Abstract
Nickel nanoparticles were fabricated by ablating a bulk Ni target with pulsed 337-nm laser radiation in distilled water. Transmission electron microscope images of the removed material show spherical particles with two size scales: tens of nm and hundreds of nm. Phase explosion and Rayleigh–Plateau hydrodynamic instability are suggested as being responsible for this distribution. An X-ray diffraction pattern of the ablated material demonstrates the presence of both nickel and nickel oxide.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Transition metal and magnetic nanoparticle synthesis is a field of active research because of the potential for biomedical, catalytic and other technological applications. For example, nanomagnetic materials hold promise for use in hyperthermia as a means to destroy cancer cells, for spintronic devices and also for magnetic recording [1–3], while Ni nanoparticles fabricated by laser ablation have been tested for their catalytic activity [4]. While chemical synthesis is the major approach for their production [5, 6], laser-assisted methods of nanoparticle fabrication have recently attracted attention [7–10]. For example, laser ablation in vacuum was used for fabrication of permalloy nanoparticles deposited on a substrate [11], nickel nanoparticles were fabricated by femtosecond laser ablation in vacuum [7], while excimer laser ablation of sintered pellets under different buffer gas conditions was used to fabricate iron oxide [12] and cobalt oxide [13] nanoparticle films.
Laser ablation in liquids is a simple and versatile method for nanoparticle synthesis [14]. The liquid environment works as a container for the produced particles, and varying the liquid can be used to control the chemical composition and the physical properties of the ablation products [15, 16]. Iron oxide nanorods and nanobelts were fabricated by excimer laser ablation in methanol [8], and this process has also been used to create permalloy nanoparticles as well as many nonmagnetic nanoparticles [17–20].
Nickel nanoparticles are important due to their magnetic and catalytic properties, and laser ablation in liquids has attracted interest as a method for Ni nanoparticle fabrication. Until now, all laser ablation on Ni targets has been with 532, 1064 and 1070-nm light, and the resulting nanoparticles have a single peak in their size distribution [21–24]. Since excimer lasers are widely used for industrial applications, analysis of properties of Ni nanoparticles obtained via UV ablation of Ni in liquid environments is of interest.
In the present work, we demonstrate, for the first time, that UV-pulsed laser ablation of a bulk nickel sample immersed in water can produce a bimodal size distribution of nanoparticles composed of nickel and nickel oxide. These findings are based on transmission electron microscopy and X-ray diffraction of the ablated material.
2 Experimental
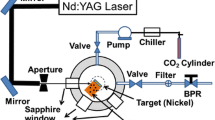
Laser ablation was performed on a 1 cm × 1 cm × 8 mm Ni block (Alfa Aesar). The target was immersed in distilled water and irradiated by a nitrogen laser operating at 337 nm, with pulse duration of 10 ns and a repetition rate of 5 Hz. The laser beam was in the TEM10 mode. The energy pulse measured just above the water was adjusted to 10 mJ. When the beam was focused on the surface of the target, two spots of ~100 µm size were formed. With 2 % reflection by the water surface, the estimated fluence at the target was about 50 J/cm2 [17]. The beaker was moved horizontally on the stage every minute in order to expose fresh nickel surface to the beam.
For the electron microscopy investigations, the ablated material was deposited on 200 mesh copper grids with carbon support and the images were obtained on a Phillips CM12 transmission electron microscope. Angle-dispersive X-ray diffraction of both the original and ablated materials was performed at beamline 12.2.2 of the Advanced Light Source at Lawrence Berkeley Laboratory [25]. The X-ray wavelength was 0.6199 nm, and the distance between the sample and the detector was about 265 mm.
3 Results and discussion
Transmission electron microscope images of the obtained particles are shown in Figs. 1 and 2. The particles are almost spherical in shape. Their diameters were determined using imaging software by calculating the average of measurements in two mutually perpendicular directions. There are two distinct sizes of particles, one with a typical size of the order of nanometers and the other with a size of the order of hundreds of nanometers. The size distributions of the particles are shown in Fig. 3 with peaks in the distributions at 6 and 100 nm.
Energy-dispersive spectroscopy results from different size particles are shown in Fig. 4. The spectrum in Fig. 4a is from a cluster of small particles, and the spectrum in Fig. 4b is from a large particle. For both cases, the presence of Ni is confirmed. One peak is located at 7.5 keV, which is identified as the peak due to Ni Kα. The other peak, which is located at 8.0 keV, is identified as the Cu Kα line [26]. It is related to the TEM support grid. The diffraction pattern of ablated material is shown in Fig. 5. Four peaks are associated with Ni and three with NiO, while the peak at 2.69 Å is not identified.
