Abstract
Micro arc oxidation method was utilized to prepare molybdenum doped TiO2 films through adding sodium molybdate into the electrolyte for the first time. The effect of sodium molybdate concentration on the morphology, crystal structure and photophysical properties of the films was investigated by field emission scanning electron microscopy, X-ray diffraction and UV–vis spectrophotometry. The results showed that with increasing of sodium molybdate concentration, the pores of films decreased in size and increased in number, more anatase phase transformed to rutile phase in the films and inter-plane spacing of the films increased. Furthermore, the absorption edges of the films shifted towards longer wavelength and the band gap energies of the films decreased with increasing the concentration of sodium molybdate. In addition, the photocatalytic degradation of methylene blue showed that the photocatalytic activity of the molybdenum doped TiO2 film was about 2.3 times that of pure TiO2 film.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, TiO2 is widely investigated as a photocatalytic material due to its low cost, high chemical and photocatalytical stability, nontoxicity and strong oxidizing power [1,2,3]. However, it is difficult to realize the practically technological application of pure TiO2 because of its wide band gap energy and easy recombination of photo-generated hole–electron pairs. Therefore, considerable efforts have been made on the modification of TiO2, such as doping TiO2 with metallic and non-metallic species [4,5,6,7], or coupling TiO2 with other semiconductors [8, 9].
To increase possibility of recycling and reuse of TiO2 photocatalyst, TiO2 photocatalytic films have been synthetized through some surface treatment technologies. As a relatively new surface treatment technology, micro arc oxidation (MAO) has attracted a great deal of attention, because the porous, thick and adherent films can be acquired on the surface of valve metals such as Al, Mg and Ti by MAO method. Furthermore, the physical and chemical properties of films can be improved through adjustment of the electrolyte composition [10,11,12]. Recently, there are only few reported investigations on producing TiO2 photocatalytic films through MAO technique. For example, Wan et al. prepared anatase TiO2 photocatalytic films by MAO of TiN films [13]. Afterward, Salami et al. employed MAO method to grow Ag-doped TiO2 photocatalytic films through adding Ag2CO3 into electrolytes [14].
As far as we know, this is the first report on preparation of the molybdenum doped TiO2 films in electrolyte containing sodium molybdate by MAO process. Furthermore, the effect of electrolyte composition on physical and chemical characteristics as well as photocatalytic activity of the molybdenum doped TiO2 films were investigated with the mechanism analysis.
2 Experimental section
2.1 Preparation of films
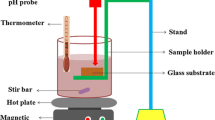
Figure 1 shows the sketch map of equipment of MAO, which consisted of an AC power supply, an electrolyte cell, a stirring system, a cooling system and an exhaust system. The pure titanium sheets with the dimensions of 20 × 20 × 2 mm were selected as anode, which was located between two stainless steel plates which were used as cathode. Prior to MAO process, the titanium sheets were polished with emery papers, degreased by acetone and then rinsed with deionised water. The positive voltage, negative voltage, frequency, duty cycle and treatment period were 400 V, 50 V, 700 Hz, 0.3 and 10 min, respectively. The electrolyte consisted of three-sodium phosphate (Na3PO4·12H2O) and sodium molybdate (Na2MoO4·2H2O) aqueous solutions with different concentrations. To compare the physical and chemical characteristics as well as photocatalytic activity of the molybdenum doped TiO2 films and those of pure TiO2 film, the electrolyte only consisted of three-sodium phosphate was utilized to prepared the pure TiO2 film. The chemical compositions and exact concentrations of the electrolytes are listed in Table 1. The temperature of electrolyte was kept under 40 °C during the MAO process. The prepared films were rinsed with deionised water and dried with hot air.
2.2 Analyses of films
The morphologies of films were characterized by field emission scanning electron microscopy (FSEM, FEI Sirion 200, FEI Company, Eindhoven, The Netherlands). The elemental composition of the films were analyzed through an energy dispersive X-ray (EDX) detector incorporated into the FSEM. X-ray diffraction (XRD, X’Pert PRO, PANalytical B.V., Almelo, Netherlands) was used to determine the crystal structure of films. The UV–vis absorption spectra of films were recorded on UV–vis spectrophotometer (UV-2550, Shimadzu, Kyoto, Japan) with an integrating sphere attachment.
