Abstract
We explored the adsorption of SO2, SOF2, and SO2F2 on Pt- or Au-doped MoS2 monolayer based on density functional theory. The adsorption energy, adsorption distance, charge transfer as well as density of states were discussed. SO2 and SOF2 exhibit strong chemical interactions with Pt-doped MoS2 based on large adsorption energy, charge transfer, and changes of electron orbitals in gas molecule. SO2 also shows obvious chemisorption on Au-doped MoS2 with apparent magnetism transfer from Au to gas molecules. The adsorption of SO2F2 on Pt–MoS2 and SOF2 on Au–MoS2 exhibits weaker chemical interactions and SO2F2 losses electrons when adsorbed on Pt-MoS2 which is different from other gas adsorption. The adsorption of SO2F2 on Au–MoS2 represents no obvious chemical interaction but physisorption. The gas-sensing properties are also evaluated based on DFT results. This work could provide prospects and application value for typical noble metal-doped MoS2 as gas-sensing materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, 2D materials have experienced rapid development in many field such as gas sensor, battery, catalytic materials, supercells, energy storage materials, etc. [1,2,3]. Layered transition metal dichalcogenides (TMDs) have unique structure and properties with widespread concern and MoS2 monolayer is the most typical one [4]. For bulk phase of MoS2, it has an indirect bandgap of about 1.2 eV, but the bandgap value increases as the number of layers decreases and reaches nearly 1.9 ev with conversion from indirect bandgap to direct bandgap [5]. MoS2-based Field-Effect Transistor (FET) devices have experience a rapid development process after the first report by Radisavljevic of FET device with 1 × 108 current on/off ratio [6]. For MoS2 and its series of modified materials, they have excellent gas sensitive properties due to their high-specific surface area, favorable adsorption properties to gas molecules.
MoS2 monolayer used as gas-sensing materials has been first reported by Li et al. Single and multi-layers of MoS2 were deposited on an SiO2/Si substrate and showed high sensitivity to NO in the concentration from 0.3 to 2 ppm at room temperature [7]. MoS2-based FET device could also be an excellent chemical gas sensor to detect triethylamine and other organics. The sensor acted as a n-type character and showed high sensitivity and selectivity to triethylamine compared with carbon nanotube [8]. The intrinsic MoS2 devices can detect arsenite down to 0.1 ppb [9]. Moreover, the number of layers can affect the sensing properties as well. For NO2 and NH3 sensing, few layers of MoS2 exhibited better sensing properties compared to monolayer and the charge transfer determined the sensitivity [10]. Not only that, MoS2-based composite materials can promote the sensitivity and selectivity to typical gases than single component of sensing materials [11,12,13,14,15,16,17,18,19].
From the first-principle researches reported by several scholars, pristine MoS2 exhibits weak interaction to most of common gases [20, 21]. To enhance the chemical interaction between typical gas molecule and MoS2 surface, doping is one of the most effective way. The doping type includes metal doping and non-mental doping and metal doping is most based on transition element. The doping of N or P can enhance the performance of MoS2 monolayer for oxygen reduction reaction to some extent [22]. In addition, transition metal doping can bring enhancement in similar realm. Zhao et al. made an elaborate discussion about the effect of doping 19 kinds of transition element on MoS2 for ORR and the results showed that Cu-embedded MoS2 monolayer performed the best [23]. Zhu et al. explored ten types of transition metal embedded monolayer MoS2 to evaluate the adsorption and gas-sensing properties to five common gas molecules including adsorption energy, net charge transfer, charge density, and density of states [24]. Kadioglu et al. [25] analyzed the effect of adding Au and Cu atoms above monolayer MoS2 to adsorb CO and H2O molecules and found that the ratio and content of Au and Cu brought different adsorption properties. Ma et al. [26] used four different transition metal atom-doped MoS2 monolayer, respectively, and compared the electron distribution when adsorbing CO and NO molecules. Therefore, the doping of MoS2 monolayer makes it more active and sensitive to typical gases than pristine monolayer.
Sulfur hexafluoride(SF6) has been used in a variety of industrial applications because of its excellent performance in insulation property. However, SF6 will react with trace water and oxygen in gas-insulated equipment when partial discharge and local overheating appear. The relatively stable products include SO2, SOF2, SO2F2, etc. [27, 28]. The severity of these insulation defects can be obtained by means of detecting the types and concentrations of these products [29, 30]. A typical effective method is to use chemical gas sensor and a series of researches demonstrated that choosing appropriate gas-sensing materials makes it more feasibility and efficiency to detect the products [31,32,33,34,35].
