Abstract
Uniform Ti-doped hematite thin films were deposited on transparent fluorine-doped tin oxide FTO coated glasses using a pulsed laser deposition method. An influence of dopant concentration on the photoelectrochemical characteristics was examined under water splitting. Photocurrent measurements indicated that 3 mol% of Ti atoms was optimal dopant concentration in hematite films produced by this method. The maximum photocurrent density of un-doped and 3 mol% Ti-doped Fe2O3 photoelectrodes was 0.67 and 1.64 mA/cm2 at 1.23 V versus RHE, respectively. The incorporation of Ti atoms into hematite photoelectrodes was found to drastically enhance the water splitting performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Solar energy to fuel conversion through photoelectrochemical (PEC) water oxidation at semiconductors is a promising approach to supply with clean and renewable energy. Solar energy comprises the largest available primary energy source, whereas the water is a ubiquitous source of hydrogen atoms [1]. The combination of water and sunlight opens the possibility to produce renewable, carbon-free and unlimited hydrogen fuel via PEC water oxidation process.
Semiconductor photocathodes used for water splitting cells should have a proper band gap energy (~2–2.2 eV), strong visible light absorption, fast transport of the photo-generated electrons and holes, low cost, etc. [2, 3]. Furthermore, photocathodes need to supply sufficient cathodic current to reduce protons to H2 and must be stable in aqueous environments. Compare to other semiconductors, hematite or α-Fe2O3 has a bandgap of ~2.2 eV, which allows utilization of nearly 40 % of the incident solar radiation. Furthermore, α-Fe2O3 is naturally abundant, cheap and has excellent photochemical corrosion in aqueous solutions. [4, 5]. However, hematite has a critical major drawback, i.e., very poor electrical conductivity and very high electron–hole recombination rate. Thus, most of the hematite photoanodes have exhibited very low PEC performance. To date, a large number of dopant ions have been introduced into α-Fe2O3 matrix attempting to improve performance including, Ti4+, Pt4+, Si4+, Zn2+, Mg2+, etc. [4–8]. These dopants have been shown to play important roles in improving the donor density, electrical conductivity, etc. However, the properties of any kind material can be strongly influenced by the dopant concentration and crystallinity [9, 10]. Therefore, the physical understanding of the PEC performance and its dependence on activators concentration is of fundamental importance.
In present study, we prepared Ti-doped hematite thin films grown directly on FTO substrates by PLD method. During the samples preparation, the targets were initially calcined at 1,000 °C (sufficiently higher than other known methods) suggesting their very high crystallinity. The PEC performances of these films were found to be strongly dependent on the Ti concentration, the parameter, which can be easily controlled during the fabrication.
2 Experimental part
2.1 Thin films deposition
All chemicals were purchased from Sigma-Aldrich Corporation and were used without further purification. Fe2O3:xTi4+ (where x = 0, 1, 3 and 5 mol%) powders were firstly prepared by a co-precipitation method. Obtained powders were pressed into pellets and calcined in air at 1,000 °C for 10 h. FTO glass (16–20 Ω cm−2) was used as a substrate for thin films deposition. Before deposition, the FTO glass was washed with acetone, ethanol and then deionized water. PLD method (248 nm KrF excimer laser, repetition rate of 10 Hz, energy density was 2 J/cm2) was used to grow 100-nm-thick Ti-doped Fe2O3 thin films on FTO substrates.
2.2 Characterization
The structures of the prepared films were examined by XRD (Bruker D8 Discover) using Cu-Kα radiation (λ = 0.15405 nm) at a 2θ scan range of 20–60o. The morphology of the films was characterized by FESEM (Hitachi S-4700). The PEC experiments were carried out in three-electrode configuration under simulated AM 1.5 (100 mW cm−2) illumination in 1 M KOH solution (pH 13.6) using Ag/AgCl as the reference electrode and a platinum foil as the counter electrode. The measured voltage was converted into the potential versus RHE. IPCE were measured using a 300 W xenon lamp (66905, Oriel Instruments) coupled with a monochromator (74004, Oriel Conerstone). Depth profiling analysis was performed on an ESCALAB 220i XL surface analysis instrument at ultrahigh vacuum (P ≤ 10−6 Torr). The depth profiling was conducted using 2 kV low energy Ar+ ions rastered over 1 × 1 mm area. The etching rate was controlled approximately at 20 nm/min. All measurements were performed at a room temperature of 22 ± 1 °C.
