Abstract
In this study, we evaluated the efficacy of sperm cryopreservation for use in larval-based propagation of Diploria labyrinthiformis and produced offspring that were maintained under controlled conditions. Gametes were collected from colonies in situ in July and August 2017 and 2018. The four largest colonies out of a total of nine appear to be senescent or produce low-quality sperm or eggs. Sperm was cryopreserved for comparison of the effects of storage time on sperm viability. We determined that cryopreserved sperm from D. labyrinthiformis is viable for at least 13 months for use in in vitro crosses, though their motility is reduced on average by 24% in comparison with fresh sperm. Using frozen sperm to fertilize freshly collected eggs led to successful fertilization, larval yields, settlement and post-settlement survival. In general, these were lower by 23%, 23%, 14% and 8%, respectively, when compared to controls fertilized with fresh sperm. Our results suggest that motility of fresh sperm is not a good indicator of the future fate of larvae because in some cases low motility led to successful settlement. We also found that not all crosses were successful, and that the direction of the cross significantly affects larval yields and settlement. Once symbionts were noticeable within the primary polyps the cryo-recruits were maintained in an ex situ nursery for observation and showed similar survival with respect to recruits produced with fresh sperm. Prior to the 2018 spawning event, Stony Coral Tissue Loss Disease (SCTLD) was detected in the studied colonies and by February 2020 seven of the nine colonies (78%) had succumbed to the disease. The sperm from these colonies was banked in a repository and since then has been used in genetic rescue projects for this species. Thus, we show that cryopreservation is a useful tool in actions designed to recover D. labyrinthiformis and can potentially be applied to other species of corals severely affected by SCTLD or in need of genetic rescue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coral reefs are undergoing major declines due to anthropogenically derived disturbances resulting in increased reef erosion rates (Perry et al. 2013) and temperature-induced coral bleaching (Hoegh-Guldberg et al. 2007; Hughes et al. 2003). In the Caribbean, coral reefs are also facing increased eutrophication (Häder et al. 2020) exacerbated by Sargassum blooms (van Tussenbroek et al. 2017) and at least 22 species of corals are experiencing high mortality associated with Stony Coral Tissue Loss Disease (SCTLD, Precht et al. 2016; Álvarez Filip et al. 2019). During the summer of 2018, an outbreak of SCTLD was detected in the Puerto Morelos Reef National Park (Álvarez-Filip et al. 2019). After a survey of 82 sites along the Mexican Caribbean, Diploria labyrinthiformis was listed as one of eleven most highly susceptible species to this disease due to significant population declines (Álvarez-Filip et al. 2019). Given that D. labyrinthiformis is a reef-building species (Weil and Vargas 2010; Chamberland et al. 2017) that provides structural complexity (Álvarez-Filip et al. 2019) such losses are of major concern making it a target species in coral reef restoration and genetic rescue programs.
Cryopreservation is potentially an excellent method for gamete conservation of endangered coral species. It also aids in genetic rescue and to prevent the loss of genetic diversity (Lin and Tsai 2012; Hagedorn and Spindler 2014; Tsai et al. 2015; Viyakarn et al. 2018; Hagedorn et al. 2019; Novak et al. 2020). This process involves the preservation of gametes, embryos, larvae, tissues or fragments by rapid freezing in the presence of cryoprotectants followed by very low temperature storage to maintain viability over a long time period (Hagedorn et al. 2006; Hagedorn and Carter 2016; Viyakarn et al. 2018). Successful examples of coral sperm and somatic cell cryopreservation exist for a growing number of species and coral life history stages (Hagedorn et al. 2012a, 2013, 2017; Lin et al. 2012; Tsai et al. 2016; Daly et al. 2018).
Sperm cryopreservation has the potential to increase the number of sexual recombinations within a coral species when spawning events occur on successive nights, lose synchrony or because a limited number of colonies are available from losses to disease, such as is the case of D. labyrinthiformis decline due to SCTLD. This species is a simultaneous hermaphrodite with a single annual gametogenic cycle of oocytes and spermatocytes released in synchronized spawning events with fertilization occurring in the water column (Fadlallah 1983; Alvarado-Chaparro et al. 2004; Weil and Vargas 2010). The timing of spawning events varies throughout the Caribbean. In Puerto Rico (Weil and Vargas 2010), Colombia (Alvarado-Chaparro et al. 2004), Bonaire (Muller and Vermeij 2011) and Bermuda (Wyers et al. 1991) spawning occurs in the spring and summer, whereas in Curaçao two peaks, in spring and autumn, have been documented (Chamberland et al. 2017). Embryonic development is fast such that within 24–48 h larvae are formed and can settle on suitable substrates (Chamberland et al. 2017).
Colonies of D. labyrinthiformis that have survived SCTLD are potentially resistant to the disease. Combining the use of cryopreserved sperm and an understanding of the reproductive biology of D. labyrinthiformis to enhance larval propagation under ex situ conditions (Chamberland et al. 2017), we can produce sexual recruits that will also be potentially resistant to SCTLD. In this study, we cryopreserved sperm from the Caribbean endemic reef-building coral D. labyrinthiformis ahead of and during the advance of SCTLD in the region. Our objectives were, first: to establish the spawning pattern of D. labyrinthiformis in the Mexican Caribbean; second: to evaluate the most common cryopreservation method on sperm viability over time in this species by following the fate of the larvae produced from fertilization with cryopreserved and fresh sperm and third: to evaluate the importance of the direction of sperm-egg crosses on fertilization, larval yield and settlement.
Materials and methods
Spawning and gamete collection
The nine Diploria labyrinthiformis colonies used in this study are located at 4.5–8 m depth (Table 1) on rocky substrates in Jardines Reef (20° N 49′ 53′′; 86° W 52′ 28′′) in the Puerto Morelos Reef National Park, Mexican Caribbean (Fig. 1a–e). Colony size was determined by measuring area in July 2018 as well as old partial mortality. In July 2019 and February 2020 percent colony mortality was recorded (Table 1).