To investigate the chemical interaction between doped MoS2 monolayer and SF6 decompositions to explore the prospective to become gas-sensing materials in this realm, we perform first-principle calculations toward adsorption properties of MoS2 with typical noble metal (Pt and Au) doping. We first obtained the most energy stable adsorption structure of each gas molecule on doped surface. Then, the adsorption properties such as adsorption energy, adsorption distance, and electron transfer were calculated based on density functional theory (DFT). To further study the chemical interactions, total density of states (TDOS) and partial density of states (PDOS) before and after adsorption were discussed. The results suggest that the doping of different transition metal could be an effective method to improve the adsorption and sensing properties of MoS2-to-SF6 decompositions.
2 Computational methods
All the first-principles calculations were carried out using Dmol3 package with density functional theory (DFT) method using linear combination of atomic orbitals (LCAO) [36, 37]. To deal with the electron exchange and correlation, Perdew–Burke–Ernzerhof function (PBE) with generalized gradient approximation (GGA) was employed [38]. We selected the double numerical plus polarization (DNP) as the atomic orbital basis set. DFT semi-core pseudopotential (DSSP) method was applied considering the relativistic effect of transition elements. It means that to reduce the quantity of calculation and increase efficiency, core electrons treatment is substituted by norm-conserving pseudopotentials. For a better description of van der Waals (VDW) interactions, the Tkatchenko and Scheffler’s (TS) method was adopted [39] and we also analyzed the adsorptions without VDW interactions. For geometric optimization, we set the convergence criteria of 1.0 × 10− 5Ha, 0.002 Ha/Å, 0.005 Å for energy tolerance, maximum force, and displacement, respectively, with a smearing of 0.005Ha for accelerating convergence. For static electronic structure calculations, a more accurate 10− 6Ha self-consistent loop energy and a large enough global orbital cut-off radius of 5.0 Å was implemented to ensure the accurate calculation of total energy. The k-point sample of Monkhorst–Pack grid was set to 3 × 3 × 1 of the Brillouin zone for geometric optimization and a more accurate k-point of 6 × 6 × 1 for static energy and electronic structure calculations [40]. All the calculations were spin polarized.
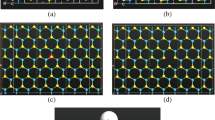
We obtained an optimized lattice parameter of 3.15 Å which is in good consistence with other researches (3.14 Å, 3.16 Å for calculation) [41, 42] and experiment results (3.15 Å, 3.16 Å) [43, 44]. We built a 4 × 4 supercell including 16 Mo and 32 S with a vacuum region of c = 15 Å for prevent the interaction from adjacent unit, as shown in Fig. 1. For transition metal-doped MoS2 models, we placed one Pt or Au atom above the top site of Mo (TMo), top site of S (TS) or hollow site (H) of the surface for geometric optimization and only the lowest energy structures were chosen for the following adsorption calculations.
We define the adsorption energy of one Pt/Au atom adsorbed on MoS2 monolayer as the following equation:
where Eone TM atom and \({E_{{\text{Mo}}{{\text{S}}_2}\;{\text{monolayer}}}}\) denote the total energy of one transition metal atom (Pt/Au) and optimized MoS2 monolayer and \({E_{{\text{Mo}}{{\text{S}}_2}{\text{-TM}}}}\) represents the total energy of one TM atom adsorbed on MoS2 monolayer.
We also define the adsorption energy of one gas molecule adsorbed on doped MoS2 monolayer as the following equation:
where Emolecule and \({E_{{\text{Mo}}{{\text{S}}_2}{\text{-TM}}}}\) denote the total energy of isolated gas molecule, respectively, and optimized doped MoS2 monolayer and \({E_{{\text{molecule}}/{\text{Mo}}{{\text{S}}_2}}}\) represents the total energy of adsorption system. We use the Hirshfeld (HI) method to define the charge transfer Qt and a negative value means that the electrons transfer from MoS2 to gas molecules, so the gas molecule has negative charge.
