Abstract
The carrier concentration of Sb-rich phase SnSb4, Ga3Sb7 and superlattice-like [SnSb4(3.5 nm)/Ga3Sb7(4 nm)]7 (SLL-7) thin films as a function of annealing temperature was investigated to explain the reason of resistance change. The activation energy for crystallization was calculated with a Kissinger equation to estimate the thermal stability. In order to illuminate the transition mechanisms, the crystallization kinetics of SLL-7 were explored by using Johnson–Mehl–Avrami theory. The obtained values of Avrami indexes indicate that a one-dimensional growth-dominated mechanism is responsible for the set transition of SLL-7 thin film. X-ray diffractometer and Raman scattering spectra were recorded to investigate the change of crystalline structure. The measurement of atomic force microscopy indicated that SLL-7 thin film has a good smooth surface. A picosecond laser pump-probe system was used to test and verify phase-change speed of the SLL-7 thin film.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Phase-change memory (PCM) has attracted increasing interest as one of the promising next-generation nonvolatile memories [1]. PCM has many outstanding properties, such as low power consumption, high scalability, good data retention, perfect fatigue performance and good compatibility with CMOS processing [2–5]. Data storage is realized through the reversible phase change excited by the Joule heating of the pulse current between the amorphous and crystalline states. The amorphous state (high resistivity) and the crystalline state (low resistivity) can be distinguished as “0” and “1” in information storage, respectively.
Phase-change material is the core of PCM. Since the pioneering research of Ovshinsky, several phase-change chalcogenide materials have been developed to improve the performance. Ge2Sb2Te5 (GST) is the most widely researched PCM material due to its good electrical and thermal property [6, 7]. However, the slow switching speed of GST in the operation of PCM devices is an important obstacle for its commercial application in mass data storage [8]. The main reason is the nucleation-dominated crystallization proceeding. To solve this problem, Sb-rich PCM materials, Al–Sb–Te [9], Si–Sb–Te [5], W–Sb–Te [10] and Sn–Sb [11], have been proved to have rapid phase-change speed due to their growth-dominated crystallization mechanism. In addition, superlattice-like (SLL) PCM materials have been paid more and more attention because of their low thermal conductivity and small grain size. Fast amorphization and crystallization of 300 ps and 1 ns were achieved in the 40-nm SLL GeTe/Sb2Te3 cells due to their fast heterogeneous crystallization and high resistivity [11]. A low RESET current of 1.5 mA and good endurance of 5.3 × 106 cycles were realized in GeTe/Sb7Te3 SLL structure [12].
In this work, Sb-rich phase-change materials SnSb4 and Ga3Sb7 were employed to fabricate SLL SnSb4/Ga3Sb7 phase-change thin films. The fast phase-change speed of SnSb4 and good thermal stability of Ga3Sb7 [13, 14] were incorporated in SLL structure. The structure, electrical and laser-induced phase-change properties SLL SnSb4/Ga3Sb7 phase-change thin films were investigated in detail. The phase-change kinetics in terms of the fundamental nucleation and growth theories were explored.
2 Experimental
SLL-7 thin films, as well as monolayer SnSb4 and Ga3Sb7 thin films, were deposited on 0.5-mm-thick oxidized Si (100) wafers using a radiofrequency (RF) magnetron sputtering system at room temperature. Prior to the growth of the SLL thin films, the deposition rates of SnSb4 and Ga3Sb7 single layers were determined by an Alpha-Step 500 profiler (Tencor Instrument). The thickness of each individual layer can be designed by controlling the deposition time. The SLL structures were obtained by sputtering the SnSb4 and Ga3Sb7 targets alternately. The total thickness of all phase-change thin films was set 50 nm. In an Ar atmosphere at a pressure of 0.2 Pa, the deposition processes were carried out with a flow of 30 sccm and an RF power of 20 W. In order to guarantee the deposition uniformity, the substrate was kept rotating automatically in 20 rpm.
