Abstract
Compared to superhydrophobic surfaces, superamphiphobic surfaces possess more potential applications but are difficult to fabricate. Herein, to address this problem, we describe a simple method to fabricate a superamphiphobic surface based on a CNTs–SiO2 hybrid material. The CNTs–SiO2 hybrid material obtained by a sol–gel approach was sprayed onto glass slides to form coatings. After surface fluorination, the sprayed coating displayed superamphiphobicity toward water and a number of organic liquids, such as dodecane. It was found that the time of fluorination slightly influenced the surface wettability of the sprayed coating. We also investigated the role of CNTs and SiO2 on superamphiphobicity establishment separately, and such information allowed us to engineer surfaces with specific wettability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Recently, superamphiphobic surfaces have immensely generated commercial and academic interest for a diverse array of applications, including nonfouling surfaces, self-cleaning surfaces, stain-free clothing and antifreezing coatings [1–5]. However, superamphiphobic surfaces are difficult to fabricate, which involve selection of a suitable surface chemistry to minimize the solid-surface energy and optimal choice of the surface texture [6–8]. Fluoropolymer/fluorocarbon materials possess one of the lowest surface energies available, which make such coatings attractive for self-cleaning surfaces. Appropriately geometrical shape is also critical in establishing superamphiphobicity. Herein, some special structures, such as the re-entrant geometry or overhanging architecture [9, 10], have to be introduced to achieve superamphiphobicity. Until now, various research groups have tried to design superamphiphobic surfaces using the methods of electrospinning, spray-coating, sol–gel methods, etching methods, and so on [11–16]. However, some of the used methods are either time-consuming or laborious, while others require special materials to realize superamphiphobicity, which greatly limit the practical applications of superamphiphobic surfaces. Thus, creating of superamphiphobic surfaces by a simple and timesaving method becomes the urgent demand and will greatly advance the applications of superamphiphobicity.
In this study, we develop a facile method to fabricate a superamphiphobic surface based on a CNTs–SiO2 hybrid material, and the designing strategy is shown in Fig. 1. The CNTs–SiO2 suspension was sprayed onto glass slides to form coatings, and after surface fluorination, the coatings displayed superamphiphobicity toward water and several organic liquids possessing much lower surface tension than that of water, such as dodecane. The whole fabrication procedure was fairly easy to be carried out and can be applied to scalable production of superamphiphobic surfaces. Moreover, tunable surface wettability can be achieved by spray-coating with varied materials. This study is expected to provide a new avenue for the basic research as well as real applications.
2 Experimental
2.1 Materials
Hydroxylic MWCNTs (30–50 nm in diameter and 50 μm in length, purity >95 wt %) were purchased from Chenguang Research Institute of Chemical Industry (Chengdu, China), and the content of hydroxyl group was 1.06 wt %, which was obtained by the chemical oxidation of raw MWCNTs. Both tetraethyl orthosilicate (TEOS) and 1H, 1H, 2H, 2H-perfluorooctyltrichlorosilane (PFOTS) were purchased from Sigma-Aldrich. Ammonia aqueous solution (25 wt %) was provided by Kaitong Chemical Reagents Company (Tianjin), and ethanol was obtained from Xilong Chemical Industry Company.
2.2 Preparation of the SiO2-CNTs hybrid material
The preparation of our SiO2-CNTs hybrid material was carried out as follows. 1 g of hydroxyl MWCNTs was ultrasonically dispersed into 100 mL of ethanol to form a homogeneous dispersion, and 5 mL of ammonia aqueous solution (25 wt %) was added dropwise to the above mixture under magnetic stirring. Subsequently, a 27.5 mL mixture of TEOS and ethanol (volume ratio 1:10) was added dropwise to the CNTs suspension. Finally, we obtained the product that we had expected after the mixture was kept stirring at room temperature for 12 h.
2.3 Fabrication of the hybrid surface using spray-coating and fluorination
The obtained SiO2-CNTs suspension was dispersed for 30 min by an ultrasonic bath, and then sprayed onto clean glass slides (we used columnar copper substrates on measurement of the surface morphology) using a spray gun (Shanghai, Lotus brand, no. 1) with 0.2 MPa nitrogen gas at room temperature. Subsequently, we placed the coatings into vacuum drying oven for 5 min (at 20 °C). After being taken out, the samples were fluorinated using CVD technique in airtight glass case that abounded with PFOTS gas. We obtained the resultant surface after placement in vacuum drying oven for 5 min (at 80 °C).