To explore the best adsorption orientation and adsorption location, we set three initial adsorption directions of SO2 (vertical with S above, parallel, vertical with S downward), two directions of SOF2 (S above and S downward), and two directions of SO2F2 (F above and O above), as shown in Fig. S1; the structure of gas molecule had been optimized using the above converge criterion and cut-off parameter and the results are shown in Table S1. All the initial distances between transition metal and S atom in molecule are set to 2.5 Å.
3 Results and discussion
3.1 Pt- and Au-doped MoS2 monolayer
The three possible structures of one Pt/Au atom adsorbed on MoS2 surface were tested to evaluate the most stable adsorption configuration. As shown in Fig. 1, based on the largest adsorption energy, the Pt atom is inclined to locate on the top of Mo site(TMo), while Au tends to be in top site of S(TS). All the adsorption energies and configurations are listed in Table S2, and Figs. S1 and S2. The lengths of bonds between Pt and S are 2.31, 2.31, and 2.32 Å, and Au and S is 2.40 Å, which is strongly accords with other study [45, 46]. The TDOS are shown in Fig. 2. The Pt-doped MoS2 structure has no magnetism with highly symmetric TDOS curve. However, due to the total magnetic moment of Au, doped MoS2 is 1.0 µB; the TDOS presents a certain asymmetry especially near Fermi-level. On account of relevant, more detailed results have been reported [45, 46]; we will not discuss it furthermore. For interactions and electronic structure, we only choose the most energy favorable adsorption configuration for each gas.
3.2 SO2 adsorption
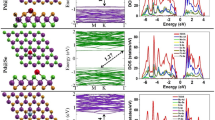
Figure 3 shows the configuration for SO2 adsorption on Pt- or Au-doped MoS2 monolayer. As to Pt–MoS2, SO2 molecule locates nearly vertical to the surface with an adsorption distance of about 2.19 Å. The structure of SO2 does not experience obvious change; only the angle of O–S–O has a small decrease to 119.0°. However, the distance between Pt and S on MoS2 surface shows an increased tendency, reaching 2.39, 2.39, and 2.42 Å, respectively, while the distance between Pt and right below Mo increases from 2.80 to 2.91 Å. For SO2 adsorbed on Au–MoS2, the plane of molecule has an angle of about 45° with MoS2 surface. The adsorption distance is 2.36 Å and the bond length of S–O increases to 1.48 Å with slight larger bond angle of 120.3°. Au becomes closer to S atom on surface by 2.36 Å. Considering other adsorption parameters, the adsorption of SO2 on Pt–MoS2 brings larger adsorption energy, but less charge transfer compared to Au–MoS2 and SO2 gains electrons from Pt–MoS2 as well as Au–MoS2.
To explore the electronic properties, we perform the TDOS and PDOS including the role of each atom orbital, as shown in Fig. 4. In Fig. 4a, the TDOS of isolated SO2 contains six peaks in spin up and spin down, respectively, with no magnetic moment. After adsorption on Pt–MoS2, the original highest peak near − 4 eV splits into two peaks located from − 6 to − 7 eV in DOS. The three original peaks from 0 to − 2 eV mix together to be a relatively wider peak and also the peak right to the Fermi-level becomes wider. Based on this phenomenon, the adsorption of SO2 on Pt–MoS2 indeed changes the electron orbitals of gas molecule. From Fig. 4d, it can be observed that the peaks of Pt and S in gas molecule near − 11, − 7, and − 6, 2 eV exhibit apparent overlap, indicating that quite strong electron orbital interaction between Pt and gas molecules. As to the adsorption on Au–MoS2, not just the changes of peak intensity and width, the SO2 processes a certain magnetism due to the asymmetry of navy curve, as shown in Fig. 4c. To have a further study of magnetic properties, we find that SO2 has a magnetic moment of 0.57 µB after adsorption and the magnetic moment of Au decrease from 0.56 to 0.16 µB which demonstrates that not only charge transfer but also magnetism transfer happens when SO2 adsorbed on Au–MoS2. In Fig. 4e, peaks overlapping appear near − 11, − 7.5, − 6, − 2, and 0 eV. S 3s orbitals contribute more than S 3p near − 11 and − 2 eV, and for other overlap region, the case is the opposite. The relatively large adsorption energy, charge transfer, and obvious change of electron orbitals of SO2 demonstrate the obvious chemical interaction between SO2 molecule and Pt–MoS2 (Au–MoS2).
