Abstract
Coral ecosystems in the central Mexican Pacific inhabit environmental conditions considered as suboptimal for reef development, such as wide ranges in temperature, low pH, and cyclonic activity. In addition, they are facing increasing nutrient and sediment inputs as a consequence of urban growth and tourism. While the global effects of anthropogenic stressors to coral communities have been described, the local response and microscale variations remain unknown. Therefore, the present study evaluates three physiological markers during 2018 (total lipid content, symbiont density, and chlorophyll a concentration) in the main reef-building coral genera (Pocillopora, Porites, and Pavona) from two coral communities: one coastal site next to a luxury touristic development with high sedimentation rates and elevated nutrient inputs from golf courses, and one at an insular MPA 6 km distant from the coast and where human activities are regulated. At each coral sampling site, nitrite, nitrate, and phosphate concentrations as well as sedimentation rates were measured. The analyses of the physiological markers showed significant differences in the lipid content and symbiont density between sites, with corals at Isla Larga presenting higher lipid content but lower symbiont density, while pigment concentration only differed across months. When assessing differences among coral genera, Pocillopora colonies presented the highest lipid content, while Pavona showed more symbionts and Porites colonies the uppermost pigment concentrations, with significant differences among genera and across the studied months. Environmental characterization showed significant differences between sites in the nitrate concentration and sedimentation rates. Generalized nonlinear models evidence that lipid concentration is related to sedimentation rates and temperatures, symbiont density to nitrite and phosphate concentrations, and pigment concentrations to nitrate and phosphate concentrations as well as sedimentation rates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coral reefs are considered as the marine ecosystems with the highest biological diversity and productivity (Reaka-Kudla 1997; Burke et al. 2011), which provide ecological and economical services to society (Spalding et al. 2017). However, they are among the most threatened ecosystems (Burke et al. 2011) as abnormal changes in seawater temperature from climate change provoke bleaching and mass mortality events that lead to their degradation, while ocean acidification reduces the available carbonate for coral calcification resulting in slower growth rates (Hoegh-Guldberg et al. 2007), endangering these systems with the risk of complete loss within the next 50 yr (Hoegh-Guldberg 2014). In addition, local impacts such as sewage discharges, overfishing, and changes in coastal areas due to agriculture and urban development are also contributing to reef decline worldwide (Carpenter et al. 2008). In particular, urban coastal development involves changes in land use that increase erosion and the input of inorganic nutrients from gardens and golf courses, which eventually reach the ocean and can change the composition of coral reefs by favoring the outgrowth of macroalgae (Fabricius and De’ath 2004; Stimson et al. 2001). Also, high inorganic nutrient concentrations reduce coral calcification, promote coral diseases, decrease the coral’s heat stress tolerance, and aggravate the effects of ocean acidification (Fabricius 2011; Prouty et al. 2017). Increasing sedimentation reduces coral growth, damages the colony’s tissue, and affects the photosynthesis of the coral’s symbionts since the suspended particles in the water reduce the amount of light that reaches the coral colonies (Fabricius 2005), promoting stress and a direct effect in the coral’s physiology that can cause short-term changes in lipid content, symbiont densities, and chlorophyll concentrations (Fabricius 2005, 2011). When this stress becomes chronic, changes in the reef metabolism and shifts in the coral community may take place (Fabricius 2005), and since coastal urban areas are expected to continue growing at accelerated rates, increasing sedimentation, high nutrient concentrations, and their effects on coral reefs are a major concern.
Coastal development has severely impacted coral reefs in the Eastern Tropical Pacific (Cortés and Reyes-Bonilla 2017). Within this region, the Central Mexican Pacific (CMP) harbors important coral communities characterized by a high coral species richness and cover (Carriquiry and Reyes-Bonilla 1997; Glynn and Ault 2000) that have been historically impacted by both strong and moderate El Niño Southern Oscillation (ENSO) events, some of which have reduced the coral cover to ~ 2% (Carriquiry et al. 2001). However, after these severe reductions, the coral community has been able to recover, providing evidence that the ecosystem can persist despite repeated disturbances (Rodríguez-Troncoso et al. 2014, 2016; pers. observ.). While corals in this area appear to acclimatize to the effects of regional stressors and of climate change, their ability to resist and recover from local anthropogenic stressors has been overlooked. The present study evaluates the physiological condition of the main reef-building coral genera in two coral communities from the Central Mexican Pacific with different levels of perturbation associated with urban development. Understanding how coral communities function and cope with local stressors is important, particularly those in areas already considered marginal for reef development and where human populations are increasing at fast rates. Moreover, this is of special importance in coral ecosystems that continue to recover from global stressors (e.g., El Niño events) and whose ability to continue doing so may be affected by local threats.
Materials and methods
Study area
The Eastern Tropical Pacific is considered a marginal region for coral reef development because of its extreme physicochemical conditions, which include a wide range of temperature fluctuations, low pH, nutrient pulses, high sedimentation rates, and cyclonic activity (Glynn 2017). In addition to these regional conditions, the CMP also presents seasonal upwellings during spring (Portela et al. 2016) and internal waves that mix the water column and reduce temperatures throughout the day (Plata and Filonov 2007). In general, temperature ranges annually from 18 to 32 °C, peaking during summer (July to September) and cooling the most at winter (January to March; Portela et al. 2016), while pH ranges from 7.72 to 8.03 (total scale; Cupul-Cortés et al. 2018).