3 Results and discussion
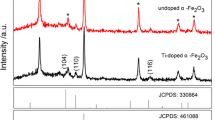
The XRD patterns of the un-doped and Ti-doped (1, 3 and 5 mol%) hematite films on the surface of FTO are shown in Fig. 1. The pure FTO substrate was also used as a reference. The XRD data for un-doped and Ti-doped hematite thin films grown on FTO substrates show that all peaks (with the exception of the FTO substrate peaks) can be indexed to the rhombohedral phase of α-Fe2O3 (JCPDS No. 33-0664) with no alien peaks of other phases. The intense peaks of the XRD pattern indicate that the α-Fe2O3 films were well crystallized. The calculated mean crystallite sizes of the prepared thin films (based on the well-known Debye–Scherrer’s equation) roughly were 57, 51, 44 and 41 nm for pure α-Fe2O3, α-Fe2O3:1 % Ti4+, α-Fe2O3:3 % Ti4+ and α-Fe2O3:5 % Ti4+ films, respectively. Thus, one can see that the mean crystallite sizes decreased with Ti4+ co-doping. Figure 2 shows the comparison on morphology of un-doped and Ti-doped hematite thin films deposited on FTO glasses. It seems that doping with different Ti4+ concentrations does not significantly change the morphology (due to uniform thin films deposition by PLD), and all samples have a nearly similar rough surface. The as-deposited thin films were about 100-nm-thick as it shown in the Fig. 2a (inset).
Figure 3 shows the photocurrent density-applied potential (J–V) scans for un-doped and Ti-doped hematite thin films. In the dark conditions, the J–V responses for all samples are similar and show no evidence of enhanced PEC activity due to the dopant. The un-doped hematite thin film yields a relatively low photocurrent density of 0.67 mA/cm2 at 1.23 V versus RHE. However, this value represents one of the highest photocurrents reported for bare hematite fabricated under standard conditions [11]. This good performance may originate from high crystallinity and interconnectivity of hematite films due to the high temperature target annealing at 1,000 °C. The Ti-doped hematite thin films show a much higher photocurrent than that of un-doped sample. Among all the compositions, the 3 mol% Ti-doped sample exhibits the highest relative PEC performance. For 3 mol% Ti-doped sample, the photocurrent density at 1.23 V versus RHE drastically increases to 1.64 mA/cm2. Thus, the photocurrent of 3 mol% Ti-doped sample is 2.4 times larger than that of the un-doped sample fabricated at the same conditions.
The incident photocurrent conversion efficiencies (IPCEs) for un-doped and Ti-doped hematite films were measured at 1.23 V versus RHE as a function of incident light wavelength, and the data are plotted in Fig. 4. Ti-doped hematite film shows enhanced IPCE values compared to the un-doped sample at all measured wavelengths, which are consistent with the J–V curves. The highest IPCE measured for Ti-doped sample is about 62 % (at a wavelength of 350 nm). The IPCE for both samples are especially low in the long-wavelength range because the quantum yield for converting photons with energies close to the hematite band edge is low. The observed high efficiency for Ti-doped sample can be attributed to higher crystallinity and improved donor density by Ti doping.
The distribution of Ti as a function of depth within the film was examined using the 3 mol% Ti-doped sample by controlled Ar+ ion etching. Figure 5 shows the XPS etching profile obtained from the 3 mol% Ti-doped hematite film. The etching time was varied from 0 to 5 min. The atomic ratio of Ti4+ was observed to be constant (~2.8 %), indicating that the surface and bulk have approximately the same Ti concentration.