3.3 SOF2 adsorption
When SOF2 adsorbed on doped MoS2, the adsorption directions for Pt–MoS2 and Au–MoS2 are the same; that is, the S atom locates beneath other atoms in molecule. An adsorption distance of 2.19 Å for Pt–MoS2 with no obvious change of bond lengths of gas molecule, but a little decrease of bond angles from 93.3° to 92.2° and 106.8° to 106.0°. The distances of Pt and nearest Mo increase to 2.89 Å, illustrating that Pt has the tendency of keeping away from the MoS2 surface when SOF2 adsorption which is similar to SO2 adsorption. As to SOF2 adsorbed on Au–MoS2, the distance between Au and adjacent S on surface maintains 2.40 Å, but the bond of Au–S shows a little inclination to the surface. Moreover, dramatic variation of gas molecule appears in the elongation of S–F2 bond which reaches 1.70 Å and changes of bond angles (105.8° to O–S–F1 and 107.7° to O–S–F2). The adsorption energy of SOF2 on Pt–MoS2 is significantly higher than Au–MoS2 (1.370–0.332 eV), but the charge transfer between gas molecule and Au–MoS2 is larger. We estimate that this phenomenon may be due to the partial consumed energy of translational motion of Au on surface. SOF2 acts as an electron acceptor when adsorption on these two kinds of doped surface (Fig. 5).
For DOS analysis, the curve of isolated SOF2 expresses several peaks with nearly the same intensity. After SOF2 adsorbed on Pt–MoS2, all the peaks experience left shift by about 3 eV. A new higher peak appears near − 8 eV which is mainly attributed to peak mixing near − 5 eV of isolated DOS curve and three peaks near − 5 eV of adsorbed SOF2 can be ascribed to peak mixing from − 3 to − 1 eV in isolated SOF2. As it can be seen, the electron orbitals of SOF2 experience certain changes when it adsorbed on Pt–MoS2. In Fig. 6d, the Pt 5d orbitals overlap with S 3 s near − 13 eV, with S 3p near − 13, − 8.5, − 7, − 5, and − 3 eV and the Pt 6p orbitals overlap with S 3p near − 0.5 eV. These overlaps indicate the orbital interaction between Pt and gas molecules. As to gas molecule adsorbed on Au–MoS2, the peaks near − 5 eV of adsorbed SOF2 experience peak mixing compared with isolated SOF2 near − 2 eV. In addition, the DOS curve of adsorbed molecule shows slight asymmetry indicating the non-zero magnetic moment of adsorbed SOF2. The calculated magnetic moment of adsorbed SOF2 is 0.31 µB which is lower than adsorbed SO2 and a decline of magnetic moment to 0.26 µB for Au atom also indicates partial magnetic transfer from Au–MoS2 to SOF2. In Fig. 6e, Au 5d orbitals overlap with S 3s near − 8.5 eV and S 3p near − 8.5 and − 5 eV, while Au 6s orbitals overlap with S 3s and S 3p near 0 eV. Based on the DOS changes of molecule before and after adsorption and overlap in electron orbital, there exists some chemical interactions between Au–MoS2 and SOF2, but these interactions are weaker compared with SO2 adsorption on Au–MoS2 due to the less adsorption energy, charge transfer, magnetism transfer and orbital overlaps.
3.4 SO2F2 adsorption
For SO2F2 adsorbed on Pt–MoS2, the distance between Pt and adjacent Mo is 2.78 Å with little change compared to the initial distance of 2.79 Å and the structure of gas molecule remains nearly unchanged except the little increase of S–O1 by 0.02 Å. When SO2F2 adsorbed on Au–MoS2, it shows a very large adsorption distance of 3.32 Å with small adsorption energy of 0.175 eV, and the structure of MoS2 also remains unchanged with only little transitional movement of Au (Fig. 7).
