Abstract
Pt NPs have attracted much attention because of their applications in many aspects such as the electronics industry, fuel cells, cancer therapy, etc. The influence of the applied electric field on the formation of platinum NPs by laser (Nd:YAG, λ = 1064 nm) ablation technique was investigated, for the first time. The TEM images showed that at higher values of the electric field, a variety of shapes like rectangular, hexagon, and rhombic were appeared besides the spherical NPs, as confirmed by SEM investigations. By increasing the applied electric field, the average size of Pt NPs decreased from 20 to 9 nm. In addition, from the XRD spectra, the mean crystalline size, crystal structure, d-spacing, and lattice parameters of NPs were calculated. On the basis of the optical absorption spectra of NPs, we observed a size-dependent blue shift of the SPR peak position when the value of the electric field increased from 0 to 20 V/cm. To identify the stretching and bending frequencies of the molecular functional groups attached to the NPs surface, Raman and FT-IR spectroscopy was applied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Metallic nanoparticles (NPs), especially platinum (Pt) NPs, have attracted much attention due to their particular attributes in many aspects; for example, they are great catalysts for the decrement of pollutant gases exhausted from the automobiles [1, 2]. In addition, for elimination of NO generated in a combustion procedure, the Pt NPs supported on a porous alumina can be applied [1]. Furthermore, Pt NPs have important industrial applications, in particular, in the electronics industry for the production of interior electrodes of multilayer ceramic capacitors and conductive thick-film circuits [2]. In fuel cells, Pt nanoparticles are the special catalyst for the generation of hydrogen from water in proton-exchange membrane fuel cells. Enzymatic platinum/multi-walled carbon nanotubes can be utilized as a glucose sensor, while Pt NPs are deposited on these carbon nanotubes. Besides, due to the slight toxic effects of Pt on human cells, it is used for cancer therapy [3].
Various factors such as the particle size, preparation methods, dispersion of the particles, and supporting materials can affect the electrocatalytic activity of Pt NPs [4]. Among the common techniques for the fabrication of colloidal Pt NPs, the pulsed laser ablation in liquid media is one of the fast, simple, and green approaches, which produces extremely stable NPs with high purity [5]. Hence, Electrical-Field-Assisted Laser Ablation in Liquids (EFLAL) has been suggested as a novel technique to fabricate microparticles and NPs with unique morphology, crystalline properties, and controlled size [6, 7]. Due to the existence of the strong correlation between the structure, shape, and size of metal NPs and the electrical, optical, and catalytic properties, the control of these parameters is essential [8]. The synthesis of NPs upon EFLAL is thoroughly investigated by various group; for instance, Liu et al. [9] prepared GeO2 micro- and nano-cubes with high-index facets and a kind of GeO2 micro- and nanospindles upon EFLAL at 532 nm using a nanosecond-pulsed Q-switched Nd: YAG laser. They found that the use of a suitable electric field can impact the shape of NPs. The work of Ismail et al. [7] involved the production of Bi2O3 NPs by the pulsed laser ablation in water under the effect of the electric field. They found that applying the electric field during laser ablation led to the increase of the Bi2O3 particle size. Sapkota et al. [10] used an excimer laser (351 nm) to ablate a solid tin target immersed in water in the presence of an external electric field. Thamir et al. [11] reported the synthesis of gold NPs by the pulsed Nd:YAG (1064 nm) laser ablation in deionized water to study the effect of the electric field on the antibacterial activity of these particles. Al-Haddad et al. [8] synthesized Au NPs by pulsed laser (Nd:YAG, λ = 1064 nm) ablation of a gold target immersed in deionized water by 300 mJ of laser energy, which a D.C electrical field was applied above the target with adjusted voltage. Due to the technological interests of Pt NPs, there are many researches on the synthesis and study of these particles during last years [12,13,14,15,16]. Up to the best of our knowledge, no papers have been published on EFLAL of Pt NPs.
In the present study, the effect of the applied electric field on the ablation products from a platinum target was considered using the laser source of nanosecond pulse durations. Both the optical and structural properties of NPs were investigated.