Location of the study site, Jardines Reef (black arrow in a) in the southern part of Puerto Morelos Reef (yellow square in b and c). The spatial distribution of tagged Diploria labyrinthiformis colonies are shown in d. The coordinates indicate the location of colony DL116. A colony of D. labyrinthiformis (e) covered by the gamete bundle collection net (f) with a closeup of gamete bundle setting (g)
In 2017, in a pilot study, the colonies were monitored for spawning, whereas in 2018, the colonies were tagged prior to spawning (Table 2, Fig. 2a–r). Spawning was monitored for 2–6 consecutive days, at one hour before sunset, starting 10 days after the full moon of each month between April and September in 2017 and May and September in 2018 (Table 2). In June 2017 no monitoring was undertaken due to unfavorable weather conditions. Gamete bundles were collected using conical-shaped nets placed over the colonies (Fig. 1f) about half an hour prior to expected spawning time when setting was visible (Fig. 1g) and left until spawning had ceased. The containers with gametes were carefully removed from the collection net, a lid was attached, and the container kept upside down during transport to the laboratory within 30 min of collection. On nights when spawning did not occur the nets were removed after 90 min.
Paired photographs of Diploria labyrinthiformis colonies monitored in this study. The photographs on the left-hand side of each pair were taken in July 2018 prior to spawning and those on the right-hand side of the pair correspond to the same colony monitored in July 2019 (a–r). Some colonies died (b, f, n), while others showed advanced symptoms of Stony Coral Tissue Loss Disease (d, h, p, r). Red arrows indicate lost tissue (d, p) and the red arrow points to the only remaining live tissue in a diseased colony (p). By 2020, only two colonies (DL119 and DL120) had survived (s, t, respectively). Scale bars: 10 cm in a, b, g–t, and 5 cm in c–f
Sperm cryopreservation
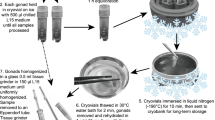
Sperm from D. labyrinthiformis were cryopreserved on four separate occasions: July and August 2017, July and August 2018. If the amount of spawn collected was minimal, gamete bundles broke during transport, or sperm motility was < 50% the sample was not cryopreserved. On each occasion, approximately 5 mL of concentrated gamete bundles were added to 25 mL of 0.45 μm filtered seawater (FSW) at 27 °C. This volume was established, based on sperm cell counts determined on two consecutive nights in July 2017, to obtain an average concentration of 5.0 × 108 fresh sperm mL−1 (Table S1). The gamete bundles were broken by gentle agitation and the buoyant eggs were separated from the sperm by passing the mixture through a 70 μm mesh nylon cell strainer (Biologix 15-1070-1). The eggs were rinsed four times with FSW and placed in separate, labeled containers.
The sperm obtained in 2017 and 2018 was cryopreserved following the Hagedorn et al. (2012a, b) protocol with modifications. The sperm was placed in cryovials (Corning) by adding equal volumes of sperm and 20% DMSO solution (D4550 SIGMA) prepared in FSW. This mixture was left in the open vial to equilibrate for 10 min without agitation. Freezing was carried out in a single stage using the ZIN cryo-rack controlled-rate freezing system: a triangular rack made of foam polyresin pieces for floatability was fitted with folded aluminum cryo-canes to hold the cryovials (Hagedorn and Carter 2016). To achieve a 20 ºC min−1 cooling rate a thick Styrofoam box (27.5 × 22.5 × 20.5 cm, L × W × H) was filled with approx. 2.9 L liquid nitrogen. Before placing the cryovials into the cryo-rack, they were closed and carefully mixed. Temperature was monitored with a thermocouple thermometer type K (Omega HH802W) with two probes that were submerged in cryovials filled with the same volume of FSW and DMSO. The rack was placed in liquid nitrogen and the temperature recorded every 15 s until the vials reached − 80 °C. The cryovials were removed from the rack, completely submerged in liquid nitrogen and left for 10 min before placing them in cryo-boxes for storage in liquid nitrogen.
Sperm motility
From each sperm sample, 10 μL aliquots were placed in a Neubauer chamber (Marienfeld 06500-30) and observed under an optical microscope (Leica DM500) at 40X to evaluate motility before and after the cryopreservation process. Pre- and post-freezing motility was determined by the same observer using at least four separate fields. Samples with motility < 50% were not cryopreserved (Hagedorn and Carter 2016).
Fertilization
To conduct in vitro fertilization, the cryovials containing sperm were removed from the liquid nitrogen and immediately placed in a water bath at 27 °C, moving them gently until they were completely thawed. Four replicate samples were used for each fertilization trial using fresh and frozen sperm. The crossing design can be seen in Table 3a, b. Sperm were thawed after being cryopreserved for 30 min, 1 month, 12 months and 13 months and used to fertilize freshly collected eggs. Positive controls consisted of fresh sperm from individual donor colonies and eggs collected from the same colonies that were used in the trials with cryopreserved sperm. Eggs without added sperm were also observed to rule out self-fertilization.
The average number of eggs used in the crosses conducted in July 2018 (321 ± 50, mean ± SD) was determined by counting at least 20 samples of 10 µL aliquots that were fixed immediately with 4% paraformaldehyde (PFA) in FSW and counted later. Due to the high number of larvae produced, the aliquots were halved for the August 2018 experiments to 5 µL aliquots (149 ± 20, SD). For the experiments, the eggs were placed in 20-ml scintillation vials with 5 mL of FSW at 27 °C to which 10 µL (= approx. 1 × 106 mL−1 final concentration) of fresh or 100 µL (= approx. 5.0 × 106 mL−1 final concentration) of thawed sperm (Hagedorn and Spindler 2014; Hagedorn et al. 2019) were added and mixed gently, but thoroughly.