To ensure whether electron orbital interaction appears between SO2F2 and doped MoS2, DOS was discussed in detail. TDOS of SO2F2 shows a symmetrical curve of several peaks with the same intensity and one higher peak near − 2.7 eV. After SO2F2 adsorbed on Pt-MoS2, we notice that two initial peaks near Fermi-level coalesce into one peak with larger intensity located near − 4.5 eV in DOS of adsorbed SO2F2 and beyond that the intensity and relative position of other initial peaks do not show a clear change. For PDOS of every orbital, it can only be seen that slight overlap between Pt 6 s and O1 2p near − 6.5 eV, Pt 5d and O1 2p near − 4.5 eV where peak mixing happens in gas molecule. As to gas adsorbed on Au–MoS2, one can see that all the peaks of gas molecule have evidently not changed including intensity and relative position, only left shift by about 4 eV. It should be noted that the left shift is only due to the different Fermi levels of isolated SO2F2 and Au–MoS2, so only the left shift could not indicate the change of electron orbitals in gas molecule. Despite of this, the high symmetry of navy curve in Fig. 8c illustrates no magnetic moment change after adsorption. As a result, the structure as well as the electron orbitals of gas molecule shows no obvious variation after adsorbed on Au–MoS2. The phenomenon indicates that only physical interaction rather than chemical adsorption appears between SO2F2 and Au–MoS2.
3.5 Summarizing of adsorption properties and forecasting of sensing application
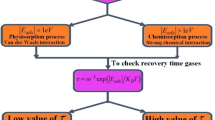
All the parameters of SO2, SOF2, and SO2F2 adsorbed on Pt–MoS2 and Au–MoS2 are listed in Table 1 and Table S4. All the adsorption energies experience a certain degree of reduction when not adopting TS method, but the values of charge transfer and adsorption distance do not change much. The interaction between SO2F2 and Au–MoS2 is mainly VDW force, and for different adsorptions, the proportion of VDW force is different. To consider the negligible role of VDW interactions, we mainly focus on the results obtained using TS method. Due to the negative value of adsorption energies, all the adsorptions are exothermic process. For a gas sensor, the sensitivity depends on both the adsorption energy and charge transfer [10]. The adsorption energy of Pt–MoS2 shows generally greater than Au–MoS2. Larger adsorption energy could bring greater adsorption amount and interaction between gases and adsorbent. For SO2 and SOF2 adsorption on Pt–MoS2, the adsorption distance (2.19 Å) is even smaller than the sum of single-bond covalent radii of Pt and S (2.26 Å) which can also indicate the strong chemical interaction between Pt–MoS2 and SO2, SOF2, and even new chemical bond formation [47]. As to SO2F2 adsorbed on Au-MoS2, the distance between Au and O1 is 3.32 Å; much larger than 1.87 Å (the sum of covalent radii of Au and O) can also prove the weak interaction between them. The adsorption energy of SO2 on Au–MoS2 is also quite large (− 0.946 eV) with the greatest charge transfer, reaching 0.222e. Charge transfer is one of the most important parameter affecting sensitivity [10]. More charge transfer with the same adsorption amount can give rise to larger sensitivity. For SO2F2 adsorbed on Pt-MoS2, the direction of charge transfer is the opposite compared with other gases which can cause the resistance change of sensor in the opposite direction. Another important parameter of a gas sensor is the recovery properties. Although larger adsorption energy and charge transfer bring greater sensitivity, the sensitivity is at the price of recovery property. If strong chemical interaction happens between gases and surface, it is difficult for desorption resulting in long recovery time. Based on the transition state theory and Van’t–Hoff–Arrhenius expression [48], the recovery time could be defined as:
where A, R, and T represent the apparent frequency factor, Boltzmann’s constant, and temperature, respectively, and Ea refers to the activation energy. For desorption process, the activation energy can be seen as the above adsorption energy Ead. If the factor A does not change much with different types of gases and different metal doping, the smaller adsorption energy could bring the shorter recovery time at the same temperature. Comparing the adsorption energy of gas molecule on Pt–MoS2 and Au–MoS2, SO2, and SOF2 is very difficult to desorb from Pt–MoS2 unless elevating the working temperature. Due to the better desorption property of Au–MoS2 than Pt-MoS2, the working temperature of Au–MoS2 can be lower than Pt–MoS2. In short, both Pt–MoS2 and Au–MoS2 will have high sensitivity to SO2, but Au–MoS2 has a better recovery property so it can work at a lower temperature than Pt–MoS2. For SOF2 sensor, the sensitivity of Pt–MoS2 will be much higher than Au–MoS2, so Pt–MoS2 is more suitable for SOF2 sensing, but the working temperature should be relatively high to guarantee the shorter recovery time. As to SO2F2 sensor, Pt–MoS2 may experiences a different direction of resistance change compared to SO2 and SOF2, and the recovery property is also better because of the very weak interaction between Au–MoS2 and SO2F2, Au–MoS2 is not suitable for SO2F2 sensing due to the low sensitivity.