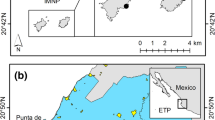
Despite these nonoptimal conditions, the coral ecosystems of this eco-region have been characterized as highly important for the species richness they harbor (Glynn and Ault 2000) and their resistance to ENSO events (Rodríguez-Troncoso et al 2016; Rodríguez-Troncoso and Cupul-Magaña 2016). The physiological response of the three main reef-building coral genera in the region (Pocillopora, Porites, and Pavona) to both high sedimentation and increment in nutrients was evaluated in two coral communities with contrasting perturbation levels, one at Islas Marietas National Park (IMNP) and one at Punta de Mita (Fig. 1). IMNP is an insular Marine Protected Area with a coral coverage of ~ 17% (Hernández-Zulueta et al. 2017) constituted by several islets and two main islands, Isla Larga (20.699167° N, 105.582111° W) and Isla Redonda (20.71394° N, 105.565° W; Fig. 1). Corals at this site have historically been affected by strong ENSO events from which they have recovered in short times (Tortolero-Langarica et al. 2017), and the protection status contributes to the regulation of human activities inside the MPA, decreasing the direct anthropogenic pressure by touristic activities (Rodríguez-Troncoso and Cupul-Magaña 2017).
In contrast, Punta de Mita (20.76° N, 105.54° W; Fig. 1) is a coastal site located next to a luxury touristic complex and with a distance of 6 km from IMNP. The coral community is less than 200 m distant from touristic development and a golf course with runoffs containing fertilizers and discharge waters. Punta de Mita harbored 87% of the total live coral cover in the CMP region, which declined after the massive bleaching and mortality event caused by the 1997–1998 ENSO (Carriquiry et al. 2001). In recent years, a slow but constant recovery has been observed in this site as live coral cover has increased to ~ 15% (pers. observ.).
Coral physiology
At IMNP (Isla Larga and Isla Redonda), a total of 54 adult colonies were tagged: 18 Pocillopora spp. colonies at Isla Larga, and 18 Porites panamensis and 18 Pavona gigantea colonies at Isla Redonda. At Punta de Mita, only adult Pocillopora spp. (n = 20) colonies were tagged as the presence of P. panamensis and P. gigantea is restricted to five adult colonies. Pocillopora colonies are located at 3–6 m depth, while Pavona and Porites are more distributed at 15–18 m. Each tagged colony was sampled (2–5 cm fragment) every 2–3 months during 2018 until an annual cycle was completed. Samples were immediately fixed using 10% seawater formaldehyde, decalcified with 10% acetic acid for ~ 12 h, and stored at room temperature until further processing.
Total lipid content was obtained from the coral tissue, which was dried for 24 h at 60 °C and weighed on an A&G® GR-200 Gemini Series Analytical Balance (0.1 mg of precision). Lipid extraction was performed according to Folch and Sloane-Stanley (1957) by adding 1 ml of a 2:1 chloroform/methanol solution to the dry tissue. To precipitate the lipids, 0.8% KCl was added to the mixture and washed with 0.5 ml from a 1:1 methanol/water solution. Precipitated lipids were centrifuged in a Heraeus Pico 17 centrifuge (Thermo Scientific®) and evaporated in a Thermo Fisher Scientific® Dry bath. Finally, the samples were dried overnight at 60 °C and weighed. Results are expressed as total lipid weight relative to dry tissue (g lipids g tissue−1).
Symbiont density was calculated using a ~ 1 cm2 tissue from each colony. The tissue was homogenized in 1.5 ml of 4% ethanol and stained with lugol. One milliliter of the resulting solution was sampled, and symbiont cells were quantified using a Neubauer hemocytometer (n = 8 counts per sample) and observed using a compound microscope (Olimpus®; Rodríguez-Troncoso et al. 2010). Photographs from all samples were taken before homogenization using a Canon® Powershot D30 camera to calculate the exact area of each tissue sample using ImageJ software (Abramoff et al. 2004); symbiont density was then expressed as cells per unit area (× 106 cells cm−2).
Chlorophyll a concentration (also referred along the manuscript as pigment concentration) was determined as described in Rodríguez-Troncoso et al. (2014). From each colony, a ~ 1 cm2 tissue sample was preserved in 1.5 ml of methanol, stored at −40 °C for 24 h, and centrifuged at 1500 g for 5 min at 4 °C using a Heraeus Pico 17 centrifuge (Thermo Scientific®). The resulting solution was analyzed in an Orion AquaMate 7000 VIS spectrophotometer (Thermo Scientific®) using a 10-cm-path-length cuvette at 750, 664, and 630 nm. Chlorophyll a concentration per cm2 was calculated with Parsons et al. (1984) equation, and exact areas were obtained as described for symbiont densities. Finally, concentrations were standardized to chlorophyll a concentration per symbiont cell with the densities previously obtained (ng Chla cell−1).
Environmental monitoring
To characterize the conditions under which coral communities develop at the studied sites, from 2016 to 2018 (including months where coral sampling was carried out) water samples were collected at each sampling site at the same depth where tagged coral colonies were located (between 5 and 16 m) to measure nitrite, nitrate, and phosphate concentrations (mg l−1) according to Strickland and Parsons (1972). Before laboratory analyses, samples were properly stored and frozen at − 20 °C. Also, sedimentation rates were estimated from May 2018 to June 2019 by installing three sediment traps at each coral site next to the tagged coral colonies (less than 1 m from distance). Collected sediments were washed and filtered with distilled water to eliminate salts and organic compounds, dried for 1 week in a Scientific Precision® 25EG Economy Oven at 60 °C, and weighed on an A&G® GR-200 Gemini Series Analytical Balance (0.1 mg of precision). Sedimentation rates were calculated using the area of the sediment trap and total sediment dry weight (g cm−2 day−1; Nava and Ramírez-Herrera 2012). Lastly, monthly temperature was recorded in situ at each site by installing HOBO® thermographs (Pendant) and programed with a 15-min interval at each site; each sensor was replaced every two months.