The carrier concentration of SnSb4, SLL-7 and Ga3Sb7 thin films annealed at different temperatures was measured with Hall instrument (Ecopia, HMS-3000). The amorphous-to-crystalline transition was investigated by in situ film resistance measurements under an Ar atmosphere. The sample temperature was measured by a Pt-100 thermocouple located at a heating stage controlled by a TP 94 temperature controller. The entire setup was connected to a computer on which the resistance and temperature of the sample were recorded by a real-time measurement program. The activation energy for crystallization was calculated by a Kissinger equation. The phase structures of SnSb4, SLL-7 and Ga3Sb7 thin films annealed at various temperatures were examined by XRD analyses using Cu Kα radiation in the 2θ range from 20° to 60°, with a scanning step of 0.01°. Raman scattering spectra were recorded in backscattering geometry at room temperature with a laser power of 20 mW. The Ar ion laser is used for excitation at a wavelength of 785 nm. In order to achieve maximum signal quality, the frequency resolution is set below 1.5 cm−1. Based on the Johnson–Mehl–Avrami (JMA) theory, the crystallization kinetics and mechanisms were studied. The surface roughness of the deposited and annealed films was evaluated by atomic force microscopy (AFM) (Bruker, Dimension Edge), which was carried out in the semi-contact mode. A picosecond laser pump-probe system was used for real-time reflectivity measurements. The light source used for irradiating the samples was a frequency-doubled model-locked neodymium yttrium aluminum garnet laser operating at 532-nm wavelength with a pulse duration of 30 ps.
3 Results and discussion
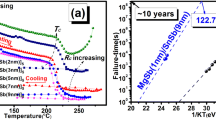
Figure 1 shows the carrier concentration and resistance of SnSb4, SLL-7 and Ga3Sb7 thin films as a function of annealing temperature. Initially, the carrier concentration of all thin films increases slowly with the increase in temperature due to a thermally assisted trap-limited conduction [15]. The carrier concentration subsequently rises abruptly as the heating temperature approached the crystallization temperature T c. T c of SnSb4, SLL-7 and Ga3Sb7 thin films increases in turn. Generally, higher crystallization temperature may lead to better thermal stability of metastable amorphous phase. The tendency is further verified in R–T curves of Fig. 1. The T c for SnSb4, SLL-7 and Ga3Sb7 thin films are 141, 209 and 291 °C, respectively. Therefore, PCM devices based on SLL-7 thin film will have a better data retention capability compared with SnSb4 layer. In addition, the resistance of amorphous SLL-7 thin film is an order of magnitude greater than that of SnSb4. Higher resistance means less free electrons which can participate in electric conduction. Figure 1 shows that the carrier concentration of SLL-7 thin film is lower than that of SnSb4 thin film, which is in agreement with the result of resistance measurement. It is well known that the higher resistivity of phase-change thin films can facilitate melting under pulse current with joule heating, which will decrease the writing current of PCM. For this reason, SLL-7 thin film-based PCM devices will have a lower power consumption.
In order to estimate the archival life stability of an amorphous phase-change material, the activation energy for crystallization E a of all thin films was calculated from a Kissinger equation [16]:
where dT/dt is the heating rate, T c is the crystallization temperature, C is a constant, E a is the activation energy for crystallization and k b is Boltzmann’s constant. The relation curves of the resistance versus temperature for all films were measured with the heating rate from 10 to 40 °C/min. The inset in Fig. 2 shows that the resistance of all SLL-7 thin films dropped more than two orders of magnitude at the crystallization temperature T c. With the increase in heating rate, the T c increases synchronously because there is insufficient time for crystallization and nucleation to take place at a faster heating rate. Figure 2 shows the Kissinger plots ln[(dT/dt)/T 2c ] versus 1/KT of SnSb4, SLL-7 and Ga3Sb7 thin films. The E a of SnSb4, SLL-7 and Ga3Sb7 thin films is 1.56, 2.86 and 3.56 eV, respectively. Most of all, the E a of SLL-7 is higher than that of GST (2.28 eV) [13]. From this point, the SLL-7 thin film has good thermal stability, which is in agreement with the result in Fig. 1.