Two hours after fertilization was initiated, each replicate was gently rinsed twice with FSW to remove excess sperm. Fertilization (%) was assessed by estimating the number of embryos undergoing cleavage (Fig. 3a) relative to the total number of eggs placed in the sample, as observed under a dissecting microscope (Leica S6E).
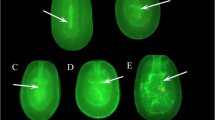
Development of Diploria labyrinthiformis sexual recruits obtained from cryopreserved sperm. Fertilization success was established with the first divisions of the resulting embryos (a), observed at 2 h post-fertilization (HPF). Settled larvae at 72 HPF on pre-conditioned substrates under white (b) and blue light (c). Primary polyps at 6 months old showing acquisition of symbionts (d). 19-month-old sexual recruits produced using fresh sperm, with 1 and 2 polyps formed, respectively (e, f). Scale bars: 200 µm in a, 1 mm in b and c, and 3 mm in d–f
Larval yield
The larvae that resulted from successful embryonic development were cultured in FSW. In July 2018, the larvae were pooled and up to 250 larvae were placed into forty-seven 2-L containers with settlement substrates. In August 2018, the larvae that resulted from the crosses were maintained in separate containers until settlement to follow the survival of known crosses. Every 24 h, total water changes were made for all treatments. Larval yield from eggs (%) was determined as the percentage of eggs resulting in larvae that were actively swimming towards the bottom of the container or searching the substrate prior to settling.
Once larvae began swimming, settlement substrates were added to each container. Settlement substrates were made with a mixture of white cement and sea sand (1:2) using circular or rectangular molds. Once dry, the substrates were conditioned for two months in an in situ nursery located in the reef lagoon. Sediment, macroalgae and other organisms were removed using a brush prior to presenting them to the larvae.
Settlement
The number of larvae from each cross that settled onto the settlement substrates (Fig. 3b) or the walls of each container was counted using a Nightsea Bluestar lamp with a BlueBlock yellow filter (Fig. 3c). Photographs were taken using a stereoscopic microscope (Leica S6E) and camera (Sony Cybershot 10.1 DSC H20). Settlement was scored two ways. Settlement yield (%) was calculated as the percentage of eggs that resulted in settled primary polyps. Settlement success (%) was calculated as the percentage of larvae that successfully converted to settled primary polyps. Subsequently, the substrates with attached primary polyps were transferred to 80-L containers with filtered (10 µm) seawater pumped in from the reef lagoon and maintained at 27.5 ± 0.3 °C (average ± SD) with constant aeration and 20% daily water changes.
Post-settlement survival
Within a week after settlement, symbionts were noticeable within the primary polyps (Fig. 3d), so they were transferred to an ex situ nursery for observation. The nursery consists of 4 × 200 L fiberglass flow through aquaria with seawater pumped from the reef lagoon that is filtered to 10 µm and temperature maintained at 27.5 ± 0.3 °C (mean ± SD). The recruits were fed Liquid Reef twice a week according to the manufacturer’s instructions for one month. After, they were fed freshly hatched Artemia nauplii alternated with Liquid Reef. Algal overgrowth was removed twice per week. Recruit survival was monitored 2 weeks post-settlement. In November, the experiment was terminated after a plague of ciliates caused significant mortality across all treatments. The few surviving recruits (Fig. 3e, f) were outplanted onto Jardines reef.
Statistical analysis
Statistical analysis was performed using Statistica 7.0 (Statsoft Inc., Tulsa, OK, USA) and Microsoft Excel (version 2013). The assumptions of homogeneity of variance and normality were taken into account with the Levene test and Shapiro–Wilk’s test, respectively. Percentage larval yield and settlement yield were transformed to arcsine values prior to analysis. Treatment means were compared using a one-way ANOVA (α = 0.05) for the sperm motility data and when testing for differences depending on the direction of crosses, which were all normally distributed. The nonparametric Kruskal–Wallis test was used for all other data, because they were not normally distributed, followed by Dunn’s multiple comparison test. Differences in all tests were considered significant when p values were < 0.05. Data are presented as means ± SE.
Results
Spawning and gamete collection
Diploria labyrinthiformis spawned in July and August in 2017 and 2018 and in September 2018 (Table 2). All colonies that were monitored in July spawned; seven in 2017 and nine in 2018, whereas in August one of six colonies spawned in 2017 and five of 11 colonies in 2018. In September no colonies spawned in 2017, although one did show setting and one colony spawned in 2018. Spawning in August 2017 and September 2018 was minimal compared to the previous months. All colonies that spawned did so 10 and 11 days after full moon (DAFM) at approximately 30 min prior to sunset. Spawning potential was variable with some colonies spawning either in July or August 2018, and three colonies spawning in both months. DL123 was the most prolific as it spawned on two consecutive days in both July and August 2018, whereas DL121 did not spawn on any of the surveyed nights.
Gamete bundles were collected from a total of eight colonies in 2017 and 14 colonies in 2018 (Table 2). The volume of gamete bundles collected varied between colonies with the lowest volumes collected from DL115 and DL117. Sperm was cryopreserved from a total of 16 colonies, five in 2017 and 11 in 2018.