Statistical analyses
First, a priori analyses were conducted to determine whether Pocillopora colonies from different morphotypes could be grouped; hence, for each physiological marker, a one-way ANOVA test was performed (Supplementary Table 1). There were no significant differences among Pocillopora species in any of the markers; therefore, subsequent analyses were carried out at the genus level. As overall data were not normal nor homoscedastic, differences between Pocillopora colonies from Isla Larga and Punta de Mita (between sites) were analyzed with a two-way univariate ANOVA with repeated measures based on permutations with the Site (S) and Month (M) as fixed factors. This statistical analysis is analogous to the multifactorial univariate ANOVA models and does not require normality and homoscedasticity, allowing the use of raw data without any transformation (Anderson et al. 2008). Differences among the main reef-building coral genera in their physiological condition were also assessed with a two-way ANOVA with repeated measures based on permutations using the physiological traits measured in the colonies from Isla Larga and Isla Redonda (IMNP) with the Genus (G) and Month (M) as fixed factors.
As the study aims to evaluate sites with contrasting perturbation levels, first, differences among sites in the anthropogenic associated stressors (nutrient concentrations and sedimentation rates) were evaluated with a two-way ANOVA based on permutations, with the sites (S) and the months (M) as fixed factors (Clarke and Gorley 2006; Anderson et al. 2008). Also, as environmental variables are not continuous along the year, as intermittent sampling was imminent due to meteorological events, we included available nutrient data from previous years (2016 and 2017) which were obtained with the same methods to secure a most representative environmental characterization, obtaining monthly nutrient values. Unfortunately, there are no previous data about the sedimentation rates in the area.
All the described ANOVAs were performed with Euclidean distance matrices, 10,000 permutations, and with a type III sum of squares. Post hoc pairwise tests were carried out when terms were significant (p < 0.05). Analyses were calculated using PRIMER ver. 6.1.11 + PERMANOVA ver.1.0.1 software (Clarke and Gorley 2006; Anderson et al. 2008).
Finally, to test the effects of environmental conditions in the corals’ physiological condition, generalized nonlinear models (GLZ) were performed with each physiological trait from each genus with the environmental variables measured at their site of collection (Green and Silverman 1994). Overall models were constructed by scaling sedimentation rates and temperature to match nutrient concentrations using Statistica ver 8.0 software (StatSoft, 2007). Results were considered significant when p < 0.05 (Zar 2010).
Results
Coral physiology
Differences between sites (Punta de Mita and Isla Larga) in the corals’ physiological traits were evaluated in Pocillopora colonies. Corals at Isla Larga exhibited higher lipid content but lower symbiont density and pigment concentrations than corals at Punta de Mita (Table 1), with statistical differences found in the site x month interaction in the lipid content and the symbiont density (Table 2). Differences in pigment concentration were only observed among months (Table 2). Pairwise comparisons between sites showed differences from March to September with a reduction in the coral’s lipid content during these months (Fig. 2a; Supplementary Table 2). Symbiont density showed differences (higher values) only during January in corals from Punta de Mita (Fig. 2b; Supplementary Table 2). As for pigment concentration, differences were observed between the cold and warm months (January-March and September-November, respectively) with the first presenting the lowest Chlorophyll a content (Fig. 2c). Also, pigment concentrations in June (the transition month between seasons) differed from the other sampling months (Supplementary Table 2).
Differences among coral genera were assessed between colonies tagged at Isla Larga and Isla Redonda (IMNP) since as described in the Materials and methods section, Punta de Mita harbors only few Pavona gigantea colonies and Porites panamensis colonies. Pocillopora colonies presented the highest lipid content, Pavona corals exhibited the most elevated symbiont densities, and Porites corals exhibited the uppermost pigment concentrations (Table 1; Fig. 3). Significant differences among genera at the genus x month interaction were observed only in the pigment concentration per symbiont cell (Table 2; Fig. 3), particularly between Pocillopora and Pavona colonies during the warm months (September–November; Fig. 3c; Supplementary Table 3). Significant differences in the other physiological markers were observed separately at the genus or month level, respectively. Pocillopora colonies significantly differed in their lipid content from both Porites and Pavona colonies, and at the month level, the lipid content that colonies presented during the cold months (January–March) was higher than the rest of the year (Fig. 3a; Supplementary Table 3). Finally, Pavona colonies significantly differed from the other genera in their symbiont density, and at the month level, the symbiont density in tagged coral colonies during the cold months (January-March) was significantly higher than the warm months (Fig. 3b; Supplementary Table 3).
Environmental monitoring
Nutrient concentrations measured from May 2016 to August 2018 were the following: nitrite ranged from < 0.007 to 2.17 mg l−1, nitrate from < 0.1 to 0.7 mg l−1, and phosphate from < 0.009 to 0.168 mg l−1 (Fig. 4a–c, respectively), while sedimentation rates ranged between 1.039 and 549.532 mg cm−2 d−1 (Fig. 4d). The univariate permutational ANOVA analysis showed significant differences in the interaction (site x month) only in the nitrate concentration, while sedimentation significantly differed only at the Site level (Table 3). Pairwise comparisons in nitrate concentrations evidence differences between Isla Redonda and the other sites (Isla Larga and Punta de Mita). In particular, Isla Redonda exhibited the highest nitrite and nitrate recorded values (Fig. 2a, b), while Punta de Mita presented the highest recorded phosphate concentration (Fig. 4c). Finally, while the three sites present different sedimentation rates (Fig. 4b, d; Supplementary Table 4), Isla Larga recorded the highest values (> 100 mg cm−2 d−1), while Isla Redonda the lowest (< 19 mg cm−2 d−1; Fig. 4d).
