Abstract
Stray grains were found during the preparation of single crystal superalloy AM3 by seeding technique and the researching on the competitive growth of bi-crystal. Stray grains were mainly observed at the diverging 〈001〉 corner of the mold wall. Therefore, increasing orientation deviation angle would intensify the possibility of the formation of stray grains. This was because the solute was inclined to enrich at the diverging 〈001〉 corner of mold wall, leading to the relatively large undercooling, and accordingly resulted in the formation of stray grains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the elimination of grain boundary where the crack source is prone to appear, nickel-based single crystal superalloy is endowed with characteristics such as excellent creep rupture life, low creep rate and sound resistance against thermal fatigue, owing to which it has became the preferred material for blades of advanced aircraft engines and industrial gas turbines [1, 2]. Single-crystal superalloys are frequently prepared with the method of directional solidification. Because of the anisotropy of the interfacial tension, crystals are prone to grow along the preferred orientation during the directional solidification [3]. However, due to the influence of solidification kinetics, the preferred orientation is not completely parallel to the axial direction in the actual growth; besides, the preferred orientation needed by actual castings is not always parallel to the heat flow direction owing to special requirements [4]. When there exists a deviation angle between the preferred orientation and the heat flow direction, stray grains, which are different from the original grains in orientation, are apt to appear during the process of directional solidification. Utilizing numerical simulation, Yang et al. [5, 6] have found that at the place where the dendrite direction of the grains and the axial direction deviate from each other, stray grains are more likely to form near the inner wall of the crucible. The integrity of single crystals is destroyed by the appearance of stray grains which will directly affect the usability of castings.
A large number of researches have been conducted on the nucleation to growing up of stray grains forming during the process of directional solidification of single crystal superalloys. Jackson et al. [7] hold the opinion that there are mainly two types of formation mechanism for stray grains in castings, i.e., the heterogeneous nucleation and the dendrite fragment nucleation. During the smelting of superalloys, some refractory inclusion particles might become the nucleation core, i.e., the heterogeneous nucleation. While in the process of preparing single-crystal superalloys with a seeding technique, there might be fragment dendrites which are not melted at the remelting interface. With the convective currents of the melt, these dendrites are transferred to the front of the interface to nucleate. However, this mechanism of the nucleation is questioned by the research findings of Stanford et al. [8]. The crystal orientation becomes harder to be controlled with the enhancement of the alloying of superalloys and the addition of some refractory elements; inevitably, solidification defects and stray grains are more likely to appear [9]. The shape variation of blades is more complicated with the ever-increasing requirements for the service temperature of blades. Thus, stray grains are prone to come into being at places where cross sections change owing to the abrupt alteration of solidification parameters [10–12]. Stray grains have a growth competition with original grain as soon as they have nucleation. And original grain will be eliminated if stray grains grow up in a dominant position; otherwise, stray grains will disappear. It has been found by Stanford et al. [13] that stray grains whose orientation is stochastic almost distribute evenly around the specimen in the vicinity of 2 mm away from the remelting interface of seed crystals, whereas, only a small number of stray grains can remain in the process of directional solidification afterwards. Therefore, it is indicated that the competitive growth mechanism also plays a significant role in the formation of stray grains.
Stray grains were found during both preparation of single-crystal superalloys with a seeding technique and the research on the competitive growth mechanism. In this article we consider in some detail the location and morphology of stray grains, and the nucleation conditions are analyzed in detail as well, which can lay certain experimental and theoretical foundation for the study on how to avoid the formation of stray grains in the future preparation of single crystals.
2 Experiment
Nickel-based single-crystal superalloy AM3 was employed in the experiments. The nominal composition (mass fraction, %) of it is Ni-7.82Cr-5.34Co-2.25Mo-4.88W-6.02Al-1.94Ti-3.49Ta. The single-crystal superalloy was produced through a self-developed directional solidification furnace with dual-zone resistance heating and liquid-metal cooling (LMC), whose temperature gradient was high and reached 360 K/cm, which was estimated by combining the constituent undercooling theory and the definition of the temperature gradient. During the preparing of the single-crystal superalloy with a seeding technique, the seed grains were placed at the bottom of the master alloy and the furnace was preheated to 1650 °C at a certain rate then the temperature was made to last for 20 minutes, so that the melt could be sufficiently homogenized.