Sperm motility
Overall, the motility of frozen sperm, considering all storage times, was significantly lower (p = 0.004) by an average of 24% compared to that of fresh sperm (Fig. 4a). For sperm that was collected from 5 colonies in 2017, the average motility of fresh sperm was 83% ± 4.4 and for sperm frozen for 30 min motility was 55% ± 5.0 and 62% ± 13.2 after 12 months of cryopreservation. In July 2018, sperm from four colonies DL118, DL119, DL122 and DL123 was cryopreserved. Motility of fresh sperm was 100% for all four colonies whereas after cryopreservation for 30 min motility was 82.5% ± 6.3. In August 2018, the sperm motility of the three colonies DL117, DL120 and DL123 was lower at 56.7% ± 3.3 for fresh sperm and 57.5% ± 16 after 30 min of cryopreservation. From here on, sperm and eggs are designated as S and E, respectively, followed by the colony number used in the cross. For example, sperm and eggs from colony DL123 are identified as S123 and E123, respectively.
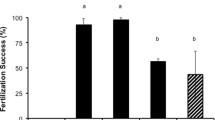
Box plots of percent motility (a), fertilization (b), larval yield (c), settlement yield (d), settlement success (e) and post-settlement survival (f) of Diploria labyrinthiformis using fresh and cryopreserved sperm that had been stored for 30 min, one month and 12–13 months (> 1 year). Results obtained in 2017 and 2018 have been combined. Larval yield refers to the percent conversion of eggs to swimming larvae. Settlement yield is defined as the percent conversion of eggs to settled primary polyps whereas settlement success is defined as the percentage of larvae that resulted in primary polyps
Fertilization
Eggs incubated without sperm did not initiate cleavage and began to disintegrate within 24 h. In general, using frozen sperm to fertilize freshly collected eggs resulted in 23% lower fertilization considering all storage times compared with fresh sperm (Fig. 4b). However, the differences are not significant (p = 0.3).
Fertilization percentages from individual crosses are given in Table 3. All three crosses involving E118 resulted in consistently higher fertilization than was observed for the three crosses involving E123 for both fresh and cryopreserved sperm. However, fertilization percentages were significantly higher for 30 min cryopreserved sperm (p = 0.0075) but not for fresh sperm (p = 0.05) or sperm cryopreserved for 12 months (p = 0.9).
Using fresh sperm, the individual cross S120 × E117 had higher fertilization (90%) in comparison with the reverse cross (S117 × E120) at 30%. Fertilization using 30 min cryopreserved sperm of the cross S120 × E117 reduced to 10% (Table 3b). Fertilization was significantly higher for all four crosses involving E117 compared to E120 using sperm that had been cryopreserved for 1 month (p < 0.0000001) and 13 months (p = 0.00001).
Larval yields
The overall conversion efficiency of eggs to swimming larvae was 23% lower for those produced with cryopreserved sperm considering all storage times, when compared to fresh sperm (Fig. 4c). Significant differences were detected in larval yield when fertilized with fresh sperm versus those fertilized with sperm cryopreserved (p = 0.012). Using Dunn’s multiple comparison test significant differences were found when comparing fresh sperm with sperm cryopreserved for 30 min (p = 0.02) or for at least 12 months (p = 0.025), but not for sperm cryopreserved for one month (p = 0.15).
Larval yields from individual crosses are given in Table 3. All three crosses involving E118 resulted in consistently higher larval yields than the three crosses involving E123 using both fresh and cryopreserved sperm. Larval yields were significantly higher for fresh sperm (p = 0.038) and for 30 min (p = 0.0005) but not for 12 month cryopreserved sperm (p = 0.5).
Similarly, all eleven crosses involving E117 had consistently higher larval yields than the eleven crosses involving E120. Although the crosses S117 × E120 and S120 × E117 using fresh sperm showed no significant differences in larval yield (p = 0.55), after cryopreservation the larval yields involving E120 were significantly lower when compared with E117 for sperm cryopreserved for 30 min (p < 0.001), 1 month (p < 0.00001) and 13 months (p < 0.0001).
Settlement yields
In general, settlement yield, calculated as the proportion of eggs that converted to settled primary polyps, was 14% lower for those fertilized with frozen sperm, considering all storage times, when compared to fresh sperm (Fig. 4d). Significant differences were detected in settlement yield when fertilized with fresh sperm versus those fertilized with sperm cryopreserved (p = 0.007). Using Dunn’s multiple comparison test significant differences were found when comparing fresh sperm with sperm cryopreserved for 1 month (p = 0.034) or for at least 12 months (p = 0.0008), but not for sperm cryopreserved for 30 min (p = 0.21).
Settlement yields from individual crosses are given in Table 3. All three crosses involving E118 resulted in consistently higher settlement yields than the three crosses involving E123 using both fresh and cryopreserved sperm. Settlement yields were significantly higher for fresh sperm (p = 0.011) and for 30 min (p = 0.0012) but not for 12 month cryopreserved sperm (p = 0.33).
All eleven crosses that involved E120 resulted in few or no larvae and low settlement rates or settlement failure, whereas the eleven crosses involving E117 had significantly higher settlement yields. Although the crosses S117 × E120 and S120 × E117 using fresh sperm showed no significant differences in settlement yield (p = 0.38), cryopreservation significantly reduced settlement yields involving E120 when compared with E117 when frozen for 30 min (p = 0.012), 1 month (p = 0.0016) and 13 months (p < 0.0001).
Settlement success
Settlement success, calculated as the percentage of larvae that settled to form primary polyps, was 17% lower for those larvae produced with frozen sperm considering all storage times when compared to fresh sperm (Fig. 4e). However, the differences are not significant (p = 0.065). Most of the crosses had high settlement success, even when larval yields or settlement yields were low (Table 3).
The settlement success from individual crosses is given in Table 3. All three crosses involving E118 resulted in consistently higher settlement success than the three crosses involving E123 using both fresh and cryopreserved sperm. Settlement success was significantly higher for fresh sperm (p = 0.001) and for 30 min (p = 0.002) but not for 12 month cryopreserved sperm (p = 0.47).