2 Experimental section
A Q-switched Nd:YAG laser device (Q-PLUS model from Spectrum. A. T. N. Ltd) with the first (1064 nm) harmonic wavelength, a pulsewidth of 7 ns, a repetition rate of 10 Hz, and laser shots number of 9000 pulses were used as the ablation source. Each pulse had a fluence of 1.5 J/cm2 to ablate the bulk target normal to its surface. Prior to beginning the experiment, to obtain the clean metal surfaces, the platinum rectangular-shaped target (1 mm thickness, 99.99% in purity) was polished with a 1500-grade emery paper. Then, it was purified with absolute ethanol, acetone, and distilled water using ultrasonication for 30 min to eliminate the pollution from its surface. After that, the target was initially fixed to the bottom of a rectangular Pyrex vessel with dimensions 8 × 5.5 × 4.5 cm3, and it was covered with 30 ml of distilled water, as the liquid environment. The depth of the solution layer above the target was ∼ 6 mm. A steady electric field was produced using a potential difference between two gold (99.99% purity) electrodes (2 × 2.6 cm2), which were positioned inside, and parallel to the two sides of the Pyrex vessel. The distance between two electrodes was 20 mm. The platinum target was placed in the center between the gold electrodes. During the ablation phenomena, two electrodes were immersed in distilled water. By applying the potential differences of 0, 10, 20, 30, and 40 V to the gold electrodes, the electric fields with magnitudes of 0, 5, 10, 15, and 20 V/cm were created. The laser beam was focused by an objective lens to a point approximately 6 mm in diameter, with a focal length of 100 mm, onto the plate target. In addition, the spot size of the laser beam on the plate target surface was calculated to be 40 μm for 1064 nm.
During the laser ablation, the Pt target and distilled water were retained at room temperature. In addition, the ablation process was typically fixed, as 15 min for all the experiments. Figure 1 shows a schematic illustration of the experimental setup of EFLAL technique. Properties of Pt colloids were studied through the spectroscopic and microscopic techniques. The absorption spectra of the samples were immediately characterized by an UV–Vis–NIR absorption spectrophotometer (Varian cary 500 scan) operated in the range of 175–3300 nm. All spectra were measured at room temperature in a quartz cell with a 10 mm optical path. X-ray diffraction (XRD) patterns were measured on an STOE X-ray diffractometer with Cu Kα radiation (λ = 1.54060 Å). The suspensions were dropped onto Si substrates and dried over a long period of time (72 h) for XRD and scanning electron microscopy (SEM) characterization. A drop of the suspension of the product was placed on the carbon-coated copper grids for the transmission electron microscopy (TEM) analysis. The shape, size distribution, and selected area electron diffraction (SAED) patterns of the particles were investigated by a Philips-EM 208S TEM operating at 100 kV. Furthermore, SEM images were taken on a KYKY-EM 3200 microscope. The surface of prepared metal NPs was considered by a Fourier transform infrared (FTIR) spectrophotometer Nexus 870 (Thermo Nicolet USA) in the mid-IR region (4000 − 400 cm−1), while the Raman spectra were obtained using an FT–Raman system (Thermo Nicolet 960 USA). The 633 nm line from a 1 mW He–Ne laser was used as the excitation source.
3 Results and discussion
The laser ablation of the Pt target by a high-intensity laser pulses within the nanosecond range can cause the fast increase of the surface temperature and the evolution from evaporation to explosive boiling. This process results in the formation of the ionized plasma containing of liquid droplets in nanoscale, vapor, free electrons, etc.
