Abstract
Outbreaks of crown-of-thorns starfish (Acanthaster spp.) represent a major threat to coral reef ecosystems throughout the Indo-Pacific, and there is significant interest in whether no-take marine reserves could moderate the frequency or severity of outbreaks. Herein, we investigate whether the incidence and severity of sublethal injuries among juvenile Pacific crown-of-thorns starfish (Acanthaster cf. solaris, max diameter = 45 mm) differs between areas that are open versus closed to fishing, between microhabitats (i.e. dead coral substratum versus live coral) and with body size. The majority (180 out of 200) of juvenile starfish had conspicuous injuries, presumably caused by predation. The incidence of injuries in juvenile starfish was negatively related to body size, but links between body size and severity of injuries were only evident in individuals collected from dead coral microhabitats. Small (3 mm radius) starfish from dead coral microhabitats had injuries to 68.06% of arms, compared to 12.00% of arms in larger (12 mm radius) starfish from the same microhabitat. Juvenile starfish associated with dead coral habitats had a higher incidence (95 vs. 87% respectively) and severity (i.e. the percentage of injured arms; 21 vs. 6%) of injuries, compared to those associated with live corals. Interestingly, there was no difference in the incidence or severity of injuries between areas that are open versus closed to fishing. Our results show that small juvenile A. cf. solaris are extremely vulnerable to sublethal, if not lethal, predation, and predation risk declines as they grow and change their microhabitat. Predation during and immediately following settlement is, therefore, likely to have a major influence on population dynamics and ontogenetic changes in microhabitat use for A. cf. solaris.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crown-of-thorns starfishes (CoTS, Acanthaster spp.) exhibit boom-and-bust population dynamics (Antonelli and Kazarinoff 1984; Bradbury et al. 1985; Pratchett et al. 2014; Condie et al. 2018). Given their diet of reef-building or scleractinian corals (Chesher 1969; Branham et al. 1971), CoTS have a major influence on coral reef ecosystems during population booms or outbreaks (e.g. Chesher 1969; De’ath et al. 2012; Baird et al. 2013). While CoTS outbreaks are increasingly considered to be a natural phenomenon (Pratchett et al. 2018), debate continues about the extent to which human activities, such as fishing (e.g. Endean 1969; Sweatman 2008; Vanhatalo et al. 2017) or runoff from catchments with intensive agriculture (Brodie et al. 2005, 2017; Fabricius et al. 2010), contribute to increased frequency or severity of outbreaks (Pratchett et al. 2014). One of the earliest and foremost hypotheses to explain population outbreaks of CoTS is the predator removal hypothesis (Endean 1969), suggesting that declines in the abundance of predators, and concomitant increases in survivorship of CoTS, may cause or exacerbate population outbreaks (e.g. Endean 1969; Dulvy et al. 2004; Sweatman 2008; Cowan et al. 2017a; Vanhatalo et al. 2017). Declines in the abundance of predatory organisms is generally ascribed to direct exploitation (Endean 1969), but might also occur due to trophic cascades (Dulvy et al. 2004) or general degradation of reef ecosystems (Mendonça et al. 2010; Cowan et al. 2017a). For example, Dulvy et al. (2004) showed that when densities of predatory fishes declined along a fishing intensity gradient by 61% at the most heavily fished sites in Fiji, CoTS densities increased by three orders of magnitude. Similarly, CoTS outbreaks were shown to have occurred 3.75 times more often on midshelf reefs of Australia’s Great Barrier Reef (GBR) that were open to fishing (albeit restricted) compared to those that were closed to fishing (no-take reefs) (Sweatman 2008).
The initial formulation of the predator removal hypothesis (sensu Endean 1969) was focussed on predators that were capable of killing adult CoTS. Endean (1969) suggested that giant triton (Charonia tritonis) were one of the few predators that attack and completely consume healthy adult starfish, albeit smaller individuals. Excessive harvesting of giant triton, therefore, may have allowed for increased densities and larger sizes of CoTS (Endean 1969), which together would have greatly increased reproductive output (Babcock et al. 2016). Subsequent studies have revealed many more reef organisms (including fishes and invertebrates) that will feed on healthy adult CoTS (reviewed by Cowan et al. 2017a), though it is still unclear to what extent they actually kill adult starfish. Nonetheless, predation may contribute to reductions in population size (and may moderate outbreaks) in several ways, including (1) direct reductions in local densities of larvae, juveniles or adults; (2) reducing individual size and fecundity through partial predation; or (3) disrupting normal feeding or spawning behaviour. Moreover, predation rates on CoTS may be particularly pronounced during early life history stages (including settlement and early post-settlement stages) and have an important influence on population dynamics (Wilmes et al. 2018).