4 Conclusions
To explore the adsorption behavior and estimate the gas-sensing properties of Pt- and Au-doped MoS2 monolayer, density functional theory was used to calculate the adsorption energy, charge transfer, adsorption distance, and density of states. For SO2, both Pt–MoS2 and Au–MoS2 exhibit relatively large adsorption energy and charge transfer with strong chemical interactions due to the obvious change of electron orbitals in gas molecule and orbital interactions between Au on surface and S in molecule. More than that, SO2 adsorption will introduce magnetism transfer from Au–MoS2 to molecule to some extent. For SOF2, the adsorption energy of Pt–MoS2 is much greater than that of Au–MoS2, but the charge transfer comparison is the opposite. The adsorption of SOF2 also brings different levels of changing in electron orbitals of gas molecule on both Pt–MoS2 and Au–MoS2 and magnetism transfer on Au–MoS2. As to SO2F2, the interactions between gas molecule and surface are weaker than the above two gases. The SO2F2 acts as an electron donor when adsorbed on Pt–MoS2 which is different from other gases. No chemical interactions are found when SO2F2 adsorbed on Au–MoS2 for the reason that electron orbitals of gas molecule remain constant with little orbital interactions between Au and SO2F2. Moreover, the application possibility of Pt–MoS2 and Au–MoS2 using as gas-sensing material to detect these three types of gases was estimated using the conventional transition state theory. This study could provide fundamental basis for typical noble metal-doped MoS2 as gas-sensing material to detect typical decompositions of sulfur hexafluoride in gas-insulated equipment for achieving industrial application.
References
C. Tan, X. Cao, X.J. Wu, Q. He, J. Yang, X. Zhang et al., Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 117(9), 6225–6331 (2017)
M. Xu, T. Liang, M. Shi, H. Chen, Graphene-like two-dimensional materials. Chem. Rev. 113(5), 3766–3798 (2013)
Q. Fu, X. Bao, Surface chemistry and catalysis confined under two-dimensional materials. Chem. Soc. Rev. 46(7), 1842–1874 (2017)
M. Chhowalla, H.S. Shin, G. Eda, L.J. Li, K.P. Loh, H. Zhang, The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 5(4), 263–275 (2013)
K.F. Mak, C. Lee, J. Hone, J. Shan, T.F. Heinz, Atomically thin MoS2: a new direct-gap semiconductor. Phys. Rev. Lett. 105(13), 136805 (2010)
B. Radisavljevic, A. Radenovic, J. Brivio, I.V. Giacometti, A. Kis, Single-layer MoS2 transistors. Nat. Nanotechnol. 6(3), 147–150 (2011)
H. Li, Z. Yin, Q. He, H. Li, X. Huang, G. Lu et al., Fabrication of single and multilayer MoS2 film based field effect transistors for sensing NO at room temperature. Small. 8(1), 63–67 (2012)
F.K. Perkins, A.L. Friedman, E. Cobas, P.M. Campbell, G.G. Jernigan, B.T. Jonker, Chemical vapor sensing with monolayer MoS2. Nano Lett. 13(2), 668–673 (2013)
P. Li, D. Zhang, Y.E. Sun, H. Chang, J. Liu, N. Yin, Towards intrinsic MoS2 devices for high performance arsenite sensing. Appl. Phys. Lett. 109(6), 063110 (2016)
D.J. Late, Y.K. Huang, B. Liu, J. Acharya, S.N. Shirodkar, J. Luo et al., Sensing behavior of atomically thin-layered MoS2 transistors. Acs Nano. 7(6), 4879–4891 (2013)
D. Sarkar, X. Xie, J. Kang, H. Zhang, W. Liu, J. Navarrete et al., Functionalization of transition metal dichalcogenides with metallic nanoparticles: implications for doping and gas-sensing. Nano Lett. 15(5), 2852–2862 (2015)