After that, the nucleation of the nanoscale cavitation bubbles along with the release of energy and creating Pt NPs takes place [10, 17]. In the EFLAL technique, by applying the electric field to the plasma plume, it is possible to control the metal NPs formation. Indeed, the electric charge distributions in the plasma plume can be affected by the applied electric fields. In addition, the electric fields result in the production of charged nanodroplets in the plasma plume and reduction of the recombination of electrons with ions. Then, the size distribution of nanodroplets in the liquid state, due to the Rayleigh instability, may be affected by the charge of nanodroplets. This is because of the electrostatic interactions. Then, these nanoscale size liquid droplets are transported by the electric field at the metal electrodes in a procedure that is similar to the electrophoresis [6, 10]. Overall, the applied electric fields upon the laser ablation can influence the composition and morphology of the final metal NPs [17]. In this research, the color of the colloidal solution changed from colorless before irradiation to yellowish brown after irradiation at different values of applied electric field, as illustrated in Fig. 2. Figure 3 shows the UV–Vis absorption spectra of colloidal Pt NPs synthesized at different values of electric field. The surface plasmon resonance (SPR) peak of the samples prepared at 0, 5, 10, 15, and 20 V/cm are located at about 239, 236, 231, 221, and 220 nm, in the UV region, respectively. The presence of these peaks in the absorption spectra is indicating the metallic nature of Pt NPs. In addition, the SPR peaks are along with a long tail towards the longer wavelengths. This probably reveals the production of inhomogeneous sizes, particle coagulation, and very small particles [18,19,20].
Figure 4 illustrates the correlation between the maximum absorption wavelength (the SPR peak position) and the applied electric field during the laser ablation.
It is obvious that with increasing the applied electric field to 20 V/cm, the SPR peak position is blue-shifted. The blue shift (the absorbance at shorter wavelengths) in SPR peaks can be attributed to the change in the size of NPs, which is an effect of quantum confinement [21,22,23]. In general, the size-tunable electrical and optical features of small nanostructures can be due to a quantum mechanical phenomenon known as quantum confinement.
On the other hand, the blue shift in the SPR peak position ensures the decrease in the mean particle size, which is matched as obtained from the size distribution histograms, as shown in Figs. 5 and 6 [21, 24].
The reason for this is the same as has been explained earlier. Figures 5 and 6 show the TEM images and corresponding the size distribution histograms of Pt NPs prepared under different electric fields of 0, 5, 10, 15, and 20 V/cm. These histograms fitted to the log-normal distribution are obtained by measuring the diameter of more than 300 particles, using the Microstructure Measurement software. The average sizes of NPs at the applied electric fields of 0, 5, 10, 15, and 20 V/cm are about 20, 16, 14, 12, and 9 nm, respectively. By increasing the applied electric field, the average size value decreases, as reported in the previous works [10, 17]. Figure 7 reveals the variation of the average size, as a function of the applied electric field. About the physical mechanism of decreasing the mean size of NPs with the rise of the electric field strength, several processes taken place during EFLAL such as plume expansion along with the evolution of charge distribution, trapped electrons by nanodroplets, and ionization of the atoms can cause this mechanism.
The plasma plume includes the free electrons, ions, vapor, and nanodroplets. It is important to say that the electric charge distribution in a plume can be influenced by the applied electric field, which can decrease the electron–ion recombination rate in the plume. Hence, increasing the applied electric field to the plume enhances the rate of trapped electrons by nanodroplets followed by the increase in the charge of nanodroplets.
Following the electrostatic interaction, the size of nanodroplets can be reduced by increasing their charge due to the Rayleigh instability. These phenomena will tend to decrease the mean size of NPs with the increase of the applied electric field [10, 17]. In EFLAL mechanism, during the nanosecond laser pulse, platinum NPs transform from a solid phase to a vapor phase in the high-temperature plasma plume through the thermal mechanisms. Increasing the laser intensity enhances the rate of ablation and causes the higher temperatures. After that, the homogeneous nucleation of particles can occur.
The vapor phase cannot transform directly to the stable phase at the high-temperature, high-pressure, and high-density plasma state.
Hence, the growing platinum particles are in a metastable phase. In this condition, there should exist a minimum surface energy to specify the equilibrium shape when we investigate the equilibrium of a small crystallite with its ambient phase. By applying a proper electric field, the surface electrostatic potential of the produced crystallite will enforce a preferred orientation for the growing crystalline planes and the nuclei–nuclei interactions in coalescences. This can result in the early formation of Pt NPs from a primary state [25]. Overall, the growth of the crystalline planes can be stabilized and enhanced by applying an appropriate electric field, which causes the formation of NPs with the final various morphologies [26].