2.3 Evaluation of photocatalytic activity
Photocatalytic activities of the films were measured by monitoring photodegradation of methylene blue (MB) in aqueous solution. The films were immersed into 10 mL of the aqueous MB solution (10 mg dm−3) for 30 min prior to irradiation to reach adsorption/desorption equilibrium. An ultraviolet germicidal lamp (40 W) with a maximum irradiation peak at 365 nm was used as light source and the irradiation time was 300 min. The degradation rate of MB was calculated from the height of peak at the wavelength of 664 nm in UV–vis spectra recorded on UV–vis spectrophotometer.
3 Results and discussion
3.1 Morphology of films
The morphology of the films synthesized in different electrolytes is shown in Fig. 2. Many crater-like holes can be observed on the surface of the films, these holes were residual discharge channels which produced by plasma discharge during the MAO process [15]. Among all the films, the film prepared in electrolyte A had relatively smooth surface and the pores with the largest size and the smallest number (Fig. 2a). Furthermore, with increasing of sodium molybdate concentration, the pores of films decreased in size and increased in number (Fig. 2b–e). After addition of sodium molybdate, the concentrations of sodium and molybdate ions increase continually, which will lead to a higher solution conductivity. The sparks on the surface of films during MAO process may become denser and smaller with increasing of solution conductivity. Because the changing tendency of size and number of pores on the surface of films prepared by MAO process is consistent with that of sparks on the surface of films [16]. Therefore, after cooling of the discharge channels, the pores on the surface of films become denser and smaller with increasing of sodium molybdate concentration.
The element percentage of films synthetized in the electrolyte C and E is presented in Table 2. It can be found that molybdenum element appeared in the films when sodium molybdate was added into the electrolyte and the percentage of molybdenum increased with increasing of sodium molybdate concentration, which is due to the fact that more molybdate ions will move to the surface of anode under the influence of electric field and participate in the formation of film when the concentration of sodium molybdate increases.
3.2 XRD analysis of films
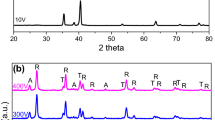
XRD patterns of the films prepared in the electrolyte A, B and E are presented in Fig. 3. The Ti peaks (JCPDS card No. 44-1294) were detected from the titanium substrate. Furthermore, it can be found that the anatase (JCPDS card No. 71-1167) and rutile (JCPDS card No. 76-1939) phases formed in the films. The percentages of anatase and rutile in the films prepared in the electrolyte A, B and E were calculated using formula as below:
where f A is the ratio of anatase, I A and I R are the intensities of diffraction peaks of anatase (1 0 1) and rutile (1 1 0) crystal plane, respectively. The obtained results are shown in Table 3. The percentages of anatase and rutile in the film prepared in the electrolyte A were similar to those in the film prepared in the electrolyte B. When the concentration of sodium molybdate increased to 4.84 g L−1 (electrolyte E), the percentage of anatase decreased whereas the percentage of rutile increased obviously. The current density of the electrolytic cell increases with increasing the electrolyte concentration, which results that more heat will be produced on the surface of anode. Therefore, more anatase which is a metastable phase will transform to thermodynamically stable rutile phase.
Figure 4 shows the enlarged XRD patterns of anatase (1 0 1) crystal plane of the films prepared in the electrolyte A, B and E, respectively. As is seen, a small shift of characteristic peaks toward lower diffraction angle is observed with increasing the concentration of sodium molybdate. The accurate characteristic peaks angles and inter-plane spacing of anatase (1 0 1) and rutile (1 1 0) crystal plane of the films prepared in the electrolyte A, B and E were obtained by XRD data and are presented in Table 4, which also indicates that the characteristic peaks angles decreased and inter-plane spacing increased with increasing the concentration of sodium molybdate. Utilizing more concentrated sodium molybdate will result that more molybdate anions are drawn toward the anode surface and participate in the formation of films. Therefore, more molybdenum atoms will dope into the TiO2 lattice under the role of violent spark discharge, which results in the decreasing of characteristic peaks angles and the increasing of inter-plane spacing.