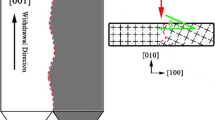
The prepared specimen was sectioned and polished, then etched with a corrosive, i.e., a mixed solution in a proportion of HNO3:HF:glycerol = 1:2:3. Subsequently, by means of an optical microscope, the corrosion of the specimen was observed. With a RIGAKU X-ray diffractometer (D/Max-2400), X-ray diffraction analysis was employed for the identification of the crystal orientation with a Cu target of 1.5406-nm wavelength. By rotating a sample along its surface axis during θ -scanning process to ensure the normal axis of the crystals plane to across the diffraction plane multiply, the deviation angle between the crystal orientation and the surface axis is obtained, as shown in Fig. 1. The deviation angle can be deduced form θ=(θ 2−θ 1)/2 and the detailed method and mechanism employed here are described in Ref. [14].
3 Results
3.1 The formation of stray grains during the preparation for single crystal
Figure 2 shows the stray grain morphology observed when single-crystal superalloy was prepared by means of seeding technique whose preferred orientation 〈001〉 was 18° away from the axis. The withdrawal rate was 100 μm/s and specimen diameter was 1.8 mm. It can be found that the formation of the stray grain started from the specimen edge where the 〈001〉 diverging from the mold wall, and appears at a distance from the remelting interface. The newly born stray grain was different from original crystal grain in dendritic morphology, and it grew in a dominating position so as to eliminate the original crystal grain in the process of subsequent solidification.
Figure 3 shows the quenched interface morphology of crystal with the preferred orientation 〈001〉 is 35° away from the axis. The withdrawal rate is 100 μm/s and its specimen diameter is 4 mm. At the dendrite growth divergence end of the specimen edge, there appeared stray grain, whose preferred orientation was different from that of the original dendrite crystals. The primary dendrite growth direction of the stray grains was not parallel to that of the heat flow. However, compared with the original crystal grain, it has a smaller deviation angle of about 20°, which was determined by the X-ray diffraction analysis technology. It could be noticed that the competitive growth of the two crystal grains mainly depended on the dendrite branches to make the grain boundary move.
3.2 The formation of the stray grains during the competitive growth
Figure 4 shows that the stray grain appeared during the competitive growth of the bi-crystal with the withdrawal rate of 6 μm/s. Two seed crystals with their preferred growth directions are far away from that of maximum temperature gradient in a divergent way and the crystal grows with a cellular interface. Not far from the remelting interface, stray grain appeared and its preferred orientation was different from that of the seed grains. The stray grain has a relative small deviation angle between the cellular growth direction and the heat flow direction. During the subsequent directional solidification, the stray grain was obviously growing in the dominating position and expanding on the whole interface continually. When the withdrawal rate was increased to 100 μm/s, the crystal grew with the developed dendrite morphology, while the stray grain was not observed and the divergent interface was filled with the dendrites branching from the two grains.
As shown in Fig. 5, stray grain was observed in the bi-crystal with converge dendrites. The withdrawal rate is 100 μm/s and the crystal growth with developed dendrites interface. No stray grain was observed at grain boundary of the converge dendrite; in contrast, the stray grain appeared at the specimen edge of the divergence dendrite end, as shown in Fig. 5. There were no stray grains observed at the position of crystal grain A whose preferred orientation was parallel to the heat flow. In accordance with the previous study [15] on the evolution of dendrite morphology, the preferred orientation 〈001〉 of the stray grains was not in the same plane as that of the seed crystal grains A and B. It could be found that the area of the stray grains increased continually and the crystal grain B was eliminated in the process of solidification.