Coral physiology and environmental conditions
In general, both the lipid content and the symbiont density decreased during the warm months in all coral colonies analyzed (September–November; Figs. 2a, b, 3a, b). In contrast, pigment concentrations were the highest during the warm months (Figs. 2c, 3c). At all cases, the most noteworthy changes were between warm and cold seasons, with the transition occurring in June which exhibited differences with the rest of the sampling months (Figs. 2, 3; Supplementary Tables 2, 3).
The GLZ models of each physiological trait showed different significant relations between the tagged coral colonies and the environmental variables measured. Total lipid content was significantly related to sedimentation rates and sea temperature (Table 4) and symbiont density was significantly related to nitrite and phosphate concentrations, while pigment concentration was significantly related to nitrate and phosphate concentrations and sedimentation rates (Table 4).
Discussion
Coral ecosystems typically develop in shallow tropical and subtropical seas (Veron 2000) with high light availability, low turbidity, warm temperatures (usually from 18 to 28 °C), and low nutrient concentrations (0.067 mg l−1 NO3 and 0.022 mg l−1 PO3; Kleypass et al. 1999) and therefore are characterized as oligotrophic conditions. However, coral ecosystems distributed along the Eastern Tropical Pacific (ETP) are influenced by conditions considered as marginal, such as wide temperature fluctuations, low pH, and seasonal upwellings (Glynn 2017); furthermore, the CMP is located on the Northern limit of the ETP, and consequently, the studied coral communities are influenced by the convergence of two coral ecoregions, the Western Mexico and Revillagigedo islands ecoregion and the Gulf of California ecoregion (Veron 2015). This convergence characterizes the CMP as an oceanographic transition zone between subtropical and temperate conditions under the influence of colder and lower in pH water from the California Current, the more saline water from the Gulf of California, and the warmer water from the Mexican coastal current (Portela et al. 2016). At the local scale, coral communities in the CMP are also under the effects of seasonal upwellings and internal waves that cause daily fluctuations in temperature of up to 5 °C (Plata and Filonov 2017). In addition to these environmental variables, our results showed that in particular, the coral communities evaluated in this study are exposed to local anthropogenic stressors such as high phosphate concentrations above the typical ranges of oligotrophic coral reefs at all sites (Fig. 4), which is a characteristic of eutrophic marine ecosystems (Karydis 2009; CONAGUA 2016). Also, elevated phosphate concentrations (Fig. 4c) as the ones recorded at Punta de Mita confirm an abnormal and constant runoff of nutrients attributed to fertilizers used in the nearby golf course. Furthermore, oligotrophic reefs also are characterized by low sedimentation rates between 1 and 10 mg cm2 d−1 (Todd et al. 2010) and in our study, both Isla Larga and Punta de Mita exhibited higher values which have been considered detrimental for reef development (Fabricius 2005; Fig. 4d). In fact, both eutrophication and sedimentation have degraded reefs located nearby coastal areas (Fabricius 2011), and they can aggravate the effects of ocean acidification through chemical bioerosion of the coral skeleton, compromising the reef structure (Prouty et al. 2017). However, this is not a nonoptimal or detrimental condition for the coral ecosystems in the Central Mexican Pacific. At the physiological level, an abnormal increase in nutrient concentrations and sedimentation cause a reduction in their lipid content, rupture of symbiosis and bleaching, and higher susceptibility to diseases (Fabricius 2005, 2011; Gil et al. 2016; Humanes et al. 2017), decreasing their ability to recover from natural disturbances. In addition, sedimentation rates above 100 mg cm−2 damage the coral’s exposed tissue leading to mortality within just a few days (Phillip and Fabricius 2003). However, during our study, no tissue damage or mortality was recorded in the studied coral colonies, including the ones at Isla Larga with the highest sedimentation rates. Also, since each site presented at least high levels of one of the analyzed stressors (Fig. 4), it would be expected that the organisms present a change in their physiological response that may be observed as a decrease on biomarkers such as lipid content and symbiont density, and while the results show significant differences between Punta de Mita and Isla Larga in these two physiological markers (Table 2; Fig. 2), they are attributed to a seasonal rather than a stress response (Figs. 2, 3 and Supplementary Table 2). Also, pigment concentration increased during the rainy season, when terrestrial runoff increases the amount of nutrients that reach the sea (Fig. 4), with nutrient concentrations higher than the ones observed during spring months, when upwellings are present in the area (Portela et al. 2016).
Specifically, elevated nitrogen concentrations in synergy with high sedimentation reduce the coral colony’s energetic reserves as the animal invests its energy to mitigate and recover from the damage caused by these stressors (Fabricius 2005; Wooldridge 2014). Contrary to this, Pocillopora corals at Isla Larga exhibited the highest lipid content (Fig. 2a) even though this site presented the “worst conditions” such as the highest nitrate concentrations and monthly sedimentation > 150 mg cm−2 day−1 (Fig. 4b, d). Healthy coral tissue is typically comprised of 30–40% of lipids (Ward 1995), and in all the analyzed colonies, lipid content was approximately 20% of the coral tissue (Table 1) and during spring months, lipid content was above the 40% (Figs. 2, 3). Furthermore, the lipid values observed in this study are higher than the ones previously recorded for nutrient-enriched conditions where lipids did not surpass the 600 µg cm−2 in Pocillopora colonies (Achituv 1994) and are above the ones previously recorded in the region (less than 20% of total lipid content; Rodríguez-Troncoso et al. 2010). Hence, these conditions may not be considered as marginal for the coral communities in the CMP as the organisms are able to accumulate energetic reserves that may be used in other physiological processes such as reproduction. In fact, lipid content was significantly related to temperature but not to nitrite, nitrate, and phosphate (Table 4), and a reduction in the energetic reserves during warm months (Figs. 2a, 3a) is consistent with the reproductive period reported not only for Pocillopora, colonies, but also for Porites, and Pavona corals (Santiago-Valentín et al 2018), and a seasonal reduction in the lipid content can be attributed to the gamete maturation, which is a high energy demand process (Harrison 2011).