Y. Niu, R. Wang, W. Jiao, G. Ding, L. Hao, F. Yang, X. He, MoS2 graphene fiber based gas sensing devices. Carbon 95, 34–41 (2015)
H. Yan, P. Song, S. Zhang, J. Zhang, Z. Yang, Q. Wang, A low temperature gas sensor based on Au-loaded MoS2 hierarchical nanostructures for detecting ammonia. Ceram. Int. 42(7), 9327–9331 (2016)
N. Yue, J. Weicheng, W. Rongguo, D. Guomin, H. Yifan, Hybrid nanostructures combining graphene–MoS2 quantum dots for gas sensing. J. Mater. Chem. A 4(21), 8198–8203 (2016)
D.H. Baek, J. Kim, MoS2 gas sensor functionalized by Pd for the detection of hydrogen. Sensors Actuators B Chem. 250, 686–691 (2017)
D. Zhang, J. Wu, P. Li, Y. Cao, Room-temperature SO2 gas-sensing properties based on a metal-doped MoS2 nanoflower: an experimental and density functional theory investigation. J. Mater. Chem. A 5(39), 20666–20677 (2017)
D. Zhang, Y.E. Sun, P. Li, Y. Zhang, Facile fabrication of MoS2-modified SnO2 hybrid nanocomposite for ultrasensitive humidity sensing. ACS Appl. Mater. Interfaces 8(22), 14142–14149 (2016)
D. Zhang, C. Jiang, P. Li, Y.E. Sun, Layer-by-layer self-assembly of Co3O4 nanorod-decorated MoS2 nanosheet-based nanocomposite toward high-performance ammonia detection. ACS Appl. Mater. Interfaces 9(7), 6462–6471 (2017)
D. Zhang, Z. Wu, P. Li, X. Zong, G. Dong, Y. Zhang, Facile fabrication of polyaniline/multi-walled carbon nanotubes/molybdenum disulfide ternary nanocomposite and its high-performance ammonia-sensing at room temperature. Sensors Actuators B Chem. 258, 895–905 (2018)
S. Zhao, J. Xue, W. Kang, Gas adsorption on MoS2 monolayer from first-principles calculations. Chem. Phys. Lett. 595, 35–42 (2014)
A. Shokri, N. Salami, Gas sensor based on MoS2 monolayer. Sensors Actuators B Chem. 236, 378–385 (2016)
H. Zhang, Y. Tian, J. Zhao, Q. Cai, Z. Chen, Small dopants make big differences: enhanced electrocatalytic performance of MoS2 monolayer for oxygen reduction reaction (ORR) by N- and P-doping. Electrochim. Acta 225, 543–550 (2017)
Z. Wang, J. Zhao, Q. Cai, F. Li, Computational screening for high-activity MoS2 monolayer-based catalysts for the oxygen reduction reaction via substitutional doping with transition metal. J. Mater. Chem. A 5(20), 9842–9851 (2017)
Y. Fan, J. Zhang, Y. Qiu, J. Zhu, Y. Zhang, G. Hu, A DFT study of transition metal (Fe, Co, Ni, Cu, Ag, Au, Rh, Pd, Pt and Ir)-embedded monolayer MoS2 for gas adsorption. Comput. Mater. Sci. 138, 255–266 (2017)
Y. Kadioglu, G. Gökoğlu, O. Aktürk, Molecular adsorption properties of CO and H2O on Au-, Cu-, and AuxCuy-doped MoS2 monolayer. Appl. Surf. Sci. 425, 246–253 (2017)
D. Ma, W. Ju, T. Li, X. Zhang, C. He, B. Ma et al., The adsorption of CO and NO on the MoS2 monolayer doped with Au, Pt, Pd, or Ni: A first-principles study. Appl. Surf. Sci. 383, 98–105 (2016)
F.Y. Chu, SF6 decomposition in gas-insulated equipment. IEEE Trans. Electr. Insul. (5), 693–725 (1986)
J. Tang, F. Liu, X. Zhang, Q. Meng, J. Zhou, Partial discharge recognition through an analysis of SF6 decomposition products part 1: decomposition characteristics of SF6 under four different partial discharges. IEEE Trans. Dielectr. Electr. Insul. 19(1), 29–36 (2012)
J. Tang, F. Liu, Q. Meng, X. Zhang, J. Tao, Partial discharge recognition through an analysis of SF6 decomposition products part 2: feature extraction and decision tree-based pattern recognition. IEEE Trans. Dielectr. Electr. Insul. 19(1), 37–44 (2012)
