Abstract
Many late transition binary alloy nanoparticles (NPs) have been fabricated through a wide variety of techniques. Various steps are involved in the fabrication of such NPs. Here, we used a simple and green route to fabricate solid-solution Rh–Pd and Rh–Pt bimetallic alloy NPs through femtosecond laser irradiation in a solution without any chemicals like reducing agents. X-ray diffraction (XRD) peaks of NPs obtained in the solutions with different ratios of Rh–Pd and Rh–Pt ions monotonically varied from the position of pure Rh to those of Pd and to Pt which respectively indicated that these NPs were alloy. Composition of fabricated NPs was fully tuned over the entire range of Rh1−x –Pd x , and Rh1−x –Pt x with varying the mixing ratio of metal ions in the solution. Studies of Rh–Pd and Rh–Pt solid-solution system suggest that the alloy formation occurs through the nucleation of Rh and then followed by the diffusion of Rh, Pd and Rh, Pt to form a homogeneous alloy. The variety of average size of the alloy NPs for different compositions could be attributed to different reduction rate and surface energies of metal ions. Our result implies that femtosecond laser irradiation in aqueous solution is one of the potential methodologies to form multimetallic solid-solution alloy NPs with fully tunable composition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, much more attention has given on metal nanoparticles (NPs) due to their unique electrical, optical, and catalytic properties, which are not achievable for isolated atoms or bulk solids [1, 2]. Among the large number of possible elemental combinations, pure rhodium (Rh), palladium (Pd), and platinum (Pt) as well as their bimetallic and trimetallic alloy NPs with controlled composition, size, and shape are of special interests as three-way catalysts for automobile emission control [3]. Bimetallic NPs composed of two different metal elements exhibits outstanding properties in comparison with pure metal NPs. For example, it is reported that the catalytic activity of bimetallic Au–Pt [4–6], Au–Pd [6, 7], Au–Rh [6], and Ag–Rh [8] alloy NPs is higher than that of their corresponding pure metal ones. This is due to the facts that the combination of different metals can provide different properties not realized by pure metal materials. Moreover, the chemical composition of alloy surface is somewhat different from the interior because of the different surface tension characteristics of the constituent elements. Enhanced catalytic activity of Rh–Pd alloy NPs was found in CO oxidation by O2 compared to those of pure Rh and Pd [9]. For Pt–Rh bimetallic NPs, enhanced catalytic property for NO x reduction, CO chemisorption, and hydrogenation were investigated [10, 11]. It was also reported that catalytic activity of CO oxidation was controlled by tuning the composition of Rh–Pt bimetallic NPs [12].
To fabricate bimetallic Pt–Rh colloidal NPs, different strategies such as colloid synthesis in polymer solutions using borohydrite-reduction, NaY-supported clusters using ion-exchange method, pulsed laser ablation, and polyol synthesis have been reported [10, 13–18]. Such chemical methods (except for the pulsed laser ablation) usually need high temperature reduction in organic solvents with high boiling point to fabricate NPs. Hileman et al. [19] reported the synthesis of immiscible Au–Rh, Au–Pt, Pt–Rh, and Pd–Rh alloy NPs using NaBH4 reduction of metal salts in aqueous solution in the presence of a polymeric surface stabilizer at room temperature. Among them, the Au–Pt system has been especially studied in past for catalytic activities including CO oxidation, hydrogen evolution, electro-oxidation, and various hydrogenations [19] using chemical methods. Moreover, chemical synthesis involves complex procedure and often employ highly reactive chemical such as NaBH4, which may cause environmental and biological hazards. In addition to that, addition of a dispersants is not suitable for practical applications. On the contrary, we have developed femtosecond laser irradiation in solution as a novel method to fabricate metal NPs and their alloy NPs. Recently, solid-solution Au–Ag [20] and Au–Pt [21, 22] alloy NPs were fabricated only by using femtosecond laser irradiation in aqueous solution. The advantages of this method over others are a pure, simple, and convenient one-step procedure to form spherical-shape crystallized-NPs without any reducing agents and heat treatments.