4 Discussion
The formation and growing up of the stray grains are observed both during the preparation for single crystals and the competitive growth of the bi-crystal grains. A number of mechanisms have been proposed for the formation of these unwanted grains: (1) Pinching-off of secondary dendrites in the semi-solid region by convective currents in the melted-back seed. These fragments are considered to be transported ahead of the dendrite tips to nucleate defect grains in liquid that has been subject to solutal undercooling [7, 16]. (2) During the smelting process, the refractory compounds which are not fully melted could be as the heterogeneous nucleation core during the direction solidification [17]. (3) Nucleation of solid at the mould surface when introducing the alloy charge on top of the seed within the mould cavity. Pouring molten alloy into a colder mould may allow rapid nucleation of chill grains on the mould wall. Some of these grains may survive the equilibration time without melting or being overgrown in the early stages of directional solidification. (4) Owning to the solute interaction, bending or possible detachment can happen to dendrite arms. Accordingly, stray grains with different preferred orientation from that of the original one will appear [18]. As for the stray grains forming under mechanism (1), the broken dendrite fragments are transferred to the interface of the front because of melt convection, thus the stray grains may nucleate at any location of the interface front and the appearing position is supposed to have nothing to do with the crystal orientation. However, according to the experimental results of this paper, the stray grains mainly appear at the edge of the specimen and are easily observed when the preferred orientation is away from the axis. The stray grains nucleation position appears very regularly and is related to the crystal orientation. Therefore, the stray grains not originate from the fragments of the secondary dendrites. Stray grains are only observed after a certain directional solidification distance instead of at the remelting interface. In addition, the experimental material in this paper is single-crystal superalloy AM3 and there exist no refractory carbides. The melt can be guaranteed to be fully melted when keeping heat at 1650 °C for 20 minutes. The dendritic bending is not observed in the experiment described in the literature [18]. Accordingly, the experimental phenomenon of the stray grains formation in this paper cannot be explained by the above four stray grain nucleation mechanisms.
The two main characteristics for the formation of stray grains in this paper are: first, appearing at the edge of the specimen, and second, appearing at the divergence end of dendrite crystal. Due to the effects of heat conduction, heat of the specimen edge is conducted faster than that of the specimen center. In addition, during the directional solidification, the solidification interface of specimen is not flat, as shown in Fig. 6. When the specimen grows with the dendrite interface morphology, there will appear a concave interface at the specimen edge of the preferred orientation divergence end. Although the temperature of the specimen edge is lower than that of the specimen center, the liquidus temperature at the specimen edge is also lower than that of the liquidus temperature at the specimen center; because of that the solute enrichment can decrease the freezing point of the liquid based on the solidification theory. However, there are many supplementary references that have computed solute diffusion and thereafter calculated the local undercooling [19–22]. It was found that the greater the volume of the liquid, the lower the solute interaction, the greater the local liquidus temperature and therefore the greater the local undercooling. A microscale cellular automaton finite difference (CA-FD) model, which solves for the solute diffusion-controlled growth of cubic structured alloys, was used to simulate the supercooling and stray grains nucleated by D’Souza et al. [23]. The simulated results revealed that the supercooling near the mold wall greater than that at the center, and the diverging case has the highest undercooling for dendrites with different orientations. In addition, P.D. Lee et al. [24] found that the tip undercooling was increased with the deviation angle by using the CA micromodel of dendritic growth. Owing to the solute accumulation, the undercooling degree at the specimen edge of the dendrite divergence end is relatively high. At the diverging 〈001〉 corner of mold wall with large undercooling, the dendrite is prone to branch here and thus consume the accumulated solute; otherwise, stray grains will come into being when critical undercooling degree is reached. When the two crystal grains are in the relative divergence growth and in the cellular interface morphology, the solute will accumulate in the divergence boundaries along with the solidification. However, the cellular crystal growth could not consume accumulated solute as dendrite crystal does; accordingly, it is easy to reach the critical undercooling degree and the stray grains could as well appear at the center of the specimen, as shown in Fig. 4. When the withdrawal rate is increased, the relatively divergent two grains will grow in the developed dendrite morphology, and the stray grains are not observed. Although there is a large chance to nucleate at the specimen edge based on the discussion above, the heterogeneous nucleation cannot rule out this possibility. The crystal growth process is very complex, and there are many factors which can influence the stray grain nucleation, such as the impurities at the edge of the crucible, which could be as the heterogeneous nucleation core during the direction solidification. Therefore, the stray grains formation mechanism needs further careful and scientific research.
5 Conclusions
-
(1)
When preferred orientation was diverged from the axis, the stray grains were prone to appear at the diverging 〈001〉 corner of mold wall. Accordingly, the increase of the orientation deviation could help to increase the possibility of the stray grains formation.