Figure 3 shows the XRD patterns of SnSb4, SLL-7 and Ga3Sb7 thin films annealed at various temperatures. In the as-deposited state (25 °C), no diffraction peaks were observed for SnSb4, SLL-7 and Ga3Sb7 thin films, indicating the amorphous structure. After annealing at the various temperatures (SnSb4 120 °C, SLL-7 180 °C, Ga3Sb7 280 °C), the three thin films are still in the amorphous state, which is consistent with the results of Fig. 1. With increasing temperature to over their respective T c, the obvious diffraction peaks appear. For SnSb4 thin film, the diffraction peaks of (012) and (110) belong to Sb phase except the peak (220) of SnSb phase. The diffraction peaks of GaSb (111) and Sb (110) were observed in annealed Ga3Sb7 thin film at 350 °C. In addition, it should be pointed that all the thin films of crystallization state have the diffraction peak of Sb (012). It can be concluded that Sb phase is existed in Sb-rich SnSb4, SLL-7 and Ga3Sb7 thin films.
Raman scattering spectra of SnSb4, SLL-7 and Ga3Sb7 thin films annealed at different temperatures are shown in Fig. 4. Two Raman peaks located at around 114 and 145 cm−1 are observed in all films, which is associated mainly with the Sb components [13]. Further analysis demonstrates that the two peaks belong to Raman modes of the A7 phase due to fluctuations in short-range order [17]. After annealed at higher temperature, the two Raman peaks 114 and 145 cm−1 become sharper, which manifests that some crystalline grains have formed. In addition, it is noteworthy that the position of Raman peaks 114 and 145 cm−1 of all thin films moves to higher wave number after crystallization, which can be attributed to stronger interatomic force in long-range order crystals.
In order to explore the crystallization kinetics and mechanisms, the isothermal crystallization behaviors of SLL-7 thin film were investigated by using Johnson–Mehl–Avrami (JMA) theory [11]. The two isothermal temperatures of 182.5 and 180 °C were selected for the research of crystallization behaviors, because they are lower than the T c of SLL-7 thin film. The variation of resistance and time for SLL-7 thin film was measured at isothermal 182.5 and 180 °C. The crystallization fraction χ is assumed to be proportional to resistance change. The crystallization fraction χ at any given time can be redrawn and normalized by [18, 19]:
where R 0 and R t are the resistances measured at time = 0 and t, respectively. R max and R min are the maximum and minimum resistances measured during phase-change process, respectively. The crystallization fraction χ as a function of time for SLL-7 thin film at isothermal 182.5 and 180 °C is shown in Fig. 5a. The typical S-shaped growth curves can be divided into four stages, i.e., incubation period, steady state nucleation, growth and coarsening [20]. Figure 4a shows that the embryos take more time to enter into the growth period at 180 °C than at 182.5 °C. The growth period (0.25 ≤ χ ≤ 0.75) was selected for the calculation of Avrami index because it best accords with JMA theory [21]. The fraction of crystallization χ as a function of time t can be described in JMA equation [22]:
where n is the Avrami exponent and κ is an effective rate constant. Figure 5b shows the curves of ln[−ln(1 − χ)] versus ln(t) at 182.5 and 180 °C. The Avrami exponent n determined by the slope of linearly fitted curves for 182.5 and 180 °C is 1.46 and 1.41, respectively. Based on diffusion-limited process, n ≥ 1.5 indicates the crystallization process in which grain growth occurs with nucleation. The n < 1.5 denotes one-dimensional grain growth from the nuclei. From the values of Avrami exponent, the crystallization mechanism of SLL-7 can be determined to be a one-dimensional growth-dominated phase transition. In this pattern, the nuclei generation becomes difficult as the amorphous matrix decreases with grain growth. The nucleation is not predominant, but grains grow gradually from the nuclei [14]. As is known, the nucleation needs much more time than grain growth [18]. Therefore, the crystallization will begin from rich Sb nuclei and the interfaces between different phase-change layers. This heterogeneous phase change will have a rapid speed [11].