2.4 Characterization
The surface morphology of the coating was investigated using a field-emission scanning electron microscopy (JEOL JSM–6701F FESEM), and the structural image of the hybrid material was observed with a transmission electron microscopy (FEI Tecnai F30 TEM). Fourier transform infrared spectrometer (FTIR, IFS66V/S, Bruker) was used to study the possible groups on the as-prepared material. The contact angles (CAs) and sliding angles (SAs) of water and several organic liquids on the surface were measured using a KRÜSS DSA 100 (KRÜSS) apparatus by injecting 10 mL of certain probing liquid onto the sample. The average values of contact angle (CA) and sliding angle (SA) were achieved by measuring the same sample at five different positions. The chemical composition information of the surface was acquired by X-ray photoelectron spectroscopy (XPS), which was carried out on a VGESCALAB210 X-ray photoelectron spectrometer.
3 Results and discussion
Figure 2 shows the FTIR spectra of CNTs, CNTs–SiO2 hybrid material, and SiO2. It can be seen that the characteristic peaks of CNTs are still observed after coating with SiO2 and have no chemical shifts. The characteristic peaks of SiO2 in the CNTs–SiO2 hybrid material are identical to characteristic peaks of SiO2. These results indicate that there is no chemical bond between CNTs and SiO2 in the CNTs–SiO2 hybrid material.
Figure 3a, b shows FESEM images of the sprayed SiO2-CNTs coating surface. It can be seen that a number of microscale protrusions are distributed on the surface (see Fig. 3a). CNTs wrapped around with SiO2 coating and its particles are exposed outside the coating surface, further increasing the surface roughness, as shown in Fig. 3b, c. Moreover, mass of pores are distributed among the protrusions, which can dramatically increase the trapped air within the grooves, and thus is beneficial to establish solid–liquid–air interface [17, 18]. The main reason for the resulting special structure is that the solvent is rapidly evaporated and the concentration of the suspension quickly increases when the SiO2–CNTs suspension is sprayed onto a certain substrate [19]. There is no observable change in the surface structure after modification with fluorosilane by CVD (see Fig. S1.). After surface fluorination, the peak for F1s was detected at 689.0 eV (see Fig. 2d), and XPS analysis demonstrated that the content of fluorine is up to 36.19 %. This high concentration of fluorine, when combined with the rough textures, results in the CNTs–SiO2 coating with superamphiphobicity.
The superamphiphobicity of the coating is highlighted in Fig. 4a, b. Water and hexadecane exhibit a spherical shape on the surface and easily roll off. Moreover, the bright and reflective surface visible underneath the liquid droplets is a signature of trapped air and the establishment of composite solid–liquid–air interfaces [20]. The formation of the Cassie–Baxter state enhances superamphiphobicity by promoting high apparent CA and low SA. As shown in Table 1, all the probing liquids exhibited a high CA and low SA on the surface. The superamphiphobic property of the surface can be persevered longer than 3 months without obvious changes, indicating the good long-term stability of the surface. Moreover, it was found that the fluorination time influenced the surface wettability slightly. As shown in Fig. 4c, the values of CAs and SAs for water and hexadecane change slightly as the fluoration time varying from 2 to 11 min.
a The CA and SA profiles of water and hexadecane droplets, b optical images of water, glycerol, ethylene glycol, rapeseed oil, hexadecane, and dodecane droplets placed on the superamphiphobic coating surface, c the relationship between the time of fluorination and the values of CAs and SAs of water and hexadecane
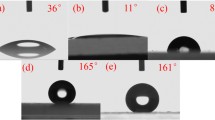
To further investigate the influence of CNTs and SiO2 on the superamphiphobic establishment separately, the pure CNTs coating and the pure SiO2 nanoparticles (circa 50 nm) coating were fabricated by spray-coating (see supporting information for details). After surface fluorination, the pure CNTs coating displayed superhydrophobicity and superoleophilicity with hexadecane, that is, CAs of 165.1° and 0° with water and hexadecane, respectively. As for the fluorinated SiO2 nanoparticles coating, it exhibited superhydrophobicity (151.1°) and oleophobicity (106.4°) with hexadecane. As shown in Fig. 5, the surface texture of the fluorinated pure CNTs (Fig. 5a, b) and SiO2 nanoparticles coatings (Fig. 5c, d) is much smoother and lacks pores that can trap air in them, when compared to CNTs–SiO2 coating.
It should be noted that the trapped air can decrease the contact area between the surface and the contact liquid and thus is beneficial to superamphiphobicity establishment. Therefore, surface texture composed of pure CNTs or pure SiO2 nanoparticles could not form superamphiphobicity, which is the synergistic action of CNTs and pure SiO2 that play a key role in establishing superamphiphobicity.
