.A. Samad, K. Liao, Graphene-clad textile electrodes for electrocardiogram monitoring. Sens. Actuators B Chem. 221, 1469–1474 (2015)
A.J. Bandodkar, C.S. Lopez, A.M.V. Mohan, L. Yin, R. Kumar, J. Wang, All-printed magnetically self-healing electrochemical devices. Sci Adv. 2, e1601465 (2016)
K.P. Zheng, S.J. Chen, L.Y. Zhu, J.Q. Zhao, X.J. Guo, Large area solution processed poly (dimethylsiloxane)-based thin film sensor patch for wearable electrocardiogram detection. IEEE Electron Dev. L. 39, 424–427 (2018)
J.-H. Moon, D.H. Baek, Y.Y. Choi, K.H. Lee, H.C. Kim, S.-H. Lee, Wearable polyimide–PDMS electrodes for intrabody communication. J. Micromech. Microeng. 20, 025032 (2010)
B. Liu, Z. Luo, W. Zhang, Q. Tu, X. Jin, Silver nanowire-composite electrodes for long-term electrocardiogram measurements. Sens. Actuators B Phys. 247, 459–464 (2016)
H.C. Jung, J.H. Moon, D.H. Baek, J.H. Lee, Y.Y. Choi, J.S. Hong, S.H. Lee, CNT/PDMS composite flexible dry electrodes for long-term ECG monitoring. IEEE T Bio-Med. Eng. 59, 1472–1479 (2012)
B.A. Reyes, H.F. Posada-Quintero, J.R. Bales, A.L. Clement, G.D. Pins, A. Swiston, J. Riistama, J.P. Florian, B. Shykoff, M. Qin, K.H. Chon, Novel electrodes for underwater ECG monitoring. IEEE T Bio-Med. Eng. 61, 1863–1876 (2014)
D. Pani, A. Dessi, J.F. Saenz-Cogollo, G. Barabino, B. Fraboni, A. Bonfiglio, Fully Textile, PEDOT:PSS based electrodes for wearable ECG monitoring systems. IEEE T Bio-Med. Eng. 63, 540–549 (2016)
Y.J. Choi, J.Y. Lee, S.H. Kong, Driver ECG measuring system with a conductive fabric-based dry electrode. IEEE Access. 6, 415–427 (2018)
N. Karim, S. Afroj, A. Malandraki, S. Butterworth, C. Beach, M. Rigout, K.S. Novoselov, A.J. Casson, S.G. Yeates, All inkjet-printed graphene-based conductive patterns for wearable e-textile applications. J. Mater. Chem. C. 5, 11640–11648 (2017)
A. Achilli, A. Bonfiglio, D. Pani, Design and characterization of screen-printed textile electrodes for ECG monitoring. IEEE Sens. J. 18, 4097–4107 (2018)
Y. Peng, S. Xiao, J. Yang, J. Lin, W. Yuan, W. Gu, X. Wu, Z. Cui, The elastic microstructures of inkjet printed polydimethylsiloxane as the patterned dielectric layer for pressure sensors. Appl. Phys. Lett. 110, 261904 (2017)
S.C. Tong, J. Sun, J.L. Yang, Printed thin-film transistors: research from China. ACS Appl. Mater. Interfaces 10, 25902–25924 (2018)
Q. Hu, H. Wu, J. Sun, D. Yan, Y. Gao, J. Yang, Large-area perovskite nanowire arrays fabricated by large-scale roll-to-roll micro-gravure printing and doctor blading. Nanoscale. 8, 5350–5357 (2016)
H. Jang, Y.J. Park, X. Chen, T. Das, M.S. Kim, J.H. Ahn, Graphene-based flexible and stretchable electronics. Adv. Mater. 28, 4184–4202 (2016)
S. Chen, K. Jiang, Z. Lou, D. Chen, G. Shen, Recent developments in graphene-based tactile sensors and E-skins. Adv. Mater. Technol. 3, 1700248 (2018)
J. Kim, J.S. Suh, Size-controllable and low-cost fabrication of graphene quantum dots using thermal plasma jet. ACS Nano 8, 4190–4196 (2014)
M. Del Rosso, C.H. Brodie, S. Ramalingam, D.M. Cabral, E. Pensini, A. Singh, C.M. Collier, Characterisation of graphene electrodes for microsystems and microfluidic devices. Sci. Rep. 9, 5773 (2019)
H. Zhang, H. Xu, X. Ni, S.L. Peng, Q. Liu, F.P. OuYang, Detection of nucleic acids by graphene-based devices: a first-principles study. J Appl Phys 115, 133701 (2014)
Y.S. Zhu, H.B. Cai, H.Y. Ding, N. Pan, X.P. Wang, Fabrication of low-cost and highly sensitive graphene-based pressure sensors by direct laser scribing polydimethylsiloxane. ACS Appl. Mater. Interfaces 11, 6195–6200 (2019)