Estimates of natural rates of predation on early life history stages of CoTS (and variation therein) are scarce, largely due to difficulties in detecting and following the fate of juvenile starfish in situ (Wilmes et al. 2018). To date, estimates of predation on newly settled CoTS (i.e. 0+ starfish in their first year post-settlement) have been derived from aquarium studies or experimental studies in which captive naïve starfish were deployed in the field (e.g. Sweatman 1995). Aquarium studies indicate that both gametes and larvae of CoTS may be heavily preyed upon by planktivorous reef fishes, such as damselfishes (Cowan et al. 2016b, 2017b). In turn, settling larvae and post-metamorphic juvenile CoTS are likely to experience strong predation pressure from benthic invertebrates (Yamaguchi 1973), and this is supported by the results of an aquarium study which investigated predation on settling CoTS larvae by polychaetes and trapeziid crabs (Cowan et al. 2016a). Field estimates of predation rates on captive-bred naïve 0+ starfish indicate that predation rates generally decrease with body size but are highly variable (Keesing and Halford 1992; Keesing et al. 1996, 2018). For instance, predation rates by epibenthic fauna were highest for small 1-month-old starfish (mean diameter = 1.1 mm) at 5.05% d−1 and decreased to 0.85% d−1 for larger 4-month-old starfish (mean diameter = 2.7 mm) (Keesing and Halford 1992). Notably, predation rates on 1-month-old starfish varied markedly among small habitat units, indicating that the composition and/or abundance of cryptofaunal predators (e.g. polychaetes and crustaceans) varied substantially within these units (Keesing et al. 1996).
Although the vulnerability of juvenile CoTS to different types of predators is expected to change as they grow (Keesing 1995), very little is known about how body size and ontogenetic shifts in microhabitat (i.e. from coralline algae encrusted pieces of dead coral to live scleractinian coral) affect predation on wild 0+ CoTS. Despite apparent evolutionary adaptations in behaviour (i.e. cryptic and nocturnal) and colouration to evade diurnal visual predators such as fishes (Yamaguchi 1973; Zann et al. 1987; Stump 1996), observations of predation on small juvenile CoTS, especially on 0+ starfish, have been limited (Endean 1969; Pearson and Endean 1969; Zann et al. 1987; Sweatman 1995). Aquarium studies indicate, however, that small juvenile CoTS (i.e. diameter < 70 mm) are vulnerable to predation, mostly by crustaceans (e.g. hermit crabs and spiny lobster—Zann et al. 1987; Hymenocera shrimp—Keesing et al. 2018).
The objective of this study was to quantify the incidence and severity of injuries in newly settled CoTS (0+ starfish) and assess whether rates of injury (and thereby predation rates) vary with zoning status (i.e. no-take marine reserves versus restricted fishing zones), microhabitat (i.e. dead coral substratum versus live scleractinian coral) and/or body size. Although sublethal predation was not witnessed per se, it is generally assumed that sublethal injuries in starfish result from predatory attacks or defensive interactions (Lawrence and Vasquez 1996). High incidence of sublethal injuries is therefore thought to reflect intense predation on starfish (McCallum et al. 1989; Bos et al. 2011; Rivera-Posada et al. 2014), such that high rates of partial injury may be a proxy for high levels of overall mortality from predation. While the frequency and extent of injuries has been used previously as proxy for predation pressure among subadult and adult CoTS (Rivera-Posada et al. 2014; Messmer et al. 2017), no such investigation has been conducted for 0+ CoTS.
Materials and methods
Sampling and definition of injured arms
A total of 200 juvenile starfish (max diameter = 45 mm) were sampled from an extensive collection of 0+ CoTS from reefs in the northern GBR during outbreaking conditions in 2015 (see Wilmes et al. 2016). All starfish were collected on SCUBA (max depth = 15 m), with searches focussing in reef slope areas where there was unconsolidated coral rubble, interspersed with patches of consolidated carbonate and live corals. All starfish were measured (diameter to the nearest mm) and preserved in 95% ethanol. During collection, we explicitly distinguished between individuals recovered from dead coral substratum (mostly coral rubble) encrusted with coralline algae, which were presumed to be feeding on coralline algae, versus those living within live (mostly branching) scleractinian coral, presumed to be feeding on coral (Wilmes et al. 2016).