All eleven crosses that involved E120 resulted in few or no larvae and low settlement rates or settlement failure, whereas the eleven crosses involving E117 had significantly higher settlement success. Although the crosses S117 × E120 and S120 × E117 using fresh sperm showed no significant differences in settlement success (p = 0.27), cryopreservation significantly reduced settlement success involving E120 when compared with E117 when frozen for 30 min (p = 0.03), and 13 months (p < 0.0001) but not for 1 month (p = 0.16).
Post-settlement survival
In general terms, post-settlement survival was significantly lower (p = 0.03) for primary polyps produced with frozen sperm considering all time periods when compared to those produced with fresh sperm (Fig. 4f). However, when comparing the post-settlement survival of the cross S117 × E120 with its reverse cross S120 × E117 no significant differences were found when using fresh sperm (p = 0.8) or sperm when frozen for 30 min (p = 0.14) or 1 month (p = 0.42). Cryopreservation significantly reduced post-settlement survival involving E120 when compared with E117 when frozen for 13 months (p = 0.022).
Individual and reverse crosses
Comparing settlement success using fresh sperm, the individual cross S118 × E123 had significantly higher success than the reverse cross S123 × E118 (p = 0.013). Using fresh sperm there was no significant difference between the crosses S117 × E120 and S120×E117 in larval yield (p = 0.55), settlement yield (p = 0.38) or settlement success (p = 0.27). There was also no significant difference in larval yield (p = 0.69) or settlement yield (p = 0.81) or settlement success (p = 0.65) for sperm from DL117 when cryopreserved for 30 min and compared to fresh sperm. However, in the reverse cross when using sperm from DL120 that had been cryopreserved for 30 min and compared with fresh sperm, there was a significant reduction in larval yield (p = 0.0003), settlement yield (p = 0.009) and settlement success (p = 0.011) with respect to fresh sperm, suggesting that the cryopreservation method had a significant negative effect on sperm from colony DL120.
Monitoring of donor colonies (2018–2020)
Tagged colonies were monitored in July and August 2018, March, July and August 2019 and February 2020 (Fig. 2a–t). Total colony size ranged from 735 cm2 to 5174 cm2; thus, all colonies exceed the reproductive minimum (Weil and Vargas 2010). The largest colony, DL121 (5174 cm2), despite having no signs of disease or partial mortality, did not spawn in July or August 2018, 2019 or 2020. This contrasts with that observed for the DL118 colony, which with only 10% living tissue, released gametes in July 2018. The eggs of this colony were highly successful when crossed with fresh or cryopreserved sperm in terms of fertilization, larval yield and settlement (Table 3a).
The monitoring undertaken in March 2019 showed that 4 colonies, which showed signs of SCTLD at the beginning of the outbreak, had died, whereas 3 colonies showed symptoms. Only two colonies, DL119 and DL120, showed no apparent signs of tissue damage from SCTLD (Table 2, Fig. 2j, l). By February 2020, all colonies had died except for DL119 and DL120, which still showed no apparent signs of tissue damage.
Discussion
In this study we evaluated the efficacy of sperm cryopreservation for use in larval propagation of Diploria labyrinthiformis, a species whose populations have been severely affected by Stony Coral Tissue Loss Disease (SCTLD) in the Mexican Caribbean. We have shown here that sperm of this species can be cryopreserved and thawed to yield viable sperm, which can be used to fertilize eggs in vitro to produce viable larvae and settled primary polyps that were maintained in ex situ aquaria.
Spawning patterns
First, we established the spawning pattern for D. labyrinthiformis to ensure the capture of gametes. In the northern section of the Mexican Caribbean D. labyrinthiformis spawning appears to be restricted to July and August at 10–11 days after full moon (DAFM). This pattern differs from other parts of the Caribbean. In Curaçao, two spawning peaks have been documented between May and October at 10–13 DAFM (Chamberland et al. 2017). Other authors report spawning in April and May at 7–10 DAFM in Puerto Rico (Weil and Vargas 2010), in May and June 5 DAFM in Colombia (Alvarado-Chaparro et al. 2004) and Bonaire (Muller and Vermeij 2011) and late July in Bermuda (Wyers et al. 1991). This species is unique among Caribbean species in that it initiates spawning prior to sunset. Here, we documented that spawning initiated at approximately 30 min before sunset, which is similar to Bonaire and Curaçao (Muller and Vermeij 2011; Chamberland et al. 2017). With D. labyrinthiformis spawning on only four days in any given year, it is best to collect gametes on as many days as possible due to variability in the individuals that spawn and the spawning volumes.
Reproductive quality
Crosses that involved eggs from colonies DL117 and DL118 suggest that these might be “super” colonies. When fertilized with sperm from any other colony tested, even after 13 months in cryopreservation, they resulted in high fertilization rates as well as high larval yield and settlement. On the other hand, the four largest colonies of D. labyrinthiformis (Table 1) may be senescent or produce low volume or low-quality spawn. The largest colony DL121 (5174 cm2) did not spawn during the two-year study, whereas colony DL115 (4700 cm2) produced a small volume of spawn (< 5 mL) that was not enough to cryopreserve. The remaining two large colonies, DL120 at 4998 cm2 and DL123 at 4557 cm2 did spawn but may have a low reproductive condition; the eggs of these colonies had lower conversion efficiencies from eggs to larvae and to settled primary polyps than the reverse crosses. This was true for DL120 eggs crossed with DL117 sperm when compared to the reverse cross and for DL123 eggs crossed with DL118 when compared to the reverse cross (n = 4, Table 3). Reproductive quality differs between colonies as shown here and is consistent with results for other species (Hagedorn et al. 2012a; Hagedorn and Carter 2016). For D. labyrinthiformis this may be more related to egg rather than sperm quality. Clear patterns could not be detected for larval yields and settlement resulting from sperm of different colonies (except for the cross S120 × E117), but differences were discerned based on the egg source.