In this letter, we report for the first time a simple and green route to synthesis surfactants free solid-solution Rh–Pd and Rh–Pt alloy NPs through femtosecond laser irradiation in solution. Although many bimetallic alloy NPs including late transition metals have been reported, very few researches focused on the synthesis of Rh based alloy NPs [19] in spite of its potential of high catalytic activities [10, 23]. Here, we used femtosecond laser irradiation in aqueous solution to fabricate Rh–Pd and Rh–Pt alloy NPs. In this process, it is thought that water molecules are initially decomposed resulting in the production of solvated electrons and hydrogen radicals, which play as strong reducing agents for metal ions [24]. Investigation into the formation of alloy NPs suggests that NPs of one metal nucleate first followed by the diffusion of other to form homogeneous alloy NPs. The effect of the fraction of Rh, Pd, and Pt ions on the composition and structure of Rh–Pd and Rh–Pt alloy NPs were also investigated.
2 Experiment
2.1 Materials
Rhodium (III) chloride trihydrate (RhCl3⋅3H2O, 99.5 %) was obtained from Wako pure chemical industries Ltd., and palladium (II) chloride (PdCl2, 99.9 %) and hydrogen hexachloroplatinate (IV) hexahydrate (H2PtCl6⋅6H2O, 99.9 %) were purchased from Sigma Aldrich. All chemicals were used without further purification.
2.2 Preparation of Rh–Pd and Rh–Pt mixed solutions
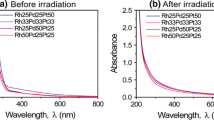
Initially, pure Rh, Pd, and Pt aqueous solutions were prepared separately by dissolving RhCl3⋅3H2O, PdCl2 and H2PtCl6⋅6H2O powder in ultra pure water with the concentration of 2.5×10−4 M. The aqueous solutions containing Rh, Pd, and Pt ions were then mixed with different molar ratio of Rh100−x –Pd x , and Rh100−x –Pt x (x=0 to 100). Samples were denoted by the molar fraction of Rh, Pd, and Pt ions in the solutions. For example, Rh100, Pd100, and Pt100 denote the solution containing 100 % of each ion. Similarly, Rh50Pd50 denotes the solution containing 50 % of Rh and 50 % Pd ions. No precipitation was observed after preparing the solution. Thereafter, 3 ml of the mixed solution was introduced into a rectangular quartz cuvette and irradiated for 30 minutes by highly intense femtosecond laser pulses at the wavelength of 800 nm generated by a chirped pulse amplification system (Spitfire Pro, Spectra-physics Co.). It is noted that the laser pulses with 100 fs pulse width (pulse energy 5.3 mJ, repetition rate 100 Hz) was focused in the solution using an aspheric lens with the focal length of 8 mm and the numerical aperture of 0.5. Figure 1 shows the UV-vis. absorption spectra of the mixed solutions with different molar ratios of Rh–Pd and Rh–Pt measured by an UV-vis. spectrophotometer (V630iRM, JASCO Co. Tokyo, Japan). The absorption spectra of the mixed solutions showed systematical change from that of pure Rh solution to Pd (or Pt) solution according to the mixing ratio of the ions.
2.3 Characterization
The size and shape of fabricated particles and corresponding selected area electron diffraction patterns were obtained by a transmission electron microscope (TEM; JEOL, JEM2000EXII) observation operated at 200 kV. TEM samples were prepared by dropping a few drops of the solution on a carbon-coated copper micro-grid (microgrid B, Okenshoji Co. Ltd.) immediately after irradiation and dried up in a vacuum chamber at room temperature. The size distribution of the fabricated particles was calculated from at least 300 particles by direct measurement of bright-field TEM images at several different places for each sample.
The crystalline structures of fabricated particles were determined by powder X-ray diffraction (XRD, RINT-V, Rigaku Co.). A computerized diffractometer equipped with a Ni-filtered Cu K α (λ=0.15406 nm) X-ray source and a goniometer with a solid state Ge detector was used to obtain XRD profiles by theta-2theta scan. The as-prepared sample was collected using a freeze drier, and then it was placed onto a single crystal silicon substrate with no diffraction peak in the scan range and subjected it for XRD measurement.
3 Results and discussion
Tiny luminescent flash and generation of bubbles were observed by naked eyes near the focal point during the laser irradiation. Chromatographic analysis (Shimadzu Co., GC-8A) had revealed that the bubbles were oxygen and hydrogen [22]. These bubbles were probably due to the decomposition of water molecules by laser induced breakdown [25]. Figure 2 shows feature of the samples after irradiation for different molar fraction of (a) Rh–Pd and (b) Rh–Pt ions. The color of the solution after 30 minutes of irradiation depends on the molar ratio of Rh, Pd, and Pt ions in the solution: colorless for Rh100 to dark brown for Pd100 and Pt100 as shown in Fig. 2.