It is interesting to compare particles in Fig. 2 with particles obtained in other laser ablation experiments. In femtosecond 780-nm laser ablation of Ni in vacuum with particles deposited on a mica support, a single peak in the range 20–60 nm was reported [7]. Ni in ethylene glycol was ablated by 532-nm laser light with nanosecond pulse duration, and only one peak in the 10-nm range was reported [21]. Laser ablation of nickel in methanol with 260-ns pulses at a wavelength of 1064 nm produced nanoparticles with ~12-nm average size [24]. In contrast to these nickel studies, the present experiment resulted in a bimodal particle size distribution. Such a distribution has been observed for many metals [27]. It has been suggested that various mechanisms are responsible for the formation of the particles of different sizes.
Phase explosion is a process of homogeneous nucleation during high-intensity pulsed laser ablation resulting in the formation of a mixture of nanoscale droplets and vapor. Phase explosion has been identified as the cause of the nanometer size particles [28, 29]. Models for the formation of larger particles during laser ablation in vacuum have been proposed based on different physical mechanisms, specifically, on spallation (mechanical disintegration), recoil pressure and hydrodynamic effects such as evaporation front instability, Raleigh–Taylor and Kelvin–Helmholtz instabilities [30–34]. Evaporation front instability occurs due to capillary wave formation on the melted surface. The wave grows because pressure in the valleys is higher than in the hills which results in a positive increment of the instability [33]. Kelvin–Helmholtz instability occurs in the presence of a velocity shear flow such as may occur at a liquid–gas interface. This has been used as an explanation for the large particles that have been observed. As the vapor plume expands laterally along the melted surface, the molten metal forms droplets with radii on the scale of tens of nanometers due to the Kelvin–Helmholtz instability [33]. Raleigh–Taylor instability can develop when a fluid with higher density is pushed against a fluid with lower density due to gravity or acceleration. This type of instability was suggested to explain the formation of droplets for the case of targets with rippled surfaces [32]. When this model is applied to laser ablation, it is thought that the ripples are formed as the result of capillary instability of the melted surface layer. For the subsequent pulse, the melt moves along valleys and hills. The resulting centrifugal acceleration may cause formation of protrusions on the liquid–vapor interface that result in droplets. Although the above mechanisms have been used to explain the presence of larger particles, quantitative estimates for all of these effects give values for droplet sizes of the order of tens of nm [33].
In fluid dynamics, droplet formation occurs due to the crown splash effect when a drop of liquid falls on a liquid layer. For Weber numbers high enough and a diameter of the drop larger than the thickness of the liquid layer (typically the ratio is about 10) after the collision, a sheet of liquid is ejected outward and upward. The ejected sheet-like jet forms a rim that eventually disintegrates into droplets. Though different mechanisms were suggested (such as Raleigh–Taylor and Richtmyer–Meshkov instabilities), there are convincing arguments that the mechanism of droplet formation is due to the Rayleigh–Plateau instability [10].
An analogy can be drawn between laser ablation with high-intensity nanosecond pulses and crown splash droplet formation. We suggest that large particles are generated because of the spatial inhomogeneity of the laser beam intensity resulting in hydrodynamic ejection of the melt in the periphery of the irradiated spot. Most of the observations of the bimodal distributions were during ablation by a Gaussian beam [35]. Hence, there is spatial inhomogeneity on the scale of 10–100 microns in the lateral direction on the target’s surface. For other laser profiles, the effect would be similar since temperature across the surface will not be uniform. Due to the inhomogeneity of the laser intensity, there will be a temperature distribution across the target surface, with the highest temperature at the center of the irradiated spot. Hence, when phase explosion occurs, in the center, a ring of melt gets an impulse directed from the center to the periphery that results in formation of a liquid jet. The large particles are formed as a result of Rayleigh–Plateau instability developed in the jet, similar to disintegration of a jet of liquid into a spray of droplets. The Rayleigh–Plateau instability results in droplets with sizes comparable to the lateral dimension of the jet—in this case, the depth of the melted layer. Since the melted layer depth is of the order of microns, the size of the resulting particles can be in the range from hundreds of nanometers to microns.