To test for variation in the incidence and severity of injuries, we sampled 100 0+ starfish from each of the two microhabitats (i.e. pieces of dead coral substratum versus live coral colonies). All starfish were collected between 11 October and 15 December 2015 from 19 reefs located between 15.51 and 17.67°S (Table 1). Of the 200 0+ starfish, 76 originated from marine national park zones (no-take) and 124 from restricted fishing zones (habitat protection and conservation park zones). Starfish were photographed (Olympus OM-D E-M5) for image analysis in ImageJ1. Sampling was intentionally biased towards the largest individuals to avoid confusion between newly forming versus regenerating arms. Because several 0+ starfish were severely injured and regeneration of lost body arms is prioritised over somatic growth in starfish (Lawrence and Lane 1982; Diaz-Guisado et al. 2006), maximum radius was chosen, over diameter, as a more robust indicator for body size at the time of injury. For each starfish, the number of arms was counted, and its maximum radius was measured from the centre of the aboral disc area to the tip of the longest arm (Fig. 1b). Injured arms were defined as those that were > 10% shorter than the maximum radius (Fig. 1b), following Bos et al.'s (2011) criterion for the identification of injured arms, which conformed most with our visual identification of injuries.
Statistical analyses
Incidence of sublethal injuries
The incidence of sublethal injuries was modelled as the presence/absence of injuries against the maximum radius of individuals to test whether body size affected the probability of injury incidence in 0+ starfish. Modelling was conducted using a generalised linear mixed effects model (glmmPQL function from the MASS package—Venables and Ripley 2002) with a binomial error distribution and a logit link function, conducted in R (R Core Team 2018). Reef was included as a random effect to account for variations in the incidence ~ size relationship among reefs, while explicitly testing for differences between no-take marine reserves and restricted fishing zones (habitat protection and conservation park zones). Goodness of fit was evaluated by comparing the sum of the squared Pearson’s residuals to chi-squared (lack of fit if p value < 0.05). The significance of fixed effects was based on p values (significant if p < 0.05), as provided by the summary function.
To test for variation in the incidence of different levels of sublethal injuries among 0+ starfish from different microhabitats and marine park zones, injuries were divided into five categories of severity depending on the percentage of injured arms: 0% (no arms injured), > 0–25%, > 25–50%, > 50–75% and > 75–100%. Frequencies of 0+ starfish in different injury severity categories were then compared between different microhabitats and marine park zones separately, using Pearson’s Chi-squared test of independence. As sample sizes between no-take (n = 76) and restricted fishing zones (n = 124) differed, frequency data are displayed as percentages for ease of interpretation.
Severity of sublethal injuries
The proportion of injured arms (as proxy for injury severity) was modelled against the maximum radius to test whether injury severity was related to body size in 0+ starfish. Modelling was again conducted in R, using generalised linear mixed-effects models (glmmPQL function) with a binomial error distribution and logit link function. In addition, zoning (i.e. no-take zone versus restricted fishing zone) was added in the model as an additive fixed effect, to test for an effect of zoning on the injury severity ~ size relationship. Because the dietary shift of 0+ starfish from coralline algae to scleractinian coral is size-related (size threshold ~ 8–10 mm, Yamaguchi 1974) and is accompanied by a shift in microhabitat (i.e. from dead coral substratum to live scleractinian coral), microhabitat and size are inevitably correlated. As a result, injury severity was modelled separately for 0+ starfish from different microhabitats. All models included reef (n = 19) as a random effect and goodness of fit was again evaluated by comparing the sum of the squared Pearson’s residuals to Chi–squared (lack of fit if p value < 0.05), and by inspecting diagnostic plots of residuals (see Logan 2011). The significance of fixed effects was based on p values (significant if p < 0.05), as provided by the summary function.
Results
Incidence of sublethal injuries
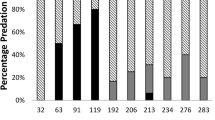
The proportion of juvenile CoTS with injuries in samples from the 19 reefs varied between 78.57 and 100% (median = 91.67%, IQR = 90.91–100%) (Table 1). The incidence of injury was negatively related to body size (p < 0.05) and most 0+ starfish showed signs of sublethal injuries (i.e. 91%); overall, only 9% were intact (Fig. 2). The incidence of injuries was slightly higher in 0+ starfish that were found among pieces of dead coral (95%), than in those associated with live coral (87%). The frequency of 0+ starfish in different injury severity categories differed significantly between microhabitats; X2 (df = 4, n = 200) = 15.358, p = 0.004. Notably, 21% of starfish found in dead coral showed signs of severe sublethal injuries (i.e. > 50% arms injured), compared to 6% of starfish found in live coral (Fig. 2). By contrast, a higher proportion of starfish associated with live coral (63%) showed no or minor signs of injury (i.e. 0–25% of arms injured), compared to 44% of individuals that were found associated with dead coral. We found no indication that the frequency of 0+ starfish in different injury severity categories differed between no-take and restricted fishing zones (Fig. 3); X2 (df = 4, n = 200) = 2.366, p = 0.669.