Figure 3 shows a representative set of bright field TEM images of particles fabricated in Rh–Pd mixed solutions with different molar fractions of Rh and Pd while Fig. 4 is for that of particles in Rh–Pt mixed solutions with different molar fractions of Rh and Pt. TEM analysis has been performed to determine the size, shape, distribution, and crystalline structure of fabricated particles. The size distribution of corresponding NPs is shown as a histogram in the left side of each image. The particle size of the dispersed NPs was obtained by an image processing program (ImageJ) from randomly chosen three or more areas for each TEM image. The average sizes with its standard deviation of Rh–Pd system shown in Fig. 3 are 3.7±2.5, 7.1±5.3, 17.3±11.3, 17.1±11.3, and 8.5±3.2 nm for Rh100, Rh75Pd25, Rh50Pd50, Rh25Pd75, and Pd100, respectively. For Rh–Pt case as shown in Fig. 4, the mean particle sizes are 6.7±4.4, 11.0±8.1, 17.6±11.6, and 14.3±6.7 nm for Rh75Pt25, Rh50Pt50, Rh25Pt75, and Pt100, respectively. The result of Rh100 (Fig. 4(a)) which is exactly the same in Fig. 3(a) is also shown for comparison. The shape of the fabricated NPs was mostly spherical but the size distribution was relatively broad (CV=∼60 %) in all samples because there were no surface stabilizers in the solutions. The colloidal solutions remained stable for few hours after irradiation depending on the fraction of Rh ions in the solution. In the absence of a dispersant, the temporary stability is achievable probably due to the electrostatic repulsion of particles formed in the high intensity laser irradiation reaction as reported by Sylvestre et al. [26]. In the case of NPs formation in Rh–Pd and Rh–Pt solutions, it is noticed from the TEM images that overall size variation of the particles fluctuated for different mixing ratio of metal ions. Although, it is hard to mention what was happening exactly during the formation of NPs due to different surface and chemical properties of Rh, Pd, and Pt atoms. The fluctuation of particle size could be due to the different atomic radius as well as surface energies of Rh, Pd, and Pt metal ions and this will able to be cleared from the following explanations. Renzas et al. [9] fabricated Rh–Pd NPs with tunable compositions for the enhancement of catalytic activity by chemical reduction process, and the maximum size of nanocrystals obtained for the composition ratio of 50:50 shows a good agreement with our results. Moreover, structure-performance relationship of Rh and Rh–Pd alloy supported catalysts [27] have reported that particle size for alloy system was larger than that of pure Rh sample. Therefore, the increase of particle size for Rh–Pd system may be because of alloying effect of two metal components, where Rh-like nucleation sites yield smaller particles, and on the contrary, Pd (and Pt)-like site yield larger particles. In addition to that, different reduction rate of the metal ions is also responsible for the fluctuation of particle size.
For the Rh–Pt case, the particle size was at its maximum fabricated in Rh25Pt75 solution. This result is consistent with earlier study reported by Cao et al., who examined thermal stability of bimetallic NPs and explained that the particle size decreases with increasing the Rh content [28]. Moreover, Park et al. also reported the various sizes of Rh–Pt bimetallic NPs for different ratio of Rh and Pt content and evaluated this size effect on the catalytic activity of CO oxidation [29]. In this case, the average particle sizes were 11, 8.5, and 5.5 nm, for the alloy composition of Rh0.47Pt0.53, Rh0.48Pt0.52, and Rh0.52Pt0.48, respectively, and the biggest particle size was found for the alloy with a high content of Pt. Recently, Yuge numerically investigated stability and electronic structure of icosahedral NPs [30] and the segregation behavior of Pt–Rh NPs based on first principle calculation combined with cluster expansion technique and Monte Carlo statistical simulation [31]. He found that Pt atoms energetically prefer surface site due to the lower surface energy of Pt compared with Rh. Moreover, he also mentioned the interatomic distance of Rh–Pt alloy NPs is larger than pure Rh NPs. Therefore, it can be predicted that the particles size becomes larger with increasing the Pt content probably due to the larger atomic radius of Pt than that of Rh.