According to Figs. 5 and 6, in all cases, the most of NPs have spherical shape. By increasing the electric field, in addition to spherical NPs, a few numbers of particles with hexagon and rectangular shapes are formed in the case of 15 and 20 V/cm, respectively. The formation of these non-spherical shapes is probably due to the aggregation (due to dipole–dipole interaction) of the primary nanocrystals via an oriented attachment mechanism [27]. The crystallographic planes usually characterize a crystal shape. After the formation of crystals under the equilibrium conditions, by the surface energies, the crystalline habits of these crystals are specified. Therefore, in the direction perpendicular to the face with the highest surface energy, the fastest growing plane takes place every time. As a consequence, the low-energy surfaces can be strengthened, while the high-energy surfaces will weaken. Hence, the final shape of the crystals depends on this evolution of the planes [8]. Therefore, the morphology of the produced NPs can be affected by the EFLAL of Pt target in distilled water. Although our work reveals that the shape of as-synthesized NPs can be changed by an applied electric field, only a few numbers of non-spherical NPs are produced, as a dependence on the applied electric field.
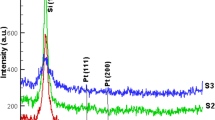
The reason probably is that the applied electric field is not enough to present the obvious correlation between the applied electric field and the shape evolution of NPs. Analysis of the SAED patterns was applied to explain the crystalline phases of the obtained Pt NPs. Figures 8 and 9 illustrate the SAED patterns of platinum NPs produced by the laser ablation in distilled water at different electric fields of 0, 5, 10, 15, and 20 V/cm, and the possible indexation of the first four rings of each pattern along with the d-spacing of the NPs. According to Figs. 8 and 9, the most of the interplanar distance from the major reflections can be related to the planes of the face-centered cubic structure of Pt NPs. The SAED results coincide with the XRD analysis in Fig. 10, which shows that these particles have a polycrystalline structure. All these reflections and planes recognized in these patterns are well in agreement with platinum PDF file No. 00-004-0802.
The corresponding XRD spectra of the samples at different values of electric field are shown in Fig. 10. The diffraction peaks are assigned to be from the (111) and (200) crystal planes of the face-centered cubic platinum (PDF Card File No. 00-004-0802). These peaks indicate that the produced NPs are pure and crystalline. The peak at 2θ = 70° is supposed to originate from the (400) planes of the Si substrate. According to the Bragg’s law, the corresponding lattice spacing (d hkl ) for the (111) reflection of NPs is calculated [28]. In addition, lattice parameters (a) are accounted from the following formula for the face-centered cubic structure:
where a is the lattice parameter; d hkl is the lattice spacing of (hkl); and h, k, and l are miller indices [28]. According to the Scherrer equation, the mean crystalline size of NPs is computed from the full-width at half-maximum (FWHM) of the (111) diffraction peak [29]. The calculated and standard parameters from the XRD patterns for NPs as a function of the applied electric field are presented in Table 1.
Figure 11 reveals the typical SEM images of platinum NPs grown under different electric fields of 0, 5, 10, 15, and 20 V/cm.
In the electric fields of 0, 5, and 10 V/cm, most of the particles are spherical in nature. At higher values of electric field (15 and 20 V/cm), the variety of shapes like rhombic, irregular, and nano-cubes are emerged besides the spherical Pt NPs, as confirmed by TEM images in Fig. 6. The growth of these Pt rhombic and nano-cubes is attributed to the crystal growth modes such as the oriented aggregation and oriented attachment by the application of a suitable electric field upon the laser ablation [27, 30]. In orientated attachment mode, through sharing a common crystallographic orientation of species and their combination at a planar interface, the NPs are self-assembled to decrease the whole surface energy [27]. Furthermore, as reported by Liu et al. [9], during the ablation of a Ge target in deionized water at different voltages of 14.5 and 32 V, GeO2 nano-cubes and spindles were observed. It is clear that the intensity of the applied electric field has a significant role during the growth of the Pt NPs, which can influence the morphology of these particles [31]. Some particles are aggregated. The aggregation is related to the Vander Waals forces between the particles [32]. It can be concluded that the laser induced plasma plume is strongly influenced by the applied electric field [9]. In addition, the formed morphology of the as-synthesized NPs depends on the applied electric field during the laser ablation process.