Severity of sublethal injuries
The median percentage of injured arms in 0+ starfish varied among reefs, ranging from 12.5% and 85.71% (median = 26.32%, IQR = 19.06–30.77%, Table 1). Injury severity was significantly related to size in 0+ starfish, especially for starfish found in dead coral (Table 2, Fig. 4). For starfish from dead coral microhabitats, the model estimates that 68.06% (CI [54.01–79.45%]) of arms were injured in small starfish (3 mm radius), compared to just 12.00% (CI [7.14–19.47%]) in larger starfish (12 mm radius). While the proportion of injured arms in starfish found in live coral tended to decline with increasing body size, this trend was not significant (Table 2, Fig. 4). Marine park zoning did not significantly affect the proportion of injured arms in 0+ starfish.
Discussion
This study shows that the incidence and severity of sublethal injuries in 0+ starfish (3–22 mm radius) decreases with increasing body size and was largely driven by changes in the extent of injuries in starfish (3–12 mm radius) from dead coral microhabitats. The incidence and severity of injuries were higher for starfish associated with dead coral than for larger starfish that had transitioned to feeding on, and living among, live corals. Although predatory attacks were not witnessed per se, differences in the incidence and severity of injuries among starfish from the two microhabitats may be explained by differences in the composition and abundance of potential predators, including cryptofaunal invertebrates (Enochs and Manzello 2012; Takada et al. 2012) and fish assemblages (e.g. Messmer et al. 2011; Komyakova et al. 2018). However, as body size and microhabitat are inevitably confounded, the reduced incidence and severity of injuries among juveniles from the live coral habitat may also, at least in part, be explained by their inherently larger size and faster growth rates (Yamaguchi 1974). Interpretation of our results is further obfuscated by the fact that sublethal injuries in starfish associated with live coral may have been carried across from the dead coral microhabitat or could have resulted from defensive interactions with coral polyps during the transition to live coral (Yamaguchi 1974). Nonetheless, coral-rubble-dominated dead coral habitats are extremely diverse environments that support high numbers of predatory cryptofaunal invertebrates and fishes (Takada et al. 2012; Enochs and Manzello 2012). Species richness of motile cryptofauna is indeed estimated to be greater in dead coral habitats than in live coral habitats (Enochs and Manzello 2012). Our results show that 0+ CoTS living on live corals have lower incidence of injuries compared to those on dead corals, suggesting that there may be increased densities of predators and higher rates of predation in rubble habitats.
These results suggest that as for other marine invertebrates and fishes, population bottlenecks (i.e. major reductions in population size) are likely to occur during settlement and the early post-settlement phase of the CoTS life cycle, when settling and newly settled individuals are smallest and most vulnerable (e.g. Gosselin and Qian 1997; Almany and Webster 2006). Indeed, the incidence of injuries in 0+ starfish (i.e. 91%) from this study was much higher than the incidence of injuries previously reported from subadult and adult CoTS populations from the GBR (i.e. 33–51%—Pearson and Endean 1969; McCallum et al. 1989; Stump 1996; Messmer et al. 2017). This suggests that the predation pressure during and immediately after settlement has a marked influence on population dynamics of crown-of-thorns starfish (see also Keesing and Halford 1992; Cowan et al. 2017a; Wilmes et al. 2018; Keesing et al. 2018).