M. Zhao, X. Cui, Y. Xu, L. Chen, Z. He, S. Yang, Y. Wang, An ordered mesoporous carbon nanosphere-encapsulated graphene network with optimized nitrogen doping for enhanced supercapacitor performance. Nanoscale 10, 15379–15386 (2018)
Y. Wu, S. Liu, K. Zhao, Z. He, H. Yuan, K. Lv, G. Jia, Chemical deposition of MnO2 nanosheets on graphene-carbon nanofiber paper as free-standing and flexible electrode for supercapacitors. Ionics 22, 1185–1195 (2016)
M. Wang, Z. Fang, K. Zhang, J. Fang, F. Qin, Z. Zhang, J. Li, Y. Liu, Y. Lai, Synergistically enhanced activity of graphene quantum dots/graphene hydrogel composites: a novel all-carbon hybrid electrocatalyst for metal/air batteries. Nanoscale 8, 11398–11402 (2016)
Y. Liu, Y. Wang, Y. Zhang, S. Liang, A. Pan, Controllable preparation of V2O5/graphene nanocomposites as cathode materials for lithium-ion batteries. Nanoscale Res. Lett. 11, 549 (2016)
Q. Zhang, W. Peng, Z. Wang, X. Li, X. Xiong, H. Guo, Z. Wang, F. Wu, Synthesis and characterization of Li4Ti5O12/graphene composite as anode material with enhanced electrochemical performance. Ionics 19, 717–723 (2013)
X. Liu, H. Zhao, X. Huang, Y. Hu, H. Gao, X. Liu, L. Shen, Fabrication of flexible graphene paper and its electrochemical properties used in lithium ion batteries. Eur. Phys. J. Appl. Phys. 66, 30301 (2014)
H. Cai, K. Han, H. Jiang, J. Wang, H. Liu, Self-standing silicon-carbon nanotube/graphene by a scalable in situ approach from low-cost Al-Si alloy powder for lithium ion batteries. J. Phys. Chem. Solids 109, 9–17 (2017)
Y. Cai, X. Cao, Z. Luo, G. Fang, F. Liu, J. Zhou, A. Pan, S. Liang, Caging Na3V2(PO4)(2)F−3 microcubes in cross-linked graphene enabling ultrafast sodium storage and long-term cycling. Adv. Sci. 5, 1800680 (2018)
F.L. Wang, H.X. Zhu, H. He, Low temperature sintering of Ag nanoparticles/graphene composites for paper based writing electronics. J. Phys. D Appl. Phys. 49, 415501 (2016)
P. He, J. Cao, H. Ding, C. Liu, J. Neilson, Z. Li, I.A. Kinloch, B. Derby, Screen-printing of a highly conductive graphene ink for flexible printed electronics. ACS Appl. Mater. Interfaces 11, 32225–32234 (2019)
N. Celik, N. Manivannan, A. Strudwick, W. Balachandran, Graphene-enabled electrodes for electrocardiogram monitoring. Nanomaterials 6, 156 (2016)
C.G. Lou, R.K. Li, Z.P. Li, T. Liang, Z.H. Wei, M.T. Run, X.B. Yan, X.L. Liu, Flexible graphene electrodes for prolonged dynamic ECG monitoring. Sensors 16, 1833 (2016)
Y.C. Yang, H.S. Hou, G.Q. Zou, W. Shi, H.L. Shuai, J.Y. Li, X.B. Ji, Electrochemical exfoliation of graphene-like two-dimensional nanomaterials. Nanoscale 11, 16–33 (2019)
P. He, J.R. Brent, H. Ding, J. Yang, D.J. Lewis, P. O'Brien, B. Derby, Fully printed high performance humidity sensors based on two-dimensional materials. Nanoscale 10, 5599–5606 (2018)
S. Yao, Y. Zhu, Nanomaterial-Enabled Dry Electrodes for Electrophysiological Sensing: A Review. JOM. 68, 1145–1155 (2016)
P. He, B. Derby, Inkjet printing ultra-large graphene oxide flakes. 2D Mater. 4, 021021 (2017)
Acknowledgements
This work was supported by the National Natural Science Foundation of China under Grants 51673214, 61804185, and the National Key Research and Development Program of China (2017YFA0206600)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, X., Luo, M., He, P. et al. Screen printed graphene electrodes on textile for wearable electrocardiogram monitoring. Appl. Phys. A 125, 714 (2019). https://doi.org/10.1007/s00339-019-3006-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-019-3006-x