Ti dopant in hematite may act in several ways. First, Ti acts as an electron donor due to the substitution of Fe3+ by Ti4+ in hematite lattice. The increased donor concentration favors to improved conductivity and decreases the carrier recombination. Second, the increased donor concentration would increase the electric field across the space charge layer resulting in higher charge separation efficiency. However, at high concentrations, Ti4+ would have defect scattering/recombination properties and finally negate the increased separation efficiency. The experimentally obtained optimum 3 mol% Ti4+ doping level may balance these competing effects and yield the best PEC performance.
4 Conclusion
In conclusion, Ti dopant was successfully introduced into hematite films at different concentration level. The Ti-doped hematite films show an improvement in efficiency for PEC water splitting compared to un-doped sample. We experimentally showed that the 3 mol% Ti-doped hematite film exhibits the highest relative PEC performance. The high photocurrent density and IPCE of 3 mol% Ti-doped hematite film can be attributed to both the favorable crystallinity and the Ti doping, which reduce the electron–hole recombination and increase the donor density in hematite films. Further work on understanding the effects of other elements doping into hematite lattice on the mechanism and PEC behavior is going on.
References
O. Zandri, B.M. Klahr, T.W. Hammann, Highly photoactive Ti-doped α-Fe2O3 thin film electrodes: resurrection of the dead layer. Energy Environ. Sci. 6, 634 (2013)
J.Y. Kim, G. Magesh, D.H. Youn, J.-W. Jang, J. Kubota, K. Domen, J.S. Lee, Single-crystalline, wormlike hematite photoanodes for efficient solar water splitting. Sci. Rep. 3, 2681 (2013)
L. Wang, C.Y. Lee, P. Schmuki, Solar water splitting: preserving the beneficial small feature size in porous α-Fe2O3 photoelectrodes during annealing. J. Mater. Chem. A 1, 212 (2013)
Y.S. Hu, A. Kleiman-Shwarsctein, A.J. Forman, D. Hazen, J.N. Park, E.W. McFarland, Pt-doped α-Fe2O3 thin films active for photoelectrochemical water splitting. Chem. Mater. 20, 3803 (2008)
J. Deng, J. Zhong, A. Pu, D. Zhang, M. Li, X. Sun, S.-T. Lee, Ti-doped hematite nanostructures for solar water splitting with high efficiency. J. Appl. Phys. 112, 084312 (2012)
A. Kay, I. Cesar, M. Gratzel, New benchmark for water photooxidation by nanostructured α-Fe2O3 films. J. Am. Chem. Soc. 128, 15714 (2006)
S. Kumari, A.P. Singh, C. Tripathi, D. Chauhan, S. Dass, R. Shrivastav, V. Gupta, K. Sreenivas, V.R. Satsangi, Enhanced photoelectrochemical response of Zn-dotted hematite. Int. J. Photoenergy 2007, 87467 (2007)
S. Mohanty, J. Ghose, Studies on some α-Fe2O3 photoelectrodes. J. Phys. Chem. Solids 53, 81 (1992)
T.S. Atabaev, Z. Piao, Y.H. Hwang, H.K. Kim, N.H. Hong, Bifunctional Gd2O3:Er3+ particles with enhanced visible upconversion luminescence. J. Alloys Compd 572, 113 (2013)
M. Ajmal, T.S. Atabaev, Facile fabrication and luminescent properties enhancement of bimodal Y2O3:Eu3+ particles by simultaneous Gd3+ codoping. Opt. Mater. 35, 1288 (2013)
Y.P. Hsu, S.W. Lee, J.K. Chang, C.J. Tseng, K.R. Lee, C.H. Wang, Effects of platinum doping on the photoelectrochemical properties of Fe2O3 electrodes. Int. J. Electrochem. Sci. 8, 11615 (2013)
Acknowledgments
This research was supported by the National Research Foundation of Korea (Grant No. 2012R1A1B3001357).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Atabaev, T.S., Ajmal, M., Hong, N.H. et al. Ti-doped hematite thin films for efficient water splitting. Appl. Phys. A 118, 1539–1542 (2015). https://doi.org/10.1007/s00339-014-8937-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-014-8937-7