While individuals that experience sublethal injuries may survive and ultimately reproduce, the regeneration of lost and/or damaged body components incurs an energetic cost. Depending on the life history stage and severity of the injuries, sublethal injuries can constrain the capacity to forage (Ramsay et al. 2001) and limit energy available for growth and reproduction (Lawrence and Lane 1982; Lawrence and Vasquez 1996; Bingham et al. 2000; Diaz-Guisado et al. 2006; Barrios et al. 2008). For instance, feeding and growth rates were demonstrated to be substantially reduced in severely injured (i.e. 6 arms lost, ~ 33% of total arms) juveniles (~ 20 mm radius, 17–27 arms) of the South American sun-star, Heliaster helianthus, while moderately injured (i.e. 3 arms lost, ~ 17%) starfish showed similar feeding and growth rates to intact ones (Barrios et al. 2008). Recovery was slow in severely injured starfish and even after 5 months, feeding rates were ~ 30% lower than those of intact starfish, resulting in comparatively low growth rates (Barrios et al. 2008). By comparison, recovery rates of injured arms in juvenile CoTS are essentially unknown and limited to a sole record of a 90-mm juvenile/subadult CoTS from the GBR, which was able to regenerate an injured arm (i.e. 4 times shorter than its average arm length) within 3 months, while increasing its overall diameter by 36 mm (Pearson and Endean 1969).
Most newly settled starfish sampled from the dead coral microhabitat (i.e. 74%) showed minor to moderate signs of sublethal injuries (i.e. > 0–50% of arms damaged), and a substantial proportion of starfish (i.e. 21%) were severely injured, with some even missing parts of their central disc (Fig. 2). Such severely injured starfish are not just extremely likely to have a reduced capacity to escape predators, but also to have a reduced capacity to forage that would likely negatively affect growth and ultimately size-related reproductive output and chances of survival. Severe sublethal injuries could even substantially delay the timing of the ontogenetic dietary shift from coralline algae to live coral, especially if injuries result in body size being reduced below the size threshold of the ontogenetic dietary shift (i.e. 8–10 mm in diameter—Yamaguchi 1974). In turn, this may have important flow-on effects on population dynamics, considering that marked delays in the ontogenetic dietary shift are likely to negatively affect individuals’ lifetime fitness and ultimately constrain population growth.
We found no evidence that the incidence and/or severity of sublethal injuries in 0+ starfish differed significantly between areas that were open versus closed to fishing (Table 2). This finding is consistent with the results of a previous study on sublethal injuries in subadult and adult starfish populations from the GBR (n = 3846), which detected no significant difference in the incidence or severity of sublethal injures among starfish from different marine park zones (Messmer et al. 2017). While coral trout (Plectropomus spp.), the primary target species on fished reefs of the GBR, has consistently been reported to be more abundant in no-take marine reserves (Williamson et al. 2004; Russ et al. 2008; Emslie et al. 2015), this piscivorous fish is unlikely to prey on 0+ CoTS. Rather, it has been suggested that declines in densities of coral trout result in increased densities of benthic carnivorous fishes, such as wrasses, and subsequent flow-on reductions in densities of coral rubble inhabiting invertebrates that prey on 0+ starfish, thereby effectively releasing predation pressure on 0+ starfish (Sweatman 2008). Effect sizes of no-take marine reserves on densities of benthic foragers are, however, small and inconsistent between inshore and offshore reefs of the GBR (Emslie et al. 2015), and evidence in support of mesopredator release or prey release is weak (Rizzari et al. 2015; Casey et al. 2017), suggesting limited top-down control on reef fish and benthic assemblages on the GBR (Emslie et al. 2015; Rizzari et al. 2015; Casey et al. 2017). Nonetheless, sublethal injuries may not be directly correlated with mortality and potential differences in survivorship of 0+, and older starfish among marine park zones may therefore simply not be detectable with this approach.
Our study results have been difficult to interpret, mostly owing to the fact that predatory attacks were not actually observed within respective microhabitats. Also, both body size at the time of injury and the severity of injuries in 0+ starfish may be underestimated, as regeneration and regrowth could have occurred since the predatory attack. Determining whether or not the observed higher frequency of CoTS outbreaks on fished midshelf reefs of the GBR is indeed related to increased survivorship of 0+ starfish that live among pieces of dead coral (Sweatman 2008), will therefore necessitate ongoing in situ measuring of settlement and post-settlement survivorship of 0+ starfish cohorts among reefs that are open and closed to fishing, across different types of habitats and starfish densities, and during different stages of the outbreak cycle. Supporting information on the identity and relative abundance of predators among habitats and between differently zoned reefs is also required. Difficulties associated with detecting 0+ starfish have greatly hindered previous attempts to monitor CoTS field populations on the GBR (Doherty and Davidson 1988; Johnson et al. 1992). However, the proven ability to detect herbivorous 0+ starfish (Zann et al. 1987, 1990; Habe et al. 1989; Wilmes et al. 2016) provides an opportunity to fill critical knowledge gaps and improve understanding and management of crown-of-thorns starfish outbreaks.