Even with apparently low-quality eggs at least some larvae were produced and settled, for example, the cross between DL120 and DL117 (Table 3b). In addition, crosses involving eggs from DL118 produced superior results in comparison with eggs from DL123 in fertilization and in the conversion of eggs to larvae and to settled primary polyps, regardless of cryopreserved sperm storage time. Thus, the reproductive condition of the large colonies may be compromised, or the colonies may be senescent, which is an important consideration in genetic rescue programs, because even when all colonies spawn almost synchronously, not all crosses will be successful. This would suggest that spawn should be collected from as many colonies as possible so as to produce as many crosses as possible to increase the likelihood of successful outcomes. When possible, spawn should also be collected on as many nights as possible because variation in reproductive traits, even within the same individual on consecutive nights, has also been documented (Hagedorn et al. 2012a).
Recent monitoring of the colonies involved in this study showed that 7 of the 9 colonies have since succumbed to SCTLD. Colony DL120 is one of the two remaining colonies along with DL119 that have, at least until the end of 2020, survived the outbreak. Our foregoing observations suggest the existence of individuals with disease-resistant genotypes to sustain the recovery of this species making these colonies key egg donors for use in future in vitro fertilization experiments. However, in July 2020 colony DL120 did not spawn, so the probability of obtaining egg donors is reduced even in apparently healthy colonies.
The loss of seven of the nine colonies of D. labyrinthiformis that were used in this study due to SCTLD will have a negative impact on the local recovery of the species, particularly through sexual reproduction due to the decrease in the reservoir of available genotypes and gamete donors (Glassom et al. 2006; Baums et al. 2013). However, the cryopreserved samples in the repository resulting from this study will help to maximize genetic variation. This is important for species ability to cope with climate change and diseases (Hoffmann and Sgrò 2011; Carlson et al. 2014; Van Oppen et Al. 2015; Hagedorn and Carter 2016; Webster et al. 2017). The identification of more surviving colonies as potential sperm and egg donors is essential to save D. labyrinthiformis from local extinction. Cryo-banking sperm from as many colonies as possible is needed to promote genetic diversity to overcome future climatic conditions (Van Oppen And Gates 2006; Darling and Côté 2018).
Sperm efficacy over time
For this study, we used the most common sperm cryopreservation method as developed by Hagedorn et al. (2012a, b). When applied to D. labyrinthiformis the cryopreservation process maintained viable sperm for all time periods that were tested (30 min, 1, 12 and 13 months). Overall, we found that sperm from D. labyrinthiformis can be cryopreserved for at least 13 months and used to cross with fresh eggs resulting in successful fertilization, larval yield, settlement and post-settlement survival. This is similar to results found for sperm of Acropora tenuis and Acropora millepora that had been cryopreserved for up to 2 years (Hagedorn et al. 2017). Although the viability of cryopreserved sperm was reduced for all time periods in comparison with fresh sperm, we found no significant differences between fresh and cryopreserved sperm for motility, fertilization, settlement success and post-settlement survival for all time points. The only exception being that we found significant differences in larval and settlement yields when sperm had been cryopreserved for at least one year.
Nevertheless, in these two cases, larvae that made it through embryonic development have a high probability of settling (Fig. 4e). This has also been found for Acropora tenuis and A. millepora (Hagedorn et al. 2017) and suggests that settlement is not dependent upon egg-sperm compatibility or larval yield, even when fertilization is low. Therefore, we suggest that initial sperm motility is not necessarily a good indicator of post-fertilization success as measured by settlement success. Even if low sperm motility leads to low larval yield, most larvae will settle. For example, some crosses such as C2-18 sperm with DL118 eggs and C2-18 sperm with DL123 eggs produced fewer larvae (37% and 25%), but of those 67% and 56% settled, respectively (Table 3a).
Some colonies were more affected by the cryopreservation process than others. Significant differences were found in the viability of D. labyrinthiformis sperm after cryopreservation as exemplified by sperm from colonies DL117 and DL120 (Table 3b). Cryopreservation had deleterious effects on DL120 sperm viability as revealed by comparing fertilization rates with fresh versus cryopreserved sperm (Table 3b). Consequently, larval and settlement yields, settlement success and post-settlement survival were also negatively affected.
A lack of fertilization could also be attributable to incompatibility between eggs and sperm even though they come from distinct colonies as exemplified by DL120, which was not compatible with any other colonies. Gamete incompatibility may vary between genotypes of the same species and this has been attributed to differences in recognition protein receptors and diffusible molecules associated with gametes, such as the guanylate cyclase receptor of sperm flagella (Zhang et al. 2019). The use of pooled sperm is recommended to overcome such potential incompatibilities (Hagedorn and Carter 2016).
Direction of crosses
We found that the direction of the cross affects larval yields, settlement yield and settlement success. Although visually there were no obvious differences in sperm motility or egg quality that could be discerned in terms of size and shape, they may be responsible for the differences based on the direction of the cross. For the crosses between sperm and eggs of the colonies DL117 and DL120 the effect of cryopreservation on sperm viability may explain the difference in results in larval yield and settlement.
The new reality of successive disturbances dictates that, to ensure a sufficient number of compatible genotypes, coral rescue plans may need to consider collecting gametes from diseased colonies of target species, as it could quite possibly be their last spawning event, as was the case for most of the colonies in the patch of D. labyrinthiformis studied here. Therefore, is it critical that we continue to cryopreserve eggs as well as coral larvae and tissues using vitrification (Feuillassier et al. 2015; Daly et al. 2018; Viyakarn et al. 2018; Cirino et al. 2019) and to locate germ cells for culturing (Shikina et al. 2012; Shikina et al. 2015; Barfield et al. 2016; Tan et al. 2020).