To analyze the crystalline structure of NPs formed in our study, selected area electron diffraction (SAED) pattern using a 300 nm aperture was employed. As a representative case, the SAED pattern of NPs obtained from the particles in the solutions of 50:50 mixing ratios for Rh–Pd and Rh–Pt systems are shown in Fig. 5. Interplanar spacing of NPs is calculated using the formula:
where r is the ring radius of the diffraction pattern, d hkl is the interplanar spacing, L is the camera length (in this case L=82.40 cm) and λ is the wavelength of the electron beam (λ=0.002507 nm). The estimated interplanar spacings of fabricated NPs in Rh–Pd and Rh–Pt mixed solutions are shown in Table 1. The interplanar spacings of NPs fabricated in the solution of Rh100, Pd100, and Pt100 well agree with the data for (111), (200), (220), and (311) planes of Rh, Pd, and Pt from Joint Committee for Powder Diffraction Standards (JCPDS) card (No. 05-0685, 46-1043 and 040802 for Rh, Pt, and Pd, respectively). Moreover, with changing the mixing ratio of Rh/Pd as well as Rh/Pt ions, the interplanar spacing changed within that of pure Rh, Pd, and Pt. Therefore, this implies that the fabricated particles are not merely alloys but solid solution. Further confirmation of alloy formation, XRD measurement was performed for each sample of Rh–Pd and Rh–Pt with different molar fractions to confirm the crystalline structure and compositions of bimetallic NPs.
Figure 6 shows a set of XRD profiles for the particles fabricated in the mixed solution with different mixing ratios of metal ions. The vertical dashed lines indicate the peak positions for (111) plane of Rh, Pd, and Pt. A single diffraction peak was detected from each sample in the measurement angle range. The XRD peak positions of NPs for different mixing ratio of Rh–Pd (Fig. 6(a)) were located between peaks of pure Rh and Pd, and the progressive shift of the (111) peak reflections indicates that the alloy composition is changing from Rh rich to Pd rich and homogeneous alloy formation occurs through interdiffusion of Rh and Pd NPs. This clearly shows that alloy compositions are fully tuned over the entire Rh100−x –Pd x . Similar trend was observed for the Rh–Pt system. This observation strongly indicates that the fabricated alloys NPs are solid solution. The lattice parameter for alloy NPs was estimated from interplanar spacing of fcc structure as follows:
where a is the lattice parameter and (hkl) is the given lattice plane. Figure 7 shows the lattice parameters of the fabricated NPs calculated by Eq. (2) as a function of Rh molar fraction for Rh–Pd and Rh–Pt systems. Here, the dotted lines are the theoretical one by Vegard’s law, the rectangles are calculated from XRD measurement and the circles with error bars are from the SAED pattern for different planes. It is clear from lattice parameter shown in Fig. 7 that the compositions of the solid solution Rh–Pd and Rh–Pt NPs are fully tunable. Such a tunable composition of alloy NPs is very important in a practical application, e.g. a catalyst in CO oxidation [8]. This report is the first report for fabrication of tunable solid solution Rh–Pd and Rh–Pd alloy NPs using femtosecond laser irradiation in solution. Such tunable alloy NPs would be more environmental friendly for practical use because of being free from any reducing agents.
Normalized powder XRD patterns of fabricated NPs in the solutions for different mixing ratio of (a) Rh–Pd and (b) Rh–Pt ions. Open circles represent experimental data while solid lines are theoretical calculation according to Vegard’s law. Vertical dashed lines indicate the peak positions for (111) plane of pure Rh, Pd, and Pt metals
Lattice parameter as a function of Rh contents for different mixing ratio of (a) Rh–Pd (b) Rh–Pt ions in solution evaluated from selected area electron diffraction (SAED) and X-ray diffraction. A circle represents the lattice parameter obtained from the SAED pattern, a square represents the XRD pattern, and a broken line represents the lattice parameter by Vegard’s law
The structural parameters obtained from XRD measurement for Rh–Pd and Rh–Pt alloy NPs for (111) plane are summarized in Table 2. The average particle sizes measured by TEM as shown in Fig. 3 and the crystalline sizes of the particles calculated by Scherrer’s equation in Table 2(a) were in a good agreement for the Rh–Pd alloy system except for 50:50 ratios. For the Rh–Pt alloy system, the calculated crystalline sizes in Table 2(b) are relatively bigger than the size measured from TEM sample. This variation of particle size might be due to the crystal growth during freeze drying and/or contribution of the small number of large particles for diffraction measurement.