References
Almany GR, Webster MS (2006) The predation gauntlet: early post-settlement mortality in reef fishes. Coral Reefs 25:19–22
Antonelli PL, Kazarinoff ND (1984) Starfish predation of a growing coral reef community. J Theor Biol 107:667–684
Babcock RC, Milton DA, Pratchett MS (2016) Relationships between size and reproductive output in the crown-of-thorns starfish. Mar Biol 163:3–9
Baird AH, Pratchett MS, Hoey AS, Herdiana Y, Campbell SJ (2013) Acanthaster planci is a major cause of coral mortality in Indonesia. Coral Reefs 32:803–812
Barrios JV, Gaymer CF, Vásquez JA, Brokordt KB (2008) Effect of the degree of autotomy on feeding, growth, and reproductive capacity in the multi-armed sea star Heliaster helianthus. J Exp Mar Bio Ecol 361:21–27
Bingham BL, Burr J, Head HW (2000) Causes and consequences of arm damage in the sea star Leptasterias hexactis. Can J Zool 78:596–605
Bos AR, Gumanao GS, van Katwijk MM, Mueller B, Saceda MM, Tejada RLP (2011) Ontogenetic habitat shift, population growth, and burrowing behavior of the Indo-Pacific beach star, Archaster typicus (Echinodermata; Asteroidea). Mar Biol 158:639–648
Bradbury R, Hammond L, Moran P, Reichelt R (1985) Coral reef communities and the crown-of-thorns starfish: evidence for qualitatively stable cycles. J Theor Biol 113:69–80
Branham JM, Reed SA, Bailey JH, Caperon J (1971) Coral-eating sea stars Acanthaster planci in Hawaii. Science 172:1155–1157
Brodie J, Fabricius K, De’ath G, Okaji K (2005) Are increased nutrient inputs responsible for more outbreaks of crown-of-thorns starfish? An appraisal of the evidence. Mar Pollut Bull 51:266–278
Brodie JE, Devlin M, Lewis S (2017) Potential enhanced survivorship of crown-of-thorns starfish larvae due to near-annual nutrient enrichment during secondary outbreaks on the central mid-shelf of the Great Barrier Reef. Australia. Diversity 9:17
Casey JM, Baird AH, Brandl SJ, Hoogenboom MO, Rizzari JR, Frisch AJ, Mirbach CE, Connolly SR (2017) A test of trophic cascade theory: fish and benthic assemblages across a predator density gradient on coral reefs. Oecologia 183:161–175
Chesher RH (1969) Destruction of Pacific corals by the sea star Acanthaster planci. Science 165:280–283
Condie SA, Plagányi ÉE, Morello EB, Hock K, Beeden R (2018) Great Barrier Reef recovery through multiple interventions. Conserv Biol 32:1356–1367
Cowan Z-L, Dworjanyn S, Caballes C, Pratchett M (2016a) Benthic predators influence microhabitat preferences and settlement success of crown-of-thorns starfish (Acanthaster cf. solaris). Diversity 8:27
Cowan ZL, Dworjanyn SA, Caballes CF, Pratchett MS (2016b) Predation on crown-of-thorns starfish larvae by damselfishes. Coral Reefs 35:1253–1262
Cowan Z, Pratchett M, Messmer V, Ling S (2017a) Known predators of crown-of-thorns starfish (Acanthaster spp.) and their role in mitigating, if not preventing, population outbreaks. Diversity 9:7
Cowan ZL, Ling SD, Dworjanyn SA, Caballes CF, Pratchett MS (2017b) Interspecific variation in potential importance of planktivorous damselfishes as predators of Acanthaster sp. eggs. Coral Reefs 36:653–661
De’ath G, Fabricius KE, Sweatman H, Puotinen M (2012) The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc Natl Acad Sci U S A 109:17995–9
Diaz-Guisado D, Gaymer CF, Brokordt KB, Lawrence JM (2006) Autotomy reduces feeding, energy storage and growth of the sea star Stichaster striatus. J Exp Mar Bio Ecol 338:73–80
Doherty PJ, Davidson J (1988) Monitoring the distribution and abundance of juvenile Acanthaster planci. In: Proceedings 6th international coral reef symposium August. vol 2, pp 131–136
Dulvy NK, Freckleton RP, Polunin NVC (2004) Coral reef cascades and the indirect effects of predator removal by exploitation. Ecol Lett 7:410–416
Emslie MJ, Logan M, Williamson DH, Ayling AM, MacNeil MA, Ceccarelli D, Cheal AJ, Evans RD, Johns KA, Jonker MJ, Miller IR, Osborne K, Russ GR, Sweatman HPA (2015) Expectations and outcomes of reserve network performance following re-zoning of the Great Barrier Reef Marine Park. Curr Biol 25:983–992