Film surface roughness has a significant impact on device performance by affecting the quality of the electrode–film interface. Phase change is accompanied by an induced stress which leads to a change of thin film surface roughness. The atomic force microscopy (AFM) images of SLL-7 thin films annealed for 5 min at 25, 150, 250 and 300 °C are shown in Fig. 6a–d, respectively. The root-mean-square surface roughness of amorphous thin films is very small (0.667 nm for as-deposited, 0.705 nm for 150 °C annealed). After crystallization, the root-mean-square surface roughness has an obvious increase (1.071 nm for 250 °C, 1.092 nm for 300 °C). The results indicate that thin films have a still good surface flatness, which will ensure the contact between the electrode and the phase-change materials. Therefore, it can be concluded that PCM devices based on SLL-7 thin film will have good reliability, which is very significant in application [14, 23].
Phase change is accompanied with resistivity as well as optical reflectivity. In order to evaluate the phase-change speed, two consecutive picosecond laser pulses with different laser fluences were used. Figure 7 shows the reflectivity evolution of SLL-7 thin films. As we can see from Fig. 7a–c, the reflectivity increases abruptly with an irradiation fluence of 7.1 mJ/cm2, corresponding to the amorphous-to-crystalline state transition. Then, a converse phase transform is achieved with a drop of optical reflectivity at a laser pulse intensity of 9.7 mJ/cm2 (Fig. 7d–f). Thus, a reversible phase change can be realized with laser pulses. The crystallization time is used as the valuation criteria of phase-change speed because the crystallization process needs more time than the amorphization one [24]. Figure 7a–c shows that the average crystallization time for SLL-7 thin films is about 6.2 ns, which is much shorter than that of GST (17.7 ns) [25]. And the average amorphization process of SLL-7 also has a faster phase-change speed (4.2 ns) than GST (16.5 ns). It is reported that the excessive Sb atoms in the Sb-rich phase-change materials would form a large number of Sb nucleus. During crystallization, the dispersed Sb nucleus can act as heterogeneous nucleating centers, which facilitated crystallization [26]. It is in agreement with the results of XRD.
4 Conclusions
In summary, SLL-7 thin films have a lower carrier concentration and Hall mobility than SnSb4 layer, which resulted in lower power consumption. The SLL-7 has a good thermal stability with the activation energy for crystallization 2.86 eV. Before and after crystallization, the surface of SLL-7 is very smooth due to smaller grain size. The Avrami exponent n is lower than 1.5, which means a one-dimensional growth-dominated phase transition for SLL-7. The phase-change times of SLL-7 are 6.2 and 4.2 s for crystallization and amorphization processes, respectively. A faster switching speed is achieved due to the heterogeneous crystallization mechanism of SLL structure.