Challenges include optimizing techniques for gamete cryopreservation of other scleractinian coral species (Viyakarn et al. 2018) and to understand the sources of variation among crosses. Tradeoffs between banking only good quality material versus banking as many genotypes as possible need to be considered. Given that SCTLD is causing high colony mortality of many species of corals (Álvarez-Filip et al. 2019), perhaps the sperm motility criterion will need to be relaxed somewhat to ensure higher genetic diversity in cryo-banking repositories, particularly for species that are threatened with local extinction. We must also establish criteria for deposition into and extraction of material from cryo-banks. A major challenge to cryopreservation is the ability to scale up efforts (Hagedorn et al. 2017). One option, particularly in remote areas, is to produce mobile cryopreservation laboratories, which are relatively inexpensive and can be easily transported to remote sites, as long as liquid nitrogen is available.
Due to the recent loss of 30% of the coral cover caused by the rapid spread of SCTLD, at least 22 coral species have been affected in Mexico and other Caribbean countries (Álvarez-Filip et al. 2019; McField et al. 2020). In the Mesoamerican Reef, the reef-building corals most affected by this syndrome are meandroid corals, such as D. labyrinthiformis (Álvarez-Filip et al. 2019; McField et al. 2020). Currently D. labyrinthiformis is listed on the International Union for Conservation Nature Red List (IUCN) Red as a species of least concern; however, due to the recent critical declines in the population of this species, our study suggests that it’s status on the IUCN Red List needs to be re-evaluated.
References
Alvarado-Chaparro E, Garcia R, Acosta A (2004) Sexual reproduction of the reef-building coral Diploria labyrinthiformis (Scleractinia:Faviidae) in the Colombian Caribbean. Rev Biol Trop 52:859–868
Álvarez-Filip L, Estrada-Saldívar N, Pérez-Cervantes E, Molina-Hernández A, González-Barrios FJ (2019) A rapid spread of the stony coral tissue loss disease outbreak in the Mexican Caribbean. PeerJ 7:e8069
Barfield S, Aglyamova GV, Matz MV (2016) Evolutionary origins of germline segregation in Metazoa: evidence for a germ stem cell lineage in the coral Orbicella faveolata (Cnidaria, Anthozoa). Proc Biol Sci 83:20152128
Baums IB, Devlin-Duarte MK, Polato NR, Xu D, Giri S, Altman NS, Ruiz D, Parkinson JE, Boulay JN (2013) Genotypic variation influences reproductive success and thermal stress tolerance in the reef building coral Acropora palmata. Coral Reefs 32:703–717
Carlson SM, Cunningham CJ, Westley PAH (2014) Evolutionary rescue in a changing world. Trends Ecol Evol 29:521–530
Chamberland VF, Snowden S, Marhaver KL, Petersen D, Vermeij MJA (2017) The reproductive biology and early life ecology of a common Caribbean brain coral, Diploria labyrinthiformis (Scleractinia: Faviinae). Coral Reefs 36:83–94
Cirino L, Wen ZH, Hsieh K, Huang CL, Leong QL, Wang LH, Chen CS, Daly J, Tsai S, Lin C (2019) First instance of settlement by cryopreserved coral larvae in symbiotic association with dinoflagellates. Sci Rep 9:18851
Daly J, Zuchowicz N, Nuñez Lendo CI, Khosla K, Lager C, Henley EM, Bischof J, Kleinhans FW, Lin C, Peters EC, Hagedorn M (2018) Successful cryopreservation of coral larvae using vitrification and laser warming. Sci Rep 8:15714
Darling ES, Côté IM (2018) Seeking resilience in marine ecosystems. Science 359:986–987
Fadlallah YH (1983) Sexual reproduction, development and larval biology in scleractinian corals: a review. Coral Reefs 2:129–150
Feuillassier L, Masanet P, Romans P, Barthélémy D, Engelmann F (2015) Towards a vitrification-based cryopreservation protocol for the coral Pocillopora damicornis L.: tolerance of tissue balls to 4.5M cryoprotectant solutions. Cryobiology 71:224–235
Glassom D, Celleirs L, Schleyer MH (2006) Coral recruitment patterns at Sodwana Bay, South Africa. Coral Reefs 25:485–492
Häder D-P, Banaszak AT, Villafañe VE, Narvarte MA, González RA, Helbling EW (2020) Anthropogenic pollution of aquatic ecosystems: emerging problems with global implications. Sci Total Environ 713:136586
Hagedorn M, Spindler R (2014) The reality, use and potential for cryopreservation of coral reefs. Adv Exp Med Biol 753:317–329
Hagedorn M, Carter V (2016) Cryobiology: principles, species conservation and benefits for coral reefs. Reprod Fertil Dev 28:1049–1060
Hagedorn M, Spindler R, Daly J (2019) Cryopreservation as a tool for reef restoration. Adv Exp Med Biol 1200:489–505
Hagedorn M, Pana R, Cox EF, Hollingsworth L, Krupp D, Lewis TD, Leong JC, Mazur P, Rall WF, MacFarlane DR, Fahy G, Kleinhans FW (2006) Coral larvae conservation: physiology and reproduction. Cryobiology 52:33–47
Hagedorn M, Carter V, Martorana K, Paresa MK, Acker J, Baums IB, Borneman E, Brittsan M, Byers M, Henley M, Laterveer M, Leong J-A, McCarthy M, Meyers S, Nelson BD, Petersen D, Tiersch T, Cuevas Uribe R, Woods E, Wildt D (2012a) Preserving and using germplasm and dissociated embryonic cells for conserving Caribbean and Pacific coral. PLoS ONE 7:e33354
Hagedorn M, Van OppenCarter MJHV, Henley M, Abrego D, Puill-Stephan E, Negri A, Heyward A, MacFarlane D, Spindler R (2012b) First frozen repository for the great barrier reef coral created. Cryobiology 65:157–158