The lattice parameters obtained from diffraction peaks of (111) plane for Rh100 (a=3.797 Å), Pd100 (3.889 Å), and Pt100 (3.918 Å) were in a good agreement with the values of pure Rh, Pd, and Pt. It is noticeable that the lattice parameter obtained for NPs varied linearly from pure Pd to Rh and from pure Pt to Rh with increasing Rh contents in the solution. In addition, the lattice parameter evaluated by diffraction techniques (SAED and XRD measurements) showed linear relationship with Rh molar fraction in the solution as shown in Fig. 7. This is strongly indicates the fabrication of solid solution Rh–Pd and Rh–Pt alloy NPs with controllable compositions by high intensity laser irradiation of solution without any reducing agents.
The formation mechanism of the Rh–Pd and Rh–Pt alloy NPs with controllable compositions without any reducing agents is attributed to the optical decomposition of water molecules [32–34]. In the optical decomposition process, reactive species such as solvated electrons and hydrogen radicals are produced through a photochemical reaction. The generation of hydrogen and oxygen gases near the focal spot strongly indicates the formation of hydrogen and hydroxyl radicals in the solution in our experiment. As a result, metal ions in the solution are reduced to neutral atoms by transient species. Therefore, most of the ions are consumed and the particles cease to grow after the particle becomes several nanometers in size. Many reports have proposed how bimetallic NPs are formed. Wynblatt et al. [35] proposed the regular solution model, where particle surface is enriched with Pt having lower surface tension in Rh–Pt system. On the contrary, according to the ionic binding model by Sachtler et al. [36], particles are enriched with Rh having lower sublimation energy in the same system. In addition, Toshima et al. [37] have proposed a core-shell model, where metal ions with higher redox potential are reduced first and then the others subsequently follow. In the case of Rh–Pt system, because Pt has higher redox potential than Rh, the model predicts that Rh will be reduced later than Pt and precipitated dominantly on the surface site of Pt core. In our case, the formation mechanism of alloy NPs containing two metal elements with different redox properties can be confirmed from linear change of the lattice parameter. Namely, tunable compositions of Rh–Pd and Rh–Pt NPs in Fig. 7 show the homogeneous alloy formation, which suggests that the particles are neither enriched by any particular element nor segregated like core-shell ones, and homogeneous alloy formation is achieved through interdiffusion between two elements promptly after simultaneous reduction of metal ions.
In this report, solid solution Rh–Pd and Rh–Pt NPs were fabricated through femtosecond laser irradiation without any reducing agents. Sizes of the fabricated particles were of the order of nanometer and the composition was fully tunable in accordance with the molar fraction of the solution. This is because of strong reducing power of solvated electrons and hydrogen radicals produced by high-intensity femtosecond laser irradiation of solutions.
4 Conclusions
In this study, solid solution Rh–Pd and Rh–Pt alloy NPs were fabricated through simultaneous reduction of Rh and Pd (or Pt) ions by using femtosecond laser irradiation with a tightly focused condition in the mixed solution of the ions. This technique is a green and simple route to synthesize metal NPs without any reducing agents. We showed the fully tunable composition of alloy NPs and, therefore, are promising candidate to control the characteristics of NPs, e.g., catalytic activity. The different size of the particles was observed for different ratio of metal ions due to the synergistic effect of metal NPs as the surface properties of the particles depending on metallic elements. On the other hand, chemical reduction has been commonly employed to synthesize Au–Rh, Au–Pt, Pt–Rh and Pd–Rh bimetallic NPs using NaBH4 reduction and PVP as a stabilizer [19]. In this study, excessive amount of reducing and stabilizing agents were consumed. This requires purification of the fabricated NPs before being used for catalytic purpose. The purification process is not simple since it requires the isolation of the NPs from the solvent by centrifugation and need to wash several times with alcohols. In contrast, our method is a much greener route to synthesize alloy NPs since no reducing or stabilizing agents is used. Our results show that the femtosecond laser irradiation in solution has a further potential to fabricate multi-metallic alloy NPs with fascinating functions. Furthermore, it should be noticed that this method can be applicable to semiconductors and hybrid NPs with controlled compositions.