Differences in symbiont density were only seen at the genus and month level (Table 2), and this physiological marker was significantly related to nitrite and phosphate concentrations (Table 4). Nutrient and sedimentation values such as the ones observed at Punta de Mita and Isla Larga are often associated with direct anthropogenic influence (Rouzé et al. 2015), and anthropogenic inputs into the ocean can disrupt the availability of nutrients; for example, an excess in nitrates unbalances de N:P ratio that the symbionts require for photosynthesis (Parkhill et al. 2001; Fabricius 2005; Rosset et al. 2017), leading to a phosphate starvation of the coral symbiont decreasing its photosynthetic efficiency, and eventually resulting in reef degradation (Parkhill et al. 2001; Wiedenmann et al. 2013). Elevated phosphate concentrations nontypical of oligotrophic reefs (Fig. 4c) suggest Pocillopora symbionts do not face unbalanced rates of inorganic nutrients, and therefore, symbiont cell division is not compromised (Fabricius 2005). On the contrary, coral colonies incremented their pigment concentrations at the end of the summer (Figs. 2, 3), when nutrient values are the highest (Fig. 4).
Since differences were found between sites, a difference in the physiological response of corals at the genus level can also be expected specially since the analyzed genera present distinct relative covers (Cupul-Magaña and Rodríguez-Troncoso 2017). In general, Pocillopora colonies presented a higher lipid content, while Porites colonies exhibited higher chlorophyll a concentrations per symbiont cell, and Pavona colonies a higher symbiont density (Table 2; Fig. 3). However, significant differences were observed in the genus x month interaction only in the chlorophyll a content per symbiont cell (Table 2), with Pavona corals differing from the other genera, even from Porites colonies, which were collected at the same site and depth (Isla Redonda; Supplementary Table 3). Also, monthly differences were present between warm and cold months in all physiological markers (Supplementary Table 3). These results suggest that overall differences in coral physiology are not a consequence from stress but a seasonal variation in the corals’ physiology and life history. In particular, differences between Pavona and Porites colonies also suggest that each genus has different annual physiological strategies at a microscale level. For example, coral colonies can develop under different environmental conditions by modulating their symbiont densities or their pigment concentrations (Fabricius 2005; Ziegel et al. 2014); hence, Porites colonies may balance their metabolic requirements by increasing the pigment concentration in their symbionts, while Pavona colonies allow the proliferation of their symbiont cells and this may be possible because nutrient enrichment, particularly of dissolved inorganic nitrogen, promotes both processes and Isla Redonda presented the highest recorded nitrogen values during the warm months and was differentiated from the other two sites (Fig. 4; Supplementary Table 4).
The observed physiological response may be then the result of increased tolerance to marginal conditions from the life history of the animal (Morgan et al. 2017; Sully and van Woesick 2019; Green et al. 2019), as corals are submitted throughout the year to nutrient concentrations nontypical of oligotrophic reefs since the region presents seasonal upwellings during winter (Portela et al. 2016) and terrestrial runoffs during the warm rainy season, which may explain why there were few differences among sites in the environmental variables analyzed (Supplementary Table 4). Even though for most environmental conditions there were no significant differences, the highest nutrient values and sedimentation rates were observed during the rainy season (June–August; Fig. 4), and since local conditions may continue to change specially because of urban growth (Merchand-Rojas 2012), a further increase in the nutrient loads into the coral communities can be expected with future changes in the patterns of terrestrial runoff due to the construction of touristic facilities and increased water discharges that contain agrochemical compounds from golf camps. Hence, we suggest that these coral communities have developed a resistance that will enable them to survive and persist even in eutrophic environments with elevated sedimentation and it can be expected that these coral communities have the ability to resist and recover from other global stressors like ENSO events. Indeed, this has been observed in the coral community at Punta de Mita, which was severely affected by the 1997–1998 El Niño event that reduced the live coral cover from 90 to 3%, and with branching corals being the sole survivors (Carriquiry et al. 2001). To date, this community has recovered 50% of its original cover (pers. observ.), and even more, corals at all the studied sites were able to resist and recover from the recent 2015–2016 ENSO event (Tortolero-Langarica et al. 2017) that caused massive bleaching and mortality in other regions (Hughes et al. 2018), suggesting that coral communities in the CMP have the capacity to acclimatize to suboptimal conditions as the limiting environment they inhabit has promoted this (Figs. 2, 3, 4) and the significant differences observed were mainly between warm and cold months (Supplementary Tables 2 and 3).
In addition, corals can also develop in nutrient-enriched environments where sediments are resuspended continuously by feeding from suspended particles (Anthony and Fabricius 2000), and this capacity of corals to obtain nutrients from suspended matter has also been suggested to favor their survival during thermal stress (Grottoli et al. 2006; Sully and van Woesick 2019). Heterotrophy can be then another process by which corals can develop in sites with elevated sedimentation rates such as Punta de Mita and even with rates > 100 mg cm−2 day−1 as the ones found at Isla Larga, the site that also presented lower monthly mean symbiont densities and pigment concentrations (Fig. 2). This extra source of nourishment may also further explain why these communities present one of the highest growth rates recorded in the Northeastern Tropical Pacific (Tortolero-Langarica et al. 2017). In fact, elevated nutrient concentrations do not always affect negatively the coral community (Wear and Thurber 2015); an increase may favor coral growth by enhancing the coral’s symbionts’ photosynthesis through cell proliferation and increase in pigment concentrations as well as coral heterotrophy nourishment as already seen in other regions (Fabricius et al. 2005; Grottoli et al. 2006; Morgan et al. 2017; Sully and van Woesick 2019). As symbiont density and pigment concentrations were significantly related to inorganic nutrients (Table 4), they may provide corals with the energy necessary to withstand other natural stressors such as the daily thermal anomalies in the region caused by internal waves (Plata and Filonov 2007; Green et al. 2019). While previous studies (Rodríguez-Troncoso et al. 2014, 2016; Tortolero-Langarica et al. 2017) along with our results suggest that corals in the region are able to cope with local stressors (including those associated with anthropogenic impacts), further analyses that incorporate gene expression, laboratory experiments in corals, and other ecological processes (such as bioerosion and grazing) are required to fully understand the mechanisms that enable corals to inhabit conditions considered as marginal for reef development and if coral communities are currently surviving close to their tolerance limits, especially when climate change and coastal development have a synergic negative effect (Prouty et al. 2017) and corals may not be able to survive future impacts.