Hagedorn M, Farrell A, Carter VL (2013) Cryobiology of coral fragments. Cryobiology 66:17–23
Hagedorn M, Carter VL, Henley EM, van Oppen MJH, Hobbs R, Spindler RE (2017) Producing coral offspring with cryopreserved sperm: a tool for coral reef restoration. Sci Rep 7:14432
Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury RH, Dubi A, Hatziolos ME (2007) Coral reefs under rapid climate change and ocean acidification. Science 318:1737–1742
Hoffmann AA, Sgrò CM (2011) Climate change and evolutionary adaptation. Nature 470:479–485
Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson JBC, Kleypas J, Lough JM, Marshall P, Nyström M, Palumbi SR, Pandolfi JM, Rosen B, Roughgarden J (2003) Climate change, human impacts, and the resilience of coral reefs. Science 301:929–933
Lin C, Tsai S (2012) The effect of chilling and cryoprotectants on hard coral (Echinopora) oocytes during short-term low temperature preservation. Theriogenology 77:1257–1261
Lin C, Wang LH, Fan TY, Kuo FW (2012) Lipid content and composition during the oocyte development of two gorgonian coral species in relation to low temperature preservation. PLoS ONE 7:e38689
McField M, Kramer P, Giró Petersen A, Soto M, Drysdale I, Craig N, Rueda Flores M (2020) 2020 Mesoamerican Reef Report Card, p 36
Muller E, Vermeij MJA (2011) Day time spawning of a Caribbean coral. Coral Reefs 30:1147
Novak BJ, Fraser D, Maloney TH (2020) Transforming ocean conservation: applying the genetic rescue toolkit. Genes 11:209
Perry CT, Murphy GN, Kench PS, Smithers SG, Edinger EN, Steneck RS, Mumby PJ (2013) Caribbean-wide decline in carbonate production threatens coral reef growth. Nat Commun 4:1402
Precht WF, Gintert BE, Robbart ML, Fura R, van Woesik R (2016) Unprecedented disease-related coral mortality in Southeastern Florida. Sci Rep 6:31374
Shikina S, Chen CJ, Liou JY, Shao ZF, Chung YJ, Lee YH, Chang FC (2012) Germ cell development in the scleractinian coral Euphyllia ancora (Cnidaria, Anthozoa). PLoS ONE 7:e41569
Shikina S, Chung YJ, Wang HM, Chiu YL, Shao ZF, Lee YH, Chang CF (2015) Immunohistochemical localization of early germ cells in a stony coral, Euphyllia ancora: potential implications for a germline stem cell system in coral gametogenesis. Coral Reefs 34:639–653
Tan ES, Izumi R, Takeuchi Y, Isomura N, Takemura A (2020) Molecular approaches underlying the oogenic cycle of the scleractinian coral, Acroporatenuis. Sci Rep 10:9914
Tsai S, Yang V, Lin C (2016) Comparison of the cryo-tolerance of vitrified gorgonian oocytes. Sci Rep 6:23290
Tsai S, Yen W, Chavanich S, Viyakarn V, Lin C (2015) Development of cryopreservation techniques for gorgonian (Junceella juncea) oocytes through vitrification. PLoS ONE 10:e0123409
Van Oppen MJ, Gates RD (2006) Conservation genetics and the resilience of reef-building corals. Mol Ecol 15:3863–3883
Van Oppen MJH, Oliver JK, Putnam HM, Gates RD (2015) Building coral reef resilience through assisted evolution. Proc Natl Acad Sci USA 112:2307–2313
Van Tussenbroek BI, Hernández Arana HA, Rodríguez-Martínez RE, Espinoza-Avalos J, Canizales-Flores HM, González-Godoy CE, Barba-Santos MG, Vega-Zepeda A, Collado-Vides L (2017) Severe impacts of brown tides caused by Sargassum spp. on near-shore Caribbean seagrass communities. Mar Pollut Bull 122:272–281
Viyakarn V, Chavanich S, Chong G, Tsai S, Lin C (2018) Cryopreservation of sperm from the coral Acropora humilis. Cryobiology 80:130–138
Webster MS, Colton MA, Darling ES, Armstrong J, Pinsky ML, Knowlton N, Schindler DE (2017) Who should pick the winners of climate change? Trends Ecol Evol 32:167–173
Weil E, Vargas WL (2010) Comparative aspects of sexual reproduction in the Caribbean coral genus Diploria (Scleractinia: Faviidae). Mar Biol 157:413–426
Wyers SC, Barnes HS, Smith SR (1991) Spawning of hermatypic corals in Bermuda: a pilot study. Hydrobiologia 216(217):109–116
Zhang Y, Chiu Y, Chen C, Ho Y, Shinzato C, Shikina S, Chang C (2019) Discovery of a receptor guanylate cyclase expressed in the sperm flagella of stony corals. Sci Rep 9:14652
Acknowledgements
This project was funded by CONACYT project number 247765 to ATB and EM. We sincerely appreciate the technical assistance of C. Morera Román, F. Negrete Soto, E. Escalante Mancera, M.A. Gómez Reali and R. Tecalco Rentería and the opportunity to learn cryopreservation techniques from Dr. Mary Hagedorn. We also wish to thank Dr. Hagedorn and two anonymous reviewers whose suggestions helped to improve the manuscript. This work was undertaken with a permit authorized by the National Aquaculture and Fisheries Commission (CONAPESCA Nº. PPF/DGOPA-003/2017).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding authors state that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topic Editor Morgan S. Pratchett
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Grosso-Becerra, M.V., Mendoza-Quiroz, S., Maldonado, E. et al. Cryopreservation of sperm from the brain coral Diploria labyrinthiformis as a strategy to face the loss of corals in the Caribbean. Coral Reefs 40, 937–950 (2021). https://doi.org/10.1007/s00338-021-02098-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-021-02098-7