For identification of any adsorbed species onto the surface of NPs, FT-IR measurements are essential [33]. Figure 12 presents the FT-IR spectra of prepared Pt NPs in distilled water without and with application of different values of electric field. Overall, the samples produced under different electric fields (0, 5, 10, 15, and 20 V/cm) demonstrate similar absorption bands. The broad absorption peaks in the range of 3425.89–3404.50 cm−1 are related to the stretching vibration OH group, indicating the existence of water molecules absorbed on the NPs surface. The infrared band at 1643.58–1626.32 cm−1 is connected with the bending vibration of OH groups of water molecules [34]. The broad bands (ʋ L ) in the 723.90–702.52 cm−1 region are proposed to be from the so-called librations, a collective normal mode involving many water molecules, while the bands observed at 2078.46–2071.33 cm−1 are caused by combination bands (combination of libration and O–H-bending vibrations) [35].
The surface chemistry and reactions occurring at the surface of platinum NPs were further identified by the Raman spectra. The Raman spectra of platinum NPs synthesized in distilled water at various applied electric fields of 0, 5, 10, 15, and 20 V/cm in the wavelength range of 3700–1500 cm−1 are illustrated in Fig. 13. In all samples, the bands in the range of 3604.50–2853.13 cm−1 correspond to the O–H stretching vibration of water molecules, interacting with the Pt NPs surface. Specifically, the bands observed at 2125.76–1868.56 cm−1 are due to the stretching vibration of Pt-H. In addition, the Raman bands in the 1592.98–1585.20 cm−1 region are attributed to the H–O–H-bending modes that is absorbed onto the NPs surface [36, 37]. It is important to say that the existence of the additional bands in the Raman spectra in Fig. 13 is being more difficult to assign.
4 Conclusion
Platinum NPs have been extensively employed in many applications such as glucose sensors, gas sensors, exhaust systems of cars, etc. We synthesized colloidal Pt NPs in distilled water by laser ablation method under different values of the electric field (0, 5, 10, 15, and 20 V/cm) for the first time. The average particle size was found to be in the range of 9–20 nm and decreased with increasing the value of the applied electric field to 20 V/cm. According to the UV–Vis absorption spectra, the reduction of the average particle size was along with the blue shift of the SPR peak position from 239 to 220 nm. From the TEM and SEM studies, the resulting NPs exhibited the spherical and non-spherical morphology as a function of the applied electric field.
XRD and SAED patterns suggested the Pt NPs present a crystalline face-centered cubic phase. Moreover, the chemical structure information of the samples was identified by the FTIR and Raman spectra.
References
F. Mafune, J. Kohno, Y. Takeda, T. Kondow, Formation of stable platinum nanoparticles by laser ablation in water. J. Phys. Chem. B. 107, 4218–4223 (2003)
L. Stepanov, A.N. Golubev, S.I. Nikitin, Y.N. Osin, A review on the fabrication and properties of platinum nanoparticles. Rev.Adv. Mater. Sci. 38, 160–175 (2014)
E. Gharibshahi, E. Saion, Influence of dose on particle size and optical properties of colloidal platinum nanoparticles. Int. J. Mol. Sci. 13, 14723–14741 (2012)
X. Qin, Z. Miao, X. Wang, Y. Fang, D. Zhang, Q. Chen, X. Shao, Synthesis of platinum nanoparticles stabilized in polyvinyl alcohol and their electrocatalytic properties. Anal. Bioanal. Electrochem. 3, 393–405 (2011)