References
M. Zhu, M.J. Xia, F. Rao, X.B. Li, L.C. Wu, X.L. Ji, S.L. Lv, Z.T. Song, S.L. Feng, H.B. Sun, S.B. Zhang, Nat. Commun. 5, 6 (2014)
T.C. Chong, L.P. Shi, X.Q. Wei, R. Zhao, H.K. Lee, P. Yang, A.Y. Du, Phys. Rev. Lett. 100, 136101 (2008)
T. Matsunaga, N. Yamada, Phys. Rev. B 69, 104111 (2004)
R.E. Simpson, P. Fons, A.V. Kolobov, T. Fukaya, M. Krbal, T. Yagi, J. Tominaga, Nat. Nanotech. 6, 501 (2011)
F. Rao, Z.T. Song, K. Ren, X.L. Zhou, Y. Cheng, L.C. Wu, B. Liu, Nanotechnology 22, 10 (2011)
T.H. Jeong, M.R. Kim, H. Seo, S.J. Kim, S.Y. Kim, J. Appl. Phys. 86, 774 (1999)
T.Y. Yang, J.Y. Cho, Y.J. Park, Y.C. Joo, Acta Mater. 60, 2021 (2012)
Y.F. Hu, X.Y. Feng, S.M. Li, T.S. Lai, S.N. Song, Z.T. Song, J.W. Zhai, Scr. Mater. 93, 4 (2014)
W. Fen, W. Long, K. Tao, S. Lin, H. Rong, Z. Jie, C.S. Guo, Appl. Phys. Lett. 103, 181908 (2013)
K. Ren, M.J. Xia, F. Rao, Z.T. Song, K.Y. Ding, X.L. Ji, L.C. Wu, B. Liu, S.L. Feng, Appl. Phys. Lett. 104, 173102 (2014)
F. Rao, Z.T. Song, K. Ren, X.L. Li, L.C. Wu, W. Xi, B. Liu, Appl. Phys. Lett. 95, 032105 (2009)
H.X. Yang, C.T. Chong, R. Zhao, H.K. Lee, J.M. Li, K.G. Lim, L.P. Shi, Appl. Phys. Lett. 94, 203110 (2009)
Y.G. Lu, Z.H. Zhang, S.N. Song, X. Shen, G.X. Wang, L.M. Cheng, S.X. Dai, Z.T. Song, Appl. Phys. Lett. 102, 241907 (2013)
Y.G. Lu, S.N. Song, Z.T.W.C. Ren, Y.L. Xiong, F. Rao, L.C. Wu, Y. Cheng, B. Liu, Scr. Mater. 66, 702 (2012)
Y.F. Hu, M.C. Sun, S.N. Song, Z.T. Song, J.W. Zhai, J. Alloy Compd. 551, 551 (2013)
M. Sun, S. Song, Z. Song, J. Zhai, G. Liang, Y. Wu, Scr. Mater. 68, 522 (2013)
J.S. Lannin, Phys. Rev. B 15, 3863 (1977)
K.F. Kao, Y.C. Chu, M.J. Tsai, T.S. Chin, J. Appl. Phys. 111, 102808 (2012)
P.H. Lee, P.C. Chang, D.S. Chao, J.H. Liang, S.C. Chang, M.J. Tsai, T.S. Chin, Thin Solid Films 520, 6636 (2012)
Y.J. Huang, T.C. Chung, C.H. Wang, T.E. Hsieh, J. Electrochem. Soc. 157, 113 (2010)
M. Avrami, J. Chem. Phys. 9, 177 (1941)
P.C. Chang, C.C. Chang, S.C. Chang, T.S. Chin, J. Non-cryst. Solids 383, 106 (2014)
S. Soeya, T. Shintani, T. Odaka, R. Kondou, J. Tominaga, Appl. Phys. Lett. 103, 053103 (2013)
Z. Zhang, S. Song, Z. Song, Y. Cheng, F. Rao, L. Wu, B. Liu, B. Chen, Y. Lu, Appl. Phys. Lett. 103, 142112 (2013)
Y.F. Hu, X.Y. Feng, S.M. Li, T.S. Lai, S.N. Song, Z.T. Song, J.W. Zhai, Appl. Phys. Lett. 103, 152107 (2013)
Y.F. Hu, S.M. Li, T.S. Lai, S.N. Song, Z.T. Song, J.W. Zhai, Scr. Mater. 69, 61 (2013)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (61474083) and the Natural Science Foundation of Jiangsu Province (BK2015020024).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hu, Y., He, Z., Zhai, J. et al. Superlattice-like SnSb4/Ga3Sb7 thin films for ultrafast switching phase-change memory application. Appl. Phys. A 121, 1125–1131 (2015). https://doi.org/10.1007/s00339-015-9470-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-015-9470-z