S. Li, J. Li, Condition monitoring and diagnosis of power equipment: review and prospective. High Volt. 2(2), 82–91 (2017)
J. Suehiro, G. Zhou, M. Hara, Detection of partial discharge in SF6 gas using a carbon nanotube-based gas sensor. Sensors Actuators B Chem. 105(2), 164–169 (2005)
H. Dai, P. Xiao, Q. Lou, Application of SnO2/MWCNTs nanocomposite for SF6 decomposition gas sensor. Phys. Status Solidi 208(7), 1714–1717 (2011)
X. Zhang, J. Zhang, Y. Jia, P. Xiao, J. Tang, TiO2 nanotube array sensor for detecting the SF6 decomposition product SO2. Sensors 12(3), 3302–3313 (2012)
S. Peng, G. Wu, W. Song, Q. Wang, Application of flower-like ZnO nanorods gas sensor detecting SF6 decomposition products. J. Nanomater. 2013, 1 (2013)
X. Zhang, L. Yu, X. Wu, W. Hu, Experimental sensing and density functional theory study of H2S and SOF2 adsorption on Au-modified graphene. Adv. Sci. 2(11), (2015)
B. Delley, An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 92(1), 508–517 (1990)
B. Delley, From molecules to solids with the DMol3 approach. J. Chem. Phys. 113(18), 7756–7764 (2000)
J.P. Perdew, K. Burke, M. Ernzerhof, Generalized gradient approximation made simple. Phys. Rev. Lett. 77(18), 3865 (1996)
A. Tkatchenko, R.A. DiStasio Jr, M. Head-Gordon, M. Scheffler, Dispersion-corrected Møller–Plesset second-order perturbation theory. J. Chem. Phys. 131(9), 094106 (2009)
H.J. Monkhorst, J.D. Pack, Special points for Brillouin-zone integrations. Phys. Rev. B 13(12), 5188 (1976)
L. Gao, Z.D. Yang, G. Zhang, A theoretical study for electronic and transport properties of covalent functionalized MoS2 monolayer. Chem. Phys. 490, 29–37 (2017)
R. Kronberg, M. Hakala, N. Holmberg, K. Laasonen, Hydrogen adsorption on MoS2-surfaces: A DFT study on preferential sites and the effect of sulfur and hydrogen coverage. Phys. Chem. Chem. Phys. 19, 16231–16241 (2017)
R.G. Dickinson, L. Pauling, The crystal structure of molybdenite. J. Am. Chem. Soc. 45(6), 1466–1471 (1923)
D. Yang, S.J. Sandoval, W.M.R. Divigalpitiya, J.C. Irwin, R.F. Frindt, Structure of single-molecular-layer MoS2. Phys. Rev. B 43(14), 12053 (1991)
P. Wu, N. Yin, P. Li, W. Cheng, M. Huang, The adsorption and diffusion behavior of noble metal adatoms (Pd, Pt, Cu, Ag and Au) on a MoS2 monolayer: a first-principles study. Phys. Chem. Chem. Phys. 19(31), 20713–20722 (2017)
J. Chang, S. Larentis, E. Tutuc, L.F. Register, S.K. Banerjee, Atomistic simulation of the electronic states of adatoms in monolayer MoS2. Appl. Phys. Lett. 104(14), 141603 (2014)
P. Pyykkö, M. Atsumi, Molecular single-bond covalent radii for elements 1–118. Chem. A Eur. J. 15(1), 186–197 (2009)
Y.H. Zhang, Y.B. Chen, K.G. Zhou, C.H. Liu, J. Zeng, H.L. Zhang, Y. Peng, Improving gas sensing properties of graphene by introducing dopants and defects: a first-principles study. Nanotechnology. 20(18), 185504 (2009)
Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of P. R. China (Project No. 51777144) and National High-tech R&D Program of China (Project No. 2015AA050204).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, D., Zhang, X., Tang, J. et al. Noble metal (Pt or Au)-doped monolayer MoS2 as a promising adsorbent and gas-sensing material to SO2, SOF2 and SO2F2: a DFT study. Appl. Phys. A 124, 194 (2018). https://doi.org/10.1007/s00339-018-1629-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-018-1629-y