S. Hamad, G.K. Podagatlapalli, V.S. Vendamani, S.V.S. Nageswara Rao, A.P. Pathak, P. Surya, S.Venugopal Tewari, Rao, Femtosecond ablation of silicon in acetone: tunable photoluminescence from generated nanoparticles and fabrication of surface nanostructures. J. Phys. Chem. C. 118, 7139–7151 (2014)
G. Compagnini, M. Sinatra, P. Russo, G.C. Messina, O. Puglisi, S. Scalese, Deposition of few layer graphene nanowalls at the electrodes during electric field-assisted laser ablation of carbon in water. Carbon. 50, 2347–2374 (2012)
R.A. Ismail, F.A. Fadhil, Effect of electric field on the properties of bismuth oxide nanoparticles prepared by laser ablation in water. J. Mater. Sci. Mater. Electron. 25, 1435–1440 (2014)
R.M.S. Al-Haddad, M.K. Hamid, T. Jumaa, Electric field effect on the synthesis of nanogold particles by PLAL. Int. J. Chem. Nat. Sci. 3, 269–274 (2015)
P. Liu, C.X. Wang, X.Y. Chen, G.W. Yang, Controllable fabrication and cathodoluminescence performance of high-index facets GeO2 micro- and nanocubes and spindles upon electrical-field-assisted laser ablation in liquid. J. Phys. Chem. C. 112, 13450–13456 (2008)
D. Sapkota, Y. Li, O.R. Musaev, J.M. Wrobel, M.B. Kruger, Effect of electric fields on tin nanoparticles prepared by laser ablation in water. J. Laser Appl. 29, 012002(1)–012002(4) (2017)
T. Jumaa, M. Chasib, M.K. Hamid, R. Al-Haddad, Effect of the electric field on the antibacterial activity of Au nanoparticles on some gram-positive and gramnegative bacteria. Nanosci. Nanotech. Res. 2, 1–7 (2014)
W.T. Nichols, T. Sasaki, N. Koshizaki, Laser ablation of a platinum target in water. III. Laser induced reactions. J. Appl. Phys. 100, 114913(1)–114913(7)
M.I.M. Palma, B. Krishnan, G.A.C. Rodriguez, T.K. Das Roy, D.A. Avellaneda, S. Shaji, Synthesis and properties of platinum nanoparticles by pulsed laser ablation in liquid. J. Nanomater. 18, 1–11 (2016)
N.T. Binh, N.D. Thanh, N.Q. Dong, N.T. Trinh, Preparation of platinum nanoparticles in solution of polyvinyl pyrrolydone (PVP) by laser ablation method”. VNU J. Sci. Math. Phys. 30, 18–24 (2014)
M. Eslamifar, Hyper rayleigh scattering and surface enhanced raman scattering studies of patinum nanoparticle suspensions. Int. J. Res. Rev. Appl. Sci. 19, 1–5 (2014)
Z. Yan, R. Bao, D.B. Chrisey, Excimer laser ablation of a pt target in water: the observation of hollow particles. Nanotechnology. 21, 145609(1)–145609(8) (2010)
Y. Li, O.R. Musaev, J.M. Wrobel, M.B. Kruger, Laser ablation in liquids of germanium in externally applied electric fields. J. Laser Appl. 28, 022004(1)–022004(4) (2016)
B. Xu, R.G. Song, P.H. Tang, J. Wang, G.Z. Chai, Y.Z. Zhang, Z.Z. Ye, Preparation of Ag nanoparticles colloid by pulsed laser ablation in distilled water. Key Eng. Mater. 373–374, 346–349 (2008)
T.M.D. Dang, T.T.T. Le, E. Fribourg-Blanc, M.C. Dang, Synthesis and optical properties of copper nanoparticles prepared by a chemical reduction method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2, 015009(1)–015009(6) (2011)
Y.H. Chen, C.S. Yeh, Laser ablation method: use of surfactants to form the dispersed Ag nanoparticles. Colloids Surf. A. Physicochem. Eng. Asp 197, 133–139 (2002)
B. Balamurugana, T. Maruyama, Evidence of an enhanced interband absorption in Au nanoparticles: size-dependent electronic structure and optical properties. Appl. Phys. Lett. 87, 143105(1)–143105(3) (2005)
R. Intartaglia, K. Bagga, F. Brandi, G. Das, A. Genovese, E. Di Fabrizio, A. Diaspro, Optical properties of femtosecond laser-synthesized silicon nanoparticles in deionized water. J. Phys. Chem. C 115, 5102–5107 (2011)