3.3 UV–vis absorption spectra of films
The UV–vis absorption spectra of films prepared in different electrolytes are shown in Fig. 5. The absorption edge of the films can be calculated from the UV–vis absorption spectra by the intercept of a tangent line to the absorption curves on the wavelength axis [17]. As is seen, the absorption edges of films synthesized in the electrolyte A, B, C, D and E were about 420, 451, 466, 486 and 508, respectively, which indicated that the absorption edges shifted gradually towards longer wavelength with increasing the concentration of sodium molybdate. Because the fundamental absorption of TiO2 is an indirect transition model [18], the band gap energies of films synthesized in different electrolytes were acquired by drawing (αhv)1/2 versus hv curves (where α is the absorbance and hv is the photon energy) and extending the straight line portion to intersect the x axis, as shown in Fig. 6, which indicated that the band gap energies of films synthesized in the electrolyte A, B, C, D and E were about 2.92, 2.71, 2.51, 2.33 and 2.18, respectively. Obviously, the band gap energies decreased gradually with increasing the concentration of sodium molybdate. The schematic diagram of band gap energy of molybdenum doped TiO2 is shown in Fig. 7. Because the orbital energy of Mo 4d is close to that of Ti 3d, the introduction of molybdenum atoms will create an impurity level in the band gap of TiO2. This impurity level locates the bottom of the conduction band of TiO2, which results that the conduction band maximum of TiO2 is modified indistinctively. Therefore, the Fermi level shifts upward and the band gap energy of TiO2 will be narrowed relatively [19, 20].
3.4 Photocatalytic activity of films
Figure 8 shows the absorbance of MB Photocatalytic degraded by the films synthesized in different electrolytes. The degradation rate of MB was calculated from the height of peak at the wavelength of 664 nm and is presented in Fig. 9, which shows that about 30, 57, 69, 65 and 63% of MB were degraded on the surface of the films prepared in the electrolyte A, B, C, D and E, respectively. It can be seen that the molybdenum doped TiO2 films had a obviously improved photocatalytic activity as compared with the pure TiO2 film. Furthermore, the photocatalytic activity of the molybdenum doped TiO2 films increased and then decreased with increasing the concentration of sodium molybdate. The molybdenum doped TiO2 film prepared in the electrolyte C exhibited the best photocatalytic activity, which was about 2.3 times that of pure TiO2 film. According to the analysis results of physical and chemical properties of the films, the variation of the photocatalytic activity of the films should be determined by the two main factors: the ratio of rutile in the films and the number of molybdenum atoms doped into the TiO2 lattice. With increasing the concentration of sodium molybdate, not only the percentage of rutile in the films increased but also more molybdenum atoms doped into the TiO2 lattice. The photogenerated electrons can transfer from the conduction band of rutile to the conduction band of anatase during the photocatalytic process of TiO2 with anatase and rutile mixed phase because the conduction band of anatase is higher than the conduction band of rutile, which promotes the separation of photogenerated electrons and holes effectively [21, 22]. Furthermore, molybdenum doping can change the distribution of the bottom of the conduction band of TiO2, which will also promote the separation of photogenerated electrons and holes effectively [19]. It can be believed that the recombination probability of photogenerated electrons and holes of TiO2 will decrease when the ratio of rutile and the number of doped molybdenum atoms increase appropriately. Therefore, the photocatalytic activity of films was improved gradually when the concentration of sodium molybdate increased from 0 to 2.42 g L−1. However, for one thing, rutile is less efficient in creating electron–hole pairs and has poorer oxygen adsorption capacity as compared with anatase due to better crystallized bulk and surface state of rutile, implying that the excess rutile in TiO2 will have a negative effect on photocatalytic activity, for another, the excess molybdenum atoms in TiO2 lattice can act as the recombination centers of photogenerated electrons and holes [23, 24]. Therefore, the photocatalytic activity of the molybdenum doped TiO2 films began to decrease when the concentration of sodium molybdate exceeded the optimal value.
4 Conclusions
Molybdenum doped TiO2 films were successfully synthesized through micro arc oxidation (MAO) method for the first time. The effects of the electrolyte composition and concentration on the morphology, phase composition and structure, photophysical properties and photocatalytic activity of the films were investigated. It was found that the pores of films decreased in size and increased in number with increasing of sodium molybdate concentration. It was also revealed that the film was composed of anatase and rutile phase and the percentage of anatase decreased whereas the percentage of rutile increased with increasing of sodium molybdate concentration. Furthermore, the inter-plane spacing of anatase (1 0 1) and rutile (1 1 0) crystal plane increased with increasing of sodium molybdate concentration, indicating that more molybdenum atoms would dope into the TiO2 lattice with increasing of sodium molybdate concentration, which resulted that the absorption edges of the films shifted gradually towards longer wavelength and the band gap energies of the films decreased gradually with increasing of sodium molybdate concentration. The results of photocatalytic experiment showed that molybdenum-doped TiO2 films had an obviously improved photocatalytic activity as compared with pure TiO2 film. Furthermore, the photocatalytic activity of the molybdenum doped TiO2 films increased and then decreased with increasing the concentration of sodium molybdate. The film prepared in the electrolyte containing three-sodium phosphate with the concentration of 19 g L−1 and sodium molybdate with the concentration of 2.42 g L−1 displayed the best photocatalytic activity due to the combined effects of appropriate rutile ratio and doped molybdenum atoms number. As a consequence, the research results in this paper will provide an appropriate method to prepare molybdenum doped TiO2 films.