References
E. Roduner, Chem. Soc. Rev. 35, 583 (2006)
S. Link, M.A. El-Sayed, Annu. Rev. Phys. Chem. 54, 331 (2003)
M.A. Newton, B. Jyoti, A.J. Dent, S. Diaz-Moreno, S.G. Fiddy, J. Evans, Chem. Phys. Chem. 5, 1056 (2004)
A. Harriman, J. Chem. Soc. Chem. Commun. (1990)
T. Yonezawa, N. Toshima, J. Mol. Catal. 83, 167 (1993)
N. Toshima, K. Hirakawa, Polym. J. 31, 1127 (1999)
A.F. Lee, C.J. Baddeley, C. Hardacre, R.M. Ormerod, R.M. Lambert, G. Schmid, H. West, J. Phys. Chem. 99, 6096 (1995)
K. Hirakawa, N. Toshima, Chem. Lett. 32, 78 (2003)
J.R. Renzas, Phys. Chem. Phys. 13, 2556 (2011)
K. Siepen, H. Bönnemann, W. Brijoux, J. Rothe, J. Hormes, Appl. Organomet. Chem. 14, 549 (2000)
C.E. Lyman, R.E. Lakis, H.G. Stenger, Ultramicroscopy 58, 25 (1995)
J.Y. Park, Y. Zhang, M. Grass, T. Zhang, G.A. Somoraji, Nano Lett. 8, 673 (2008)
N. Savastenko, H.R. Volpp, O. Gerlach, W. Strehlau, J. Nanopart. Res. 10, 277 (2008)
Y. Wang, J. Zhang, X. Wang, J. Ren, B. Zuo, Y. Tang, Top. Catal. 35, 35 (2005)
M. Harada, K. Asakura, N. Toshima, J. Phys. Chem. 98, 2653 (1994)
T. Hashimoto, K. Saijo, M. Harada, N. Toshima, J. Chem. Phys. 109, 5627 (1998)
M. Harada, H. Einaga, J. Colloid Interface Sci. 308, 568 (2007)
E. Cimini, R. Prins, J. Phys. Chem. B 101, 5277 (1997)
E.R. Essinger–Hileman, D. DeCicco, J.F. Bondi, R.E. Schaak, J. Mater. Chem. 21, 11599 (2011)
Y. Herbani, T. Nakamura, S. Sato, J. Nanomater. 2010, 154210 (2010)
J.L.H. Chau, C.Y. Chen, M.C. Yang, K.L. lin, S. Sato, T. Nakamura, C.C. Yang, C.W. Cheng, Mater. Lett. 65, 804 (2011)
T. Nakamura, Y. Herbani, S. Sato, J. Nanopart. Res. 14, 785 (2012)
R.J. Bonilla, B.R. James, P.G. Jessop, Chem. Commun. 941 (2000)
S. Pommeret, F. Gobert, M. Mostafavi, I. Lampre, J.C. Mialocq, J. Phys. Chem. A 105, 11400 (2001)
S.L. Chin, S. Legacé, Appl. Opt. 35, 907 (1996)
J.-P. Sylvestre, S. Poulin, A.V. Kabashin, E. Sacher, M. Meunier, J.H.T. Luong, J. Phys. Chem. B 108, 16864 (2004)
A.J. Dent, J. Evans, S.G. Fiddy, B. Jyoti, M.A. Newton, M. Tromp, Faraday Discuss. 138, 287 (2008)
A. Cao, G. Veser, Nat. Mater. 9, 75 (2010)
J.Y. Park, Y. Zhang, S.H. Joo, Y. Jung, G.A. Somoraji, Catal. Today 181, 133 (2012)
K. Yuge, Mater. Trans. 52, 1399 (2011)
K. Yuge, Phys. Rev. B 84, 085451 (2011)
H.H. Huang, X.P. Ni, G.L. Loy, C.H. Chew, K.L. Tan, F.C. Loh, J.F. Deng, G.Q. XU, Langmuir 12, 909 (1996)
A. Henglein, Chem. Mater. 10, 444 (1998)
T. Kempa, R.A. Farrer, M. Giersig, J.T. Fourkas, Plasmonics 1, 45 (2006)
D. Wynblatt, R.C. Ku, Appl. Surf. Sci. 65, 511 (1987)
W.M.H. Sachtler, R.A. van Santen, Appl. Surf. Sci. 3, 121 (1979)
N. Toshima, T. Yonezawa, M. Harada, K. Asakura, Y. Iwasawa, Chem. Lett. 1769 (1989)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarker, M.S.I., Nakamura, T., Herbani, Y. et al. Fabrication of Rh based solid-solution bimetallic alloy nanoparticles with fully-tunable composition through femtosecond laser irradiation in aqueous solution. Appl. Phys. A 110, 145–152 (2013). https://doi.org/10.1007/s00339-012-7467-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-012-7467-4