B. Fei, Z. Xin-Zheng, W. Zhen-Hua, W. Qiang, H. Hao, X.J. Jun Preparation and size characterization of silver nanoparticles produced by femtosecond laser ablation in water. Chin. Phys. Lett 25, 4463–4465 (2008)
H.S. Desarkar, P. Kumbhakar, A.K. Mitra, Effect of Ablation Time and Laser Fluence on the Optical Properties of Copper Nano Colloids Prepared by Laser Ablation Technique. Appl. Nanosci 2, 285–291 (2012)
P. Liu, H. Cui, C.X. Wang, G.W. Yang, From nanocrystal synthesis to functional nanostructure fabrication: laser ablation in liquid. Phys. Chem. Chem. Phys. 12, 3942–3952 (2010)
K. Abbas, I.M. Ibrahim, D.K. Naser, The effect of electric and magnetic field on silver nanoparticles prepared by pulsed laser ablation. Int. J. Sci. Eng. Res 7, 976–980 (2016)
X.Z. Lin, P. Liu, J.M. Yu, G.W. Yang, Synthesis of CuO nanocrystals and sequential assembly of nanostructures with shape-dependent optical absorption upon laser ablation in liquid. J. Phys. Chem. C 113, 17543–17547 (2009)
Y. Hu, H. Zhang, P. Wu, H. Zhang, B. Zhou, C. Cai, Bimetallic Pt–Au nanocatalysts electrochemically deposited on graphene and their electrocatalytic characteristics towards oxygen reduction and methanol oxidation. Phys. Chem. Chem. Phys. 13, 4083–4094 (2011)
L. Zhao, N. Heinig, K.T. Leung, Formation of Au-Pt alloy nanoparticles on a Si substrate by simple dip-coating at room temperature. Langmuir. 29, 927–931 (2013)
P. Liu, Y. Liang, X. Lin, C. Wang, G. Yang, A general strategy to fabricate simple polyoxometalate nanostructures: electrochemistry-assisted laser ablation in liquid. ACS Nano 5, 4748–4755 (2011)
Y. Liang, P. Liu, H.B. Li, G.W. Yang, Synthesis and characterization of copper vanadate nanostructures via electrochemistry assisted laser ablation in liquid and the optical multi-absorptions performance. Cryst. Eng. Comm 14, 3291–3296 (2012)
H.L. Aye, S. Choopun, T. Chairuangsri, Influence of solvents on characteristics of nanoparticles prepared by pulsed laser ablation on iron target. CMU. J. Nat. Sci 31, 37–42 (2014)
M.A. Gondal, Q.A. Drmosh, Z.H. Yamani, T.A. Saleh, Synthesis of ZnO2 nanoparticles by laser ablation in liquid and their annealing transformation into ZnO nanoparticles. Appl. Surf. Sci 256, 298–304 (2009)
S.L. Sharifi, H.R. Shakur, A. Mirzaei, A. Salmani, M.H. Hosseini, Characterization of cobalt oxide Co3O4 nanoparticles prepared by various methods: effect of calcination temperatures on size, dimension and catalytic decomposition of hydrogen peroxide. Int. J. Nanosci. Nanotechnol 9, 51–58 (2013)
S.Y. Venyaminov, F.G. Prendergast, Water (H2O and D2O) molar absorptivity in the 1000–4000 cm– 1 range and quantitative infrared spectroscopy of aqueous solutions. Anal. Biochem 248, 234–245 (1997)
D.M. Carey, G.M. Korenowski, Measurement of the raman spectrum of liquid water. J. Chem. Phys. 108, 2669–2675 (1998)
E.E. Fileti, M.A. Castro, S. Canuto, Calculations of vibrational frequencies, raman activities and degrees of depolarization for complexes involving water, methanol and ethanol. Chem. Phys. Lett. 452, 54–58 (2008)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moniri, S., Hantehzadeh, M.R., Ghoranneviss, M. et al. Study of the optical and structural properties of Pt nanoparticles prepared by laser ablation as a function of the applied electric field. Appl. Phys. A 123, 684 (2017). https://doi.org/10.1007/s00339-017-1311-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-017-1311-9