-
(2)
When the two crystal grains were in relative divergence growth and in cellular crystal morphology, the stray grains were observed at the divergence boundaries. However, when the withdrawal rate was increased, the crystal grew with the developed dendrite interface and the stray grains were not observed.
The stray grains nucleation was because of the solute accumulation at the divergence end of the preferred orientation and the heat was conducted faster at the specimen edge than at the specimen center, leading to higher undercooling degree and the formation of the stray grains.
References
E.W. Ross, K.S. O’Hara, in Superalloys 1996, ed. by E.A. Loria (Minerals, Metals & Materials Soc, Warrendale, 1996), p. 19
A.D. Cetel, D.N. Duhl, in Superalloys 1988, ed. by S. Reichman (TMS, Warrendale, 1988), p. 235
B. Chalmers, Principles of Solidification (Wiley, New York, 1964)
J.Z. Wang, US Patent EP01306925 (2002)
X.L. Yang, H.B. Dong, W. Wang, P.D. Lee, Mater. Sci. Eng. A 386, 129 (2004)
X.L. Yang, D. Ness, P.D. Lee, N. D’Souza, Mater. Sci. Eng. A 413–414, 571 (2005)
K.A. Jackson, J.D. Hunt, D.R. Uhlmann, Trans. Metall. Soc. AIME 236, 149 (1966)
N. Stanford, A. Djakovic, B.A. Shollock, M. McLean, N. D’Souza, Scr. Mater. 50, 159 (2004)
R.E. Napolitano, R.J. Schaefer, J. Mater. Sci. 35, 1641 (2000)
A.D. Bussac, C.-A. Gandin, Mater. Sci. Eng. A 237A, 35 (1997)
M. Rappaz, C.A. Gandin, J.L. Desbiolles, P. Thevoz, Metall. Mater. Trans. 27A, 695 (1996)
C.A. Gandin, R.J. Schaefer, M. Rappaz, Acta Mater. 44, 3333 (1996)
N. Stanford, A. Djakovic, B.A. Shollock, M. McLean, N. D’Souza, P. Jennings, in Superalloys 2004, ed. by K.A. Green, T.M. Pollock, et al. (TMS, Warrendale, 2004), p. 719
X.B. Zhao, L. Liu, Z.H. Yu, G. Liu, H.Z. Fu, Rare Met. Mater. Eng. 38, 1280 (2009)
C.B. Yang, L. Liu, X.B. Zhao, Y.F. Li, J. Zhang, H.Z. Fu, Prog. Natl. Sci.: Mater Int. 22, 407 (2012)
J.P. Gu, C. Beckermann, A.F. Giamei, Metall. Mater. Trans. A 28, 1533 (1997)
X. Huang, Y. Zhang, Y. Liu, Z. Hu, Metall. Mater. Trans. A 28, 2143 (1997)
Y.Z. Zhou, Scr. Mater. 65, 281 (2011)
P.D. Lee, J.D. Hunt, Acta Mater. 49, 1383 (2001)
R.C. Atwood, P.D. Lee, Metall. Mater. Trans. 33B, 209 (2002)
W. Wang, A. Kermanpur, P.D. Lee, M. McLean, Int. J. Cast Met. Res. 15, 269 (2002)
W. Wang, P.D. Lee, M. McLean, Acta Mater. 51, 2971 (2003)
N. D’Souza, P.A. Jennings, X.L. Yang, H.B. Dong, P.D. Lee, M. McLean, Metall. Mater. Trans. B 36, 657 (2005)
P.D. Lee, A. Chirazi, R.C. Atwood, W. Wang, Mater. Sci. Eng. A 365, 57 (2004)
Acknowledgements
This work was supported by the National High Technology Research and Development Program of China (863 Program) (No. 2012AA03A511), the National Natural Science Foundation of China (Nos. 50931004, 51101120, 51171151, 51331005), the National Basic Research Program of China (Nos. 2010CB631202, 2011CB610406).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, C., Liu, L., Zhao, X. et al. Formation of stray grains during directional solidification of a superalloy AM3. Appl. Phys. A 114, 979–983 (2014). https://doi.org/10.1007/s00339-013-8046-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-013-8046-z