In this experiment, the mass ratios of CNTs and TEOS have a great effect on the wettability of the superamphiphobic coating. As shown in Fig. 6, the anti-wettability of water and hexadecane on the surface significantly increase when the mass ratios of CNTs and TEOS are in the range from 0 to 0.4. However, once the ratios are larger than 0.4, the CAs for water and hexadecane begin to decline. Differently, the CA for water is gradually stabilized but always larger than 160° and the CA for hexadecane is decreased at all times, which finally becomes 0° as the only CNTs, i.e., superoleophilicity. Therefore, the optimum mass ratio of CNTs and TEOS (0.4) is chosen as the experimental advisement. Herein, the wettability of the resultant surface with different mass ratio of CNTs and TEOS is relevant to the corresponding surface morphology, which could be considered as the Cassie or Wenzel model. According to the relation of the mass ratios and the CAs, the crucial status of SiO2 nanoparticles and CNTs on the superamphiphobic surface can be further approved.
4 Conclusions
We developed a facile method to fabricate a superamphiphobic coating by spraying CNTs–SiO2 hybrid material followed by surface fluorination. Water and a number of extremely low surface tension liquids, such as hexadecane, displayed typical spherical shapes on the coating and could roll off the coating easily. It is found that the fluorination time affects the surface wettability slightly. Separate experiments show that surface texture composed of pure CNTs or pure SiO2 nanoparticles cannot achieve superamphiphobicity after surface fluorination. This study provides a key addition to the functional superamphiphobic materials.
References
J. Genzer, K. Efimenko, Biofouling 22, 339 (2006)
R. Blossey, Nat. Mater. 2, 301 (2003)
B. Leng, Z. Shao, G. de With, W. Ming, Langmuir 25, 2456 (2009)
L. Cao, A.K. Jones, V.K. Sikka, J. Wu, D. Gao, Langmuir 25, 12444 (2009)
D. Xiong, G. Liu, L. Hong, Chem. Mater. 23, 4357 (2011)
A.K. Kota, Y. Li, J.M. Mabry, A. Tuteja, Adv. Mater. 24, 5838 (2012)
A. Tuteja, W. Choi, G.H. McKinley, R.E. Cohen, M.F. Rubner, MRS Bull. 33, 752 (2008)
L. Cao, T.P. Price, M. Weiss, D. Gao, Langmuir 24, 1640 (2008)
M. Liu, Y. Zheng, J. Zhai, L. Jiang, Acc. Chem. Res. 43, 368 (2010)
R.T.R. Kumar, K.B. Mogensen, P. Bøggild, J. Phys. Chem. 114, 2936 (2010)
V.A. Ganesh, S.S. Dinachali, H.K. Raut, T.M. Walsh, A.S. Nair, S. Ramakrishna, RSC Adv. 3, 3819 (2013)
S. Pan, A.K. Kota, J.M. Mabry, A. Tuteja, J. Am. Chem. Soc. 135, 578 (2013)
S. Srinivasan, S.S. Chhatre, J.M. Mabry, R.E. Cohen, G.H. McKinley, Polymer 52, 3209 (2011)
Y.-C. Sheen, W.-H. Chang, W.-C. Chen, Y.-H. Chang, Y.-C. Huang, F.-C. Chang, Mater. Chem. Phys. 114, 63 (2009)
L. Gao, T.J. McCarthy, J. Am. Chem. Soc. 128, 9052 (2006)
K. Zhao, K.S. Liu, J.F. Li, W.H. Wang, L. Jiang, Scripta Mater. 60, 225 (2009)
H.-J. Butt, C. Semprebon, P. Papadopoulos, D. Vollmer, M. Brinkmann, M. Ciccotti, Soft Matter 9, 418 (2013)
A. Tuteja, W. Choi, M. Ma, J.M. Mabry, S.A. Mazzella, G.C. Rutledge, G.H. McKinley, R.E. Cohen, Science 318, 1618 (2007)
Y. Goto, H. Takashima, K. Takishita, H. Sawada, J. Colloid. Interface Sci. 362, 375 (2011)
A.B.D. Cassie, S. Baxter, Wettability of porous surfaces. Trans. Faraday Soc. 40, 546 (1944)
Acknowledgments
The authors acknowledge the financial support of National Natural Science Foundation of China (21101082, J1210001) and Fundamental Research Funds for the Central University (Lzujbky-2013-55).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Appendix A. Supplementary material
Experimental details and further information of characterization are available in this section.
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Y., Zhu, X., Zhou, X. et al. A facile way to fabricate a superamphiphobic surface. Appl. Phys. A 115, 765–770 (2014). https://doi.org/10.1007/s00339-014-8438-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-014-8438-8