Previous studies suggested that under the current climate change scenario, coral ecosystems in the Eastern Pacific may shift from being ecosystems majorly constituted by branching coral species (e.g., Pocillopora colonies) to being dominated by massive scleractinians (like Porites colonies) with the subsequent loss of reef complexity (Cabral-Tena et al. 2018), especially since branching coral species belonging to the Pocillopora genus were considered to be highly susceptible to changes in environmental conditions (Carriquiry et al. 2001). However, recently a number of studies suggest that these corals are able to cope not only with global stressors (Romero-Torres et al. 2020), but with local conditions which for specific locations could be considered as detrimental for coral development (Morgan et al. 2017; Green et al. 2019; Sully and van Woesik 2019; present study). This is important because reefs that develop under variable water conditions may have a wider resistance threshold due to the history of the coral community (Romero-Torres et al. 2020), which can make them future refuges for corals under the current climate change scenario and increasing anthropogenic pressure. However, the idea that marginal coral ecosystems have developed a bigger resistance does not imply that a continuous increase of these stressors at accelerated rates will not have a negative effect in their health. According to the Federal Mexican Ecological Criteria of Water Quality for the Protection of Marine Life (CONAGUA 2016), nutrient levels are five times above those considered as adequate for the development of marine ecosystems (NO2 ≤ 002, NO3 ≤ 0.04, and PO4 ≤ 0.002 mg l−1) even at IMNP, a Marine Protected Area, and are expected to increase with further urban growth.
Our results confirm the recent suggestion that coral ecosystems in marginal conditions may become biodiversity refuges in a changing ocean (Morgan et al. 2017; Green et al. 2019; Sully and van Woesick 2019), and therefore, their protection may be essential for the conservation of coral ecosystems. There is an urgent need for adequate management policies that regulate nutrient and sediment inputs from anthropogenic sources; unfortunately, the CMP is a luxury destination region for tourism and is expected to grow at a fast rate to fulfill increasing touristic demands (Merchand-Rojas 2012). How long will corals withstand a changing environment while facing anthropogenic threats? The answer is uncertain as so far evidence suggests that corals can maintain and develop in this suboptimal region, but surely, their ability to persist will be affected by the synergic effects of climate change and nonregulated urban development.
References
Abramoff MD, Magalhaes PJ, Ram SJ (2004) Image Processing with ImageJ Biophotonics Int 11(7):36–42
Achituv Y, Ben-Zion M, Mizrahi L (1994) Carbohydrate, lipid, and protein composition of zooxanthellae and animal fractions of the coral Pocillopora damicornis exposed to ammonium enrichment. Pac Sci 48(03):224–233
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: guide to software and statistical methods. PRIMER-E Press, Plymouth
Anthony KRN, Fabricius KE (2000) Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. J Exp Mar Biol Ecol 252:221–253
Burke L, Reytar K, Spalding M, Perry A (2011) Reefs at risk revisited. World Resources Institute, Washington DC
Cabral-Tena RA, Reyes-Bonilla H, Lluch-Cota S, Paz-García DA, Calderón-Aguilera LE, Norzagaray-López O, Balart EF (2013) Different calcification rates in males and females of the coral Porites panamensis in the Gulf of California. Mar Ecol Prog Ser 476:1–8
Carpenter KE, Abrar M, AbeyG ARB, Banks S, Bruckner A, Chiriboga A, Cortés J, Delbeek JC, DeVantier L, Edgar GJ, Edwards AJ, Fenner D, Guzmán HM, Hoeksema BW, Hodgson G, Johan O, Licuanan WY, Livingstone SR, Lovell ER, Moore JA, Obura DO, Ochavillo D, Polidoro BA, Precht WF, Quibilan MC, Reboton C, Richards ZT, Rogers AD, Sanciango J, Sheppard A, Sheppard C, Smith J, Stuart S, Turak E, Veron JEN, Wallace C, Weil E, Wood E (2008) One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science 321:560–563
Carriquiry JD, Reyes-Bonilla H (1997) Community structure and geographic distribution of the coral reefs of Nayarit. Mexican Pacific. Cienc 23(2):227–248
Carriquiry JD, Cupul-Magaña AL, Rodríguez-Zaragoza F, Medina-Rosas P (2001) Coral bleaching and mortality in the Mexican Pacific during the 1997–98 El Niño and prediction from a remote sensing approach. B Mar Sci 69(1):237–249
Clarke KR, Gorley RN (2006) Primer6: usermanual/tutorial. Primer-E, Plymouth
CONAGUA (2016) Ley federal de derechos: disposiciones aplicables en materia de aguas nacionales 2016. México.