Endean R (1969) Report on investigations made into aspects of the current Acanthaster planci (crown-of-thorns) infestations of certain reefs of the Great Barrier Reef. Fisheries Branch, Queensland Department of Primary Industries, Brisbane, p 38
Enochs IC, Manzello DP (2012) Species richness of motile cryptofauna across a gradient of reef framework erosion. Coral Reefs 31:653–661
Fabricius KE, Okaji K, De’ath G (2010) Three lines of evidence to link outbreaks of the crown-of-thorns seastar Acanthaster planci to the release of larval food limitation. Coral Reefs 29:593–605
Gosselin LA, Qian PY (1997) Juvenile mortality in benthic marine invertebrates. Mar Ecol Prog Ser 146:265–282
Habe T, Yamamoto G, Nagai A, Kosaka M, Ogura M, Sawamoto S, Ueno S, Yokochi H (1989) Studies on the conservation and management of coral reefs and the control of Acanthaster planci juveniles. Report of Grant-in-Aid for Scientific Research, Ministry of Education, Science and Culture, Japan pp 158–186
Johnson DB, Moran PJ, Baker VJ, Christie CA, Miller IR, Miller-Smith BA, Thompson AA (1992) An attempt to locate high density populations of juvenile crown-of-thorns starfish (Acanthaster planci) on the central Great Barrier Reef. Coral Reefs 11:122
Keesing JK (1995) Temporal patterns in the feeding and emergence behaviour of the crown-of-thorns starfish Acanthaster planci. Mar Freshw Behav Physiol 25:209–232
Keesing JK, Halford AR (1992) Field measurement of survival rates of juvenile Acanthaster planci: techniques and preliminary results. Mar Ecol Prog Ser 85:107–114
Keesing JK, Wiedermeyer WL, Okaji K, Halford AR, Hall KC, Cartwright CM (1996) Mortality rates of juvenile starfish Acanthaster planci and Nardoa spp. measured on the Great Barrier Reef, Australia and in Okinawa, Japan. Oceanol Acta 19:441–448
Keesing JK, Halford AR, Hall KC (2018) Mortality rates of small juvenile crown-of-thorns starfish Acanthaster planci on the Great Barrier Reef: implications for population size and larval settlement thresholds for outbreaks. Mar Ecol Prog Ser 597:179–190
Komyakova V, Jones GP, Munday PL (2018) Strong effects of coral species on the diversity and structure of reef fish communities: a multi-scale analysis. PLoS One 13:1–20
Lawrence JM, Lane JM (1982) The utilization of nutrients by post metamorphic echinoderms. In: Jangoux M, Lawrence JM (eds) Echinoderm nutrition. CRC Press, Boca Raton, pp 331–371
Lawrence J, Vasquez J (1996) The effect of sublethal predation on the biology of echinoderms. Oceanol Acta 19:431–440
Logan M (2011) Biostatistical Design and analysis using R : a practical guide. Wiley, Amsterdam, p 546
McCallum HI, Endean R, Cameron A (1989) Sublethal damage to Acanthaster planci as an index of predation pressure. Mar Ecol Prog Ser 56:29–36
Mendonça VM, Jabri MM, Ajmi I, Muharrami M, Areimi M, Aghbari HA (2010) Persistent and expanding population outbreaks of the corallivorous starfish Acanthaster planci in the Northwestern Indian Ocean: Are they really a consequence of unsustainable starfish predator removal through overfishing in coral reefs, or a response to a changing environment? Zool Stud 49:108–123
Messmer V, Jones GP, Munday PL, Holbrook SJ, Schmitt RJ (2011) Habitat biodiversity as a determinant of fish community structure on coral reefs. Ecology 92:2285–2298
Messmer V, Pratchett M, Chong-Seng K (2017) Variation in incidence and severity of injuries among crown-of-thorns starfish (Acanthaster cf. solaris) on Australia’s Great Barrier Reef. Diversity 9:12
Pearson RG, Endean R (1969) A preliminary study of the coral predator Acanthaster planci (L.) (Asteroidea) on the Great Barrier Reef. Fisheries Notes, Queensland Department of Harbours and Marine, pp 1–38
Pratchett MS, Caballes CF, Posada JAR, Sweatman HPA (2014) Limits to understanding and managing outbreaks of crown-of-thorns starfish (Acanthaster spp.). Oceanogr Mar Biol An Annu Rev 52:133–200
Pratchett MS, Lang BJ, Matthews S (2018) Culling crown-of-thorns starfish (Acanthaster cf. solaris) on Australia’s Great Barrier Reef: rationale and effectiveness. Aust Zool 40:13–24