References
T. Ohno, T. Mitsui, M. Matsumura, Chem. Lett. 32, 364 (2003)
M.M. Khan, S.A. Ansari, D. Pradhan, M.O. Ansari, D.H. Han, J. Lee, M.H. Cho, J. Mater. Chem. 2, 637 (2014)
H. Zhang, L.H. Guo, D. Wang, L. Zhao, B. Wan, A.C.S. Appl, Mater. Interfaces 7, 1816 (2015)
L.F. Cui, Y.S. Wang, M.T. Niu, G.X. Chen, Y. Cheng, J. Solid State Chem. 182, 2785 (2009)
G. Colόn, M. Maicu, M.C. Hidalgo, J.A. Navío, Appl. Catal. B 67, 41 (2006)
R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki, Y. Taga, Science 293, 269 (2001)
J.H. Sun, L.P. Qiao, S.P. Sun, G.L. Wang, J. Hazard. Mater. 155, 312 (2008)
D. Robert, Catal. Today 122, 20 (2007)
J.T. Tian, L.J. Chen, Y.S. Yin, X. Wang, J.H. Dai, Z.B. Zhu, X.Y. Liu, P.W. Wu, Surf. Coat. Technol. 204, 205 (2009)
P. Wang, T. Wu, H. Peng, X.Y. Guo, Mater. Lett. 170, 171 (2016)
Q.L. Huang, T.A. Elkhooly, X.J. Liu, R.R. Zhang, X. Yang, Z.J. Shen, Q.L. Feng, Mater. Sci. Eng. C 67, 195 (2016)
H. Tang, Y. Han, T. Wu, W. Tao, X. Jian, Y.F. Wu, F.J. Xu, Appl. Surf. Sci. 400, 391 (2017)
L. Wan, J.F. Li, J.Y. Feng, W. Sun, Z.Q. Mao, Mater. Sci. Eng. B 139, 216 (2007)
N. Salami, M.R. Bayati, F. Golestani-Fard, H.R. Zargar, Mater. Res. Bull. 47, 1080 (2012)
A.L. Yerokhin, X. Nie, A. Leyland, A. Matthews, S.J. Dowey, Surf. Coat. Technol. 122, 73 (1999)
R.F. Zhang, S.F. Zhang, Y.L. Shen, L.H. Zhang, T.Z. Liu, Y.Q. Zhang, S.B. Guo, Appl. Surf. Sci. 258, 6602 (2012)
B. Baruwati, R.S. Varma, J. Nanosci. Nanotechnol. 11, 2036 (2011)
S. Lee, C. Jeon, Y. Park, Chem. Mater. 16, 4292 (2004)
S. Wang, L.N. Bai, H.M. Sun, Q. Jiang, J.S. Lian, Powder Technol. 244, 9 (2013)
Y.Q. Gai, J.B. Li, S.S. Li, J.B. Xia, S.H. Wei, Phys. Rev. Lett. 102, 36402 (2009)
L. Shi, D. Weng, J. Environ. Sci. 20, 1263 (2008)
T. Ohno, K. Tokieda, S. Higashida, M. Matsumura, Appl. Catal. A Gen. 244, 383 (2003)
A. Sclafani, J.M. Herrmann, J. Phys. Chem. 100, 13655 (1996)
J.Q. Li, D.F. Wang, H. Liu, Z.F. Zhu, Mater. Manuf. Process 27, 631 (2012)
Acknowledgements
This work was supported by key scientific research project of Wuhan Polytechnic University (2015d7).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, Q., Cai, Q. A novel method to synthesize molybdenum doped TiO2 films with enhanced photocatalytic activity. Appl. Phys. A 123, 451 (2017). https://doi.org/10.1007/s00339-017-1063-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-017-1063-6