Cortés J, Reyes-Bonilla H (2017) Human influences on Eastern Tropical Pacific coral communities and coral reefs. Glynn PW, Manzello DP, Enochs IC(eds) Coral reefs of the Eastern tropical pacific: persistence and loss in a dynamic environment. Springer, Berlin, pp 549–563
Cupul-Cortés M, Hernández-Ayón JM, Cupul-Magaña AL, Rodríguez-Troncoso AP (2018) Variabilidad del sistema de CO2en el Parque Nacional Islas Marietas (PNIM), Bahía de Banderas. Nayarit. Simposio Internacional del Carbono en México, Sonora, pp 235–242
Cupul-Magaña AL, Rodríguez-Troncoso AP (2017) Tourist carrying capacity at Islas Marietas National Park: an essential tool to protect the coral community. Appl Geogr 88:15–23
Fabricius KE (2005) Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar Pollut Bull 50:125–146
Fabricius KE (2011) Factors determining the resilience of coral reefs to eutrophication: a review and conceptual model. Dubinsky Z, Stambler N(eds) Coral reefs: an ecosystem in transition. Springer, Berlin, pp 493–505
Fabricius KE, Death G (2001) Environmental factors associated with the spatial distribution of crustose coralline algae on the Great Barrier Reef. Coral Reefs 19:303–309
Folch JLM, Sloane-Stanley GH (1957) Simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–506
Gil MA, Goldenberg SU, Bach ALT, Mills SC, Claudet J (2016) Interactive effects of three pervasive marine stressors in a post disturbance coral reef. Coral Reefs 35:1281–1293
Glynn PW (2017) History of Eastern Pacific coral reef research. Glynn PW, Manzello DP, Enochs IC(eds) Coral reefs of the Eastern Tropical Pacific: persistence and loss in a dynamic environment. Springer, Berlin, pp 1–37
Glynn PW, Ault JS (2000) A biogeographic analysis and review of the far eastern Pacific coral reef region. Coral Reefs 19:1–23
Green PJ, Silverman BW (1994) Nonparametric regression and generalized linear models: a roughness penalty approach. Chapman & Hall/CRC Press, Boca Raton
Green RH, Lowe RJ, Buckley ML, Foster T, Gilmour JP (2019) Physical mechanisms influencing localized patterns of temperature variability and coral bleaching within a system of reef atolls. Coral Reefs 38(4):759–771
Grottoli AG, Rodrigues LJ, Palardy JE (2006) Heterotrophic plasticity and resilience in bleached corals. Nature 440(7088):1186–1189
Harrison PL (2011) Sexual reproduction of scleractinian corals. In: Dubinsky Z, Stambler N (eds) Coral Reefs: an ecosystem in transition. Springer, Berlin, pp 59–84
Hernández-Zulueta J, Rodríguez-Zaragoza FA, Araya R, Vargas-Ponce O, Rodríguez-Troncoso AP, Cupul-Magaña AL, Días-Pérez L, Ríos-Jara E, Ortiz M (2017) Multi-scale analysis of hermatypic coral assemblages at Mexican Central Pacific. Sci Mar 81(1):91–102
Hoegh-Guldberg O (2014) Coral reef sustainability through adaptation: glimmer of hope or persistent mirage? Curr Opin Environ Sustain 7:127–133
Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Bridge TC, Claar DC, Eakin M, Gilmour JP, Graham NAJ, Harrison H, Hobbs JPA, Hoey AS, Hoogenboom M, Lowe RJ, McCulloch MT, Pandolfi JM, Pratchett M, Schoepf V, Torda G, Wilson SK (2018) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359(6371):80–83
Humanes A, Ricardo GF, Willis BL, Fabricius KE, Negri AP (2017) Cumulative effects of suspended sediments, organic nutrients and temperature stress on early life history stages of the coral Acropora tenuis. Sci Rep 7:44101
Karydis M (2009) Eutrophication assessment of coastal waters based on indicators: a literature review. Glob NEST J 11(4):373–390
Kleypas JA, McManus JW, Menez LA (1999) Environmental limits to coral reef development: where do we draw the line? Am Zool 39(1):146–159
Merchand-Rojas MA (2012) The inter-state development of Puerto Vallarta and Bahía de Banderas: México. Prob Des 43(168):147–173
Morgan KM, Perry CT, Johnson JA, Smithers SG (2017) Nearshore turbid-zone corals exhibit high bleaching tolerance on the Great Barrier Reef following the 2016 ocean warming event. Front Mar Sci 4:224
Nava H, Ramírez-Herrera MT (2012) Land use and impact on coral communities along the central Pacific coast of Mexico. Environ Earth Sci 65(2012):1095–1104
Parkhill JP, Maillet G, Cullen JJ (2001) Fluorescence-based maximal quantum yield for PSII as a diagnostic of nutrient stress. J Phycol 37:517–529
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater -analysis. Pergamon Press, New York
Phillip E, Fabricius KE (2003) Photophysiological stress in scleractinian corals in response to short-term sedimentation. J Exps Mar Biol Ecol 287:57–78
Plata L, Filonov A (2007) Marea interna en la parte noroeste de la Bahía de Banderas México. Cienc Mar 33(2):197–215
Portela W, Beier E, Barton ED, Castro R, Godínez V, Palacios-Hernández E, Fiedler PC, Sánchez-Velazco L, Trasviña A (2016) Water masses and circulation in the tropical pacific off Central Mexico and surrounding areas. J Phys Oceanogr 46(10):3069–3081
Prouty NG, Cohen A, Yates KK, Storlazzi CD, Swarzenski PW, White D (2017) Vulnerability of coral reefs to bioerosion from land-based sources of pollution. J Geophys Res Oceans 122(12):9319–9331
Reaka-Kudla ML (1997) The global biodiversity of coral reefs: a comparison with rain forests. Reaka-Kudla ML, Wilson DE, Wilson EO(eds) Biodiversity II: understanding and protecting our biological resources. National Academy Press, Washington DC, pp 83–108