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing
Ramsay AK, Kaiser MJ, Richardson CA (2001) Invest in arms : behavioural and energetic implications of multiple autotomy in starfish (Asterias rubens). Behav Ecol Sociobiol 50:360–365
Rivera-Posada J, Caballes CF, Pratchett MS (2014) Size-related variation in arm damage frequency in the crown-of-thorns sea star, Acanthaster planci. J Coast Life Med 2:187–195
Rizzari JR, Bergseth BJ, Frisch AJ (2015) Impact of conservation areas on trophic interactions between apex predators and herbivores on coral reefs. Conserv Biol 29:418–429
Russ GR, Cheal AJ, Dolman AM, Emslie MJ, Evans RD, Miller I, Sweatman H, Williamson DH (2008) Rapid increase in fish numbers follows creation of world’s largest marine reserve network. Curr Biol 18:514–515
Stump RJW (1996) An investigation to describe the population dynamics of Acanthaster planci (L.) around Lizard Island, Cairns section, Great Barrier Reef Marine Park. CRC Reef Research Centre, Technical Report No. 10, Townsville, 56 pp
Sweatman HPA (1995) A field study of fish predation on juvenile crown-of-thorns starfish. Coral Reefs 14:47–53
Sweatman H (2008) No-take reserves protect coral reefs from predatory starfish. Curr Biol 18:598–599
Takada Y, Abe O, Shibuno T (2012) Variations in cryptic assemblages in coral-rubble interstices at a reef slope in Ishigaki Island, Japan. Fish Sci 78:91–98
Vanhatalo J, Hosack GR, Sweatman H (2017) Spatiotemporal modelling of crown-of-thorns starfish outbreaks on the Great Barrier Reef to inform control strategies. J Appl Ecol 54:188–197
Venables WN, Ripley BD (2002) Modern Applied statistics with S. Sringer, New York
Williamson DH, Russ GR, Ayling AM (2004) No-take marine reserves increase abundance and biomass of reef fish on inshore fringing reefs of the Great Barrier Reef. Environ Conserv 31:149–159
Wilmes J, Matthews S, Schultz D, Messmer V, Hoey A, Pratchett M (2016) Modelling growth of juvenile crown-of-thorns starfish on the northern Great Barrier Reef. Diversity 9:172–182
Wilmes JC, Caballes CF, Cowan ZL, Hoey AS, Lang BJ, Messmer V, Pratchett MS (2018) Contributions of pre- versus post-settlement processes to fluctuating abundance of crown-of-thorns starfishes (Acanthaster spp.). Mar Pollut Bull 135:332–345
Yamaguchi M (1973) Early life histories of coral reef asteroids, with special reference to Acanthaster planci (L.). In: Jones OA, Endean R (eds) Biology and geology of coral reefs. Academic Press Inc, New York, pp 369–387
Yamaguchi M (1974) Growth of juvenile Acanthaster planci (L.) in the laboratory. Pacific Sci 28:123–138
Zann L, Brodie J, Berryman C, Naqasima M (1987) Recruitment, ecology, growth and behavior of juvenile Acanthaster planci (L.) (Echinodermata: Asteroidea). Bull Mar Sci 41:561–575
Zann L, Brodie J, Vuki V (1990) History and dynamics of the crown-of-thorns starfish Acanthaster planci (L.) in the Suva area. Fiji. Coral Reefs 9:135–144
Acknowledgements
This work was funded by the Australian Government Research Training Program Scholarship, the ARC Centre of Excellence for Coral Reef Studies, James Cook University, the Australian Museum’s Lizard Island Research Station and the National Environmental Science Program. We would like to acknowledge, in particular, Daniel Schultz for his contributions made to sampling 0+ starfish in the field and constructive comments on the manuscript. We also thank the Association of Marine Park Tourism Operators for logistical support provided during field sampling and reviewers for improving the quality of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Topic Editor Simon Davy
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wilmes, J.C., Hoey, A.S., Messmer, V. et al. Incidence and severity of injuries among juvenile crown-of-thorns starfish on Australia’s Great Barrier Reef. Coral Reefs 38, 1187–1195 (2019). https://doi.org/10.1007/s00338-019-01845-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-019-01845-1