In our experiment, where a two-peak size distribution was observed, the fluence of the laser pulses was about 50 J/cm2, which is much greater than the ~5 J/cm2 fluence in the other reported experiments [21, 24]. Hence, the thickness of the melted layer in the present case was substantially higher. When a jet is in the Rayleigh–Plateau regime, the droplet size is comparable to the wavelength of the fastest growing instability which is ~4.51d, where d is the jet’s diameter. Hence, in the Rayleigh–Plateau model of particle formation during ablation, the size of the droplets scales with the melt depth, which is a function of fluence. For fluences ϕ close to the threshold fluence for melting, ϕ m , the maximum melting depth is given by:
where l T = (Dτ m )1/2 is the characteristic length for heat propagation with τ m being a time interval from the start of the pulse until the melting of the surface and D is the thermal diffusivity of the ablated material. For fluences far above the melting threshold,
where I a is the radiation intensity, τ is the pulse duration, ρ is the density, Δ H is the enthalpy of fusion of the ablated material, and ϕ a is the ablation fluence [33].
Since for Ni, the melting threshold is below 1 J/cm2, from the above formulas, the estimate for the melt depth in our experiment is ten times higher than in the citied examples. Hence, we can expect that the particles formed due to the Rayleigh–Plateau instability are approximately ten times larger as well.
The time of breakup of the jet is given by: \( t\sim 0.36 \sqrt {\rho d^{3} /\sigma } \), where σ is the surface tension of the liquid [36]. For a one-micron jet of molten nickel, as expected in the present study, the breakup time is ~100 ns. This is a reasonable characteristic time for nanosecond-pulsed laser ablation. Rayleigh–Plateau instability has been observed in other laser–matter interactions. From theoretical and molecular dynamics analysis, it was concluded that the melted nanoscale jet on a graphite substrate disintegrated via Rayleigh–Plateau instability [37]. Similar nanometer-scale Rayleigh–Plateau instability examples were demonstrated in other papers as well [37–42].
Hence, two physical mechanisms may be responsible for the double-peak distribution: phase explosion and Rayleigh–Plateau instability. It should be noted though that phase explosion produces two types of particles: nanoscale droplets and sub-nanometer clusters formed from the vapor during plume expansion. Consequently, a three-peak distribution of particle size is possible. The exact shape of the peaks depends on details of the plume evolution. For the present case of nickel ablated in water, plume quenching results in 10-nm particles. Quenching of the vapor may result in nanometer and sub-nanometer particles. These smaller particles could form agglomerates which prevent one from resolving individual particles.
4 Conclusion
Nickel in distilled water was ablated by nanosecond UV laser pulses, and the ablated particles were characterized by transmission electron microscopy, energy-dispersive spectroscopy and X-ray diffraction. The ablated material was found to be nanoparticles composed of nickel and nickel oxide. Two types of nanoparticles were observed: those with a characteristic size of ~10 nm and those with a characteristic size of ~100 nm. The bimodal distribution is explained by phase explosion (~10-nm particles) and Rayleigh–Plateau instability (~100-nm particles).
References
W. Andra, H. Nowak (eds.), Magnetism in Medicine: A Handbook (Wiley-VCH, Weinheim, 2007)
K. Iwasaki, T. Itoh, T. Yamamura, Mater. Trans. 46, 1568 (2005)
D.D. Awschalom, M.E. Flatte, Nat. Phys. 3, 153 (2007)
S. Kim, B.K. Yoo, K. Chun, W. Kang, J. Choo, M.-S. Gong, S.-W. Joo, J. Mol. Catal. Chem. 226, 231 (2005)