Rodríguez-Troncoso AP, Al Cupul-Magaña (2016) Effect of abnormal high temperature as during 2014–2015 on coral communities from the Central Mexican Pacific. Proc 13 Int Coral Reef Symp 1:291–292
Rodríguez-Troncoso AP, Carpizo-Ituarte E, Cupul-Magaña AL (2010) Differential response to cold and warm water conditions in Pocillopora colonies from the Central Mexican Pacific. J Exp Mar Bio Ecol 391(2010):57–64
Rodríguez-Troncoso AP, Carpizo-Ituarte E, Cupul-Magaña AL (2016) Physiological response to high temperature in the Tropical Eastern Pacific coral Pocillopora verrucosa. Mar Ecol 37(5):1168–1175
Rodríguez-Troncoso AP, Carpizo-Ituarte E, Pettay DT, WarnerME C-M (2014) The effects of abnormal decrease in temperature on the Eastern Pacific reef-building coral Pocillopora verrucosa. Mar Biol 161:131–139
Romero-Torres M, Acosta A, Palacio-Castro AM, Treml EA, Capata FA, Paz-García DA, Porter JW (2020) Coral reef resilience to thermal stress in the Eastern Tropical Pacific. Glob Chang Biol. https://doi.org/10.1111/GCB.15126
Rosset S, Wiedenmann J, Reed AJ, D’Angelo C (2017) Phosphate deficiency promotes coral bleaching and is reflected by the ultrastructure of symbiotic dinoflagellates. Mar Pollut Bull 118:180–187
Rouzé H, Lecellier G, Langlade MJ, Planes S, Berteaux-Lecellier V (2015) Fringing reefs exposed to different levels of eutrophication and sedimentation can support similar benthic communities. Mar Pollut Bull 92(2015):212–221
Santiago-Valentín JD, Colley SB, Glynn PW, Cupul-Magaña AL, López-Pérez RA, Rodríguez-Zaragoza FA, Benítez-Villalobos F, Bautista-Guerrero E, Zavala-Casas DA, Rodríguez-Troncoso AP (2018) Regional and species specific sexual reproductive patterns of three zooxanthellate scleractinian corals across the Eastern Tropical Pacific. Mar Ecol 39(2):e12497
Spalding M, Burke L, Wood SA, Ashpole J, Hutchinson J, zu Ermgassen P (2017) Mapping the global value and distribution of coral reef tourism. Mar Policy 82(2017):104–113
StatSoft, Inc. (2007). STATISTICA (data analysis software system), version 8.0. www.statsoft.com.
Stimson J, Larned ST, Conklin E (2001) Effects of herbivory, nutrient levels, and introduced algae on the distribution and abundance of the invasive macroalga Dictyosphaeria cavernosa in Kaneohe Bay. Hawaii Coral Reefs 19:343–357
Strickland JDH, Parsons TR (1972) A practical handbook of seawater analysis. Fisheries research board of Canada, Ottawa
Sully S, van Woesik R (2019) Turbid reefs moderate coral bleaching under climate-related temperature stress. Glob Change Biol 26(3):1367–1373
Todd PA, Ong X, Chou LM (2010) Impacts of pollution on marine life in Southeast Asia. Biodivers Conserv 19:1063–1082
Tortolero-Langarica JJA, Rodríguez-Troncoso AP, Cupul-Magaña AL, Carricart-Ganivet JP (2017) Calcification and growth rate recovery of the reef-building Pocillopora species in the northeast tropical Pacific following an ENSO disturbance. Peer J 5:e3191
Veron JEN (2000) Corals of the world. Australian Institute of Marine Science, Townsville
Veron J, Stafford-Smith M, DeVantier L, Turak E (2015) Overview of distribution patterns of zooxanthellate Scleractinia. Front Mar Sci 1:81
Ward S (1995) The effect of damage on the growth, reproduction and storage of lipids in the scleractinian coral Pocillopora damicornis (Linnaeus). J Exp Mar Biol Ecol 187(1995):193–206
Wear SL, Thurber RV (2015) Sewage pollution: mitigation is key for coral reef stewardship. Ann N Y Acad Sci 1355(2015):15–30
Wiedenmann J, D’Angelo C, Smith EG, Hunt AN, Legiret FE, Postle AD, Achterberg EP (2013) Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat Clim Chang 3(2):160–164
Wooldridge SA (2014) Assessing coral health and resilience in a warming ocean: why looks can be deceptive. Bio Essays 36:1041–1049
Zar JH (2010) Biostatistical analysis. Prentice Hall, New Jersey
Acknowledgments
VMC received a Ph.D. scholarship from the Centro Nacional de Ciencia y Tecnología while conducting the study and writing the manuscript (ID. 332939). The present research was supported by two National Geographic Society Grants (NGS-55349R-19 to APRT and EC-51496C-18 to VMC), and by the project “Restauración de Arrecifes Coralinos en el PN Islas Marietas” (PROCER/CCER/DROPC/09/2016) to ALCM. Coral sampling was performed under permit PPF/DGOPA-061/18. The authors thank the organization “Protección y Restauración de Islas y Zonas Naturales” (PROZONA A.C.) for their assistance in field operations. Also, the authors kindly thank Diana Morales de Anda and Vladimir Pérez de Silva for their assistance and advice with the statistical analyses, and the comments of Jared Johnson and two anonymous reviewers that greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interests.
Additional information
Topic Editor John A. Burt
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Martínez-Castillo, V., Rodríguez-Troncoso, A.P., Santiago-Valentín, J.D. et al. The influence of urban pressures on coral physiology on marginal coral reefs of the Mexican Pacific. Coral Reefs 39, 625–637 (2020). https://doi.org/10.1007/s00338-020-01957-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-020-01957-z