A.C.C. Yu, M. Mizuno, Y. Sasaki, H. Kondo, K. Hiraga, Appl. Phys. Lett. 81, 3768 (2002)
S. Sun, C.B. Murray, D. Weller, L. Folks, A. Moser, Science 287, 1989 (2000)
S. Amoruso, G. Ausanio, C. de Lisio, V. Iannotti, M. Vitiello, X. Wang, L. Lanotte, Appl. Surf. Sci. 247, 71 (2003)
S. Mollah, S.J. Henley, S.R.P. Silva, Nanotechnology 19, 205604 (2008)
W.T. Nichols, G. Malyavanatham, D.E. Henneke, J.R. Brock, M.F. Becker, J.W. Keto, H.D. Gliksman, J. Nanopart. Res. 2, 141 (2000)
L.V. Zhang, P. Brunet, J. Eggers, R.D. Deegan, Phys. Fluids 22, 122105 (2010)
J. Lee, M.F. Becker, J.R. Brock, J.W. Keto, R.M. Walser, IEEE Trans. Magn. 32, 4484 (1996)
T. Sasaki, X. Zeng, N. Koshizaki, Mater. Res. Soc. Symp. Proc. 526, 67 (1998)
N. Koshizaki, A. Narazaki, T. Sasaki, Scr. Mater. 44, 1925 (2001)
H. Zeng, X.-W. Du, S.C. Singh, S.A. Kulinich, S. Yang, J. He, W. Cai, Adv. Funct. Mater. 22, 1333 (2012)
S. Barcikowski, F. Mafune, J. Phys. Chem. C 115, 4985 (2010)
H. Muto, K. Yamada, K. Miyajima, F. Mafune, J. Phys. Chem. C 111, 17221 (2007)
O.R. Musaev, V. Dusevich, D.M. Wieliczka, J.M. Wrobel, M.B. Kruger, J. Appl. Phys. 104, 084316 (2008)
O.R. Musaev, A.E. Midgley, J.M. Wrobel, J. Yan, M.B. Kruger, J. Appl. Phys. 106, 054306 (2009)
T. Tsuji, T. Higuchi, M. Tsuji, Chem. Lett. 34, 476 (2005)
J. Ren, M. Kelly, L. Hesselink, Opt. Lett. 30, 1740 (2005)
J. Zhang, C.Q. Lan, Mater. Lett. 62, 1521 (2008)
R. Mahfouz, F.G.C.S. Aires, A. Brenierb, B. Jacquierb, J.C. Bertolinia, Appl. Surf. Sci. 254, 5181 (2008)
S.Z. Khan, Y. Yuan, A. Abdolvand, M. Schmidt, P. Crouse, L. Li, Z. Liu, M. Sharp, K.G. Watkins, J. Nanopart. Res. 11, 1421 (2009)
B. Jaleh, M.J. Torkamany, R. Golbedaghi, M. Noroozi, S. Habibi, F. Samavat, V.J. Hamedan, L. Albeheshti, Adv. Mater. Res. 403–408, 4440 (2012)
W.A. Caldwell, M. Kunz, R.S. Celestre, E.E. Domning, M.J. Walter, D. Walker, J. Glossinger, A.A. MacDowell, H.A. Padmore, R. Jeanloz, S.M. Clark, Methods Phys. Res. A 582, 221 (2007)
F. Alonso, P. Riente, J.A. Sirvent, M. Yus, Appl. Catal. A 378, 42 (2010)
R. Hergenröder, Spectrochim. Acta Part B 61, 284 (2006)
A. Miotello, R. Kelly, Appl. Phys. Lett. 67, 3535 (1995)
N.M. Bulgakova, A.V. Bulgakov, I.M. Bourakov, N.A. Bulgakova, Appl. Surf. Sci. 96, 197–198 (2002)
M.E. Povarnitsyn, T.E. Itina, M. Sentis, K.V. Khischenko, P.R. Levashov, Phys. Rev. B 75, 235414 (2007)
R.L. Webb, J.T. Dickinson, G.J. Exarhos, Appl. Spectrosc. 51, 707 (1997)
A.B. Brailovsky, S.V. Gaponov, V.I. Luchin, Appl. Phys. A 61, 81 (1995)
D. Bäuerle, Laser Processing and Chemistry, 3rd edn. (Springer, Berlin, 2000)
P.G. Drazin, W.H. Reid, Hydrodynamic Stability (Cambridge University Press, Cambridge, 1981)
Y. Jee, M.F. Becker, R.M. Walser, J. Opt. Soc. Am. B 5, 648 (1988)
W.A. Sirignano, C. Mehring, Prog. Energy Comb. Sci. 26, 655 (2000)
J. Fowlkes, S. Horton, M. Fuentes-Cabrera, P.D. Rack, Angew. Chem. Int. Ed. 51, 8768 (2012)
J. Lian, L. Wang, X. Sun, Q. Yu, R.C. Ewing, Nano Lett. 6, 1047 (2006)
J.J. Kaufman, G. Tao, S. Shabahang, E.-H. Banaei, D.S. Deng, X. Liang, S.G. Johnson, Y. Fink, A.F. Abouraddy, Nature 487, 463 (2012)
J.D. Fowlkes, L. Kondic, J. Diez, Y.Y. Wu, P.D. Rack, Nano Lett. 11, 2478 (2011)
Y. Wu, J.D. Fowlkes, P.D. Rack, J.A. Diez, L. Kondic, Langmuir 26, 11972 (2010)
C. Favazza, J. Trice, R. Kalyanaraman, R. Sureshkumar, Appl. Phys. Lett. 91, 043105 (2007)
Acknowledgments
This work was partially supported by National Science Foundation Contract DMR–0923166.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Musaev, O.R., Yan, J., Dusevich, V. et al. Ni nanoparticles fabricated by laser ablation in water. Appl. Phys. A 116, 735–739 (2014). https://doi.org/10.1007/s00339-014-8569-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-014-8569-y