Abstract
High-latitude coral communities are distinct from their tropical counterparts, and how they respond to recent heat wave events that have decimated tropical reefs remains unknown. In Australia, the 2016 El Niño resulted in the largest global mass coral bleaching event to date, reaching as far south as Sydney Harbour (~ 34°S). Coral bleaching was observed for the first time (affecting ca., 60% of all corals) as sea surface temperatures in Sydney Harbour remained > 2 °C above the long-term mean summer maxima, enabling us to examine whether high-latitude corals bleached in a manner described for tropical corals. Responses of the geographically cosmopolitan Plesiastrea versipora and southerly restricted Coscinaraea mcneilli were contrasted across two harbour sites, both in situ and among samples-maintained ex situ in aquaria continually supplied with Sydney Harbour seawater. While both coral taxa hosted the same species of microalgal endosymbiont (Breviolum spp; formerly clade B), only P. versipora bleached both in situ and ex situ via pronounced losses of endosymbiont cells. Both species displayed very different metabolic responses (growth, photosynthesis, respiration and calcification) and bleaching susceptibilities under elevated temperatures. Bacterial microbiome profiling, however, revealed a convergence of bacterial community composition across coral species throughout the bleaching. Corals species found in temperate regions, including the generalist P. versipora, will therefore likely be highly susceptible to future change as heat waves grow in frequency and severity unless their thermal thresholds increase. Our observations provide further evidence that high-latitude systems are susceptible to community reorganisation under climate change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change-induced warm-water thermal anomalies are increasing in frequency and intensity globally (Heron et al. 2016; Hughes et al. 2018). Such “heat wave” events combined with high solar radiation (Mumby et al. 2001) induce widespread coral bleaching by disrupting the symbiosis between coral hosts and their microalgal endosymbionts (e.g. Warner et al. 2002; Suggett and Smith 2011). Subsequent mass coral mortality can result following bleaching that in turn drives extensive changes in the structure and function of coral reefs (Pandolfi et al. 2011). The sustained 2015–2017 El Niño-Southern Oscillation (ENSO) thermal anomaly was the most severe to date and resulted in a major global mass coral bleaching event (Hughes et al. 2018), with the Great Barrier Reef experiencing its worst-ever coral bleaching and mortality (Hughes et al. 2017). In an unprecedented outcome, we observed the highest latitude coral bleaching event occurring in Sydney Harbour (33°S), the first ever for this region, as sea surface temperatures (SST) reached > 2 °C above the long-term summer maxima in February 2016 and remained above long-term average temperatures until July 2016 (U.S. National Oceanic and Atmospheric Administration (NOAA) Coral Reef Watch).
High-latitude corals persist in environments where SSTs, irradiance levels and/or aragonite saturation states are seasonally lower and much more variable when compared to the tropics (Kleypas et al. 1999; Sommer et al. 2018; Camp et al. 2018). Corals in these regions survive under marginal environmental conditions with increased stress tolerance through phenotypic plasticity and adaptations to colder waters (Beger et al. 2014; Camp et al. 2018, Tuckett and Wernberg 2018). Even so, how such high-latitude coral communities respond to future trajectories of ocean warming, accompanied with other stressors remains generally unknown (Camp et al. 2018). High-latitude coral populations from the Solitary Islands Marine Park and Lord Howe Island Marine Park (such as Isopora cuneata, Pocillopora damicornis and Acroporidae) have been suggested to exhibit lower absolute bleaching thresholds than their tropical counterparts due to differences in thermal histories (Dalton and Carroll 2011). Bleaching in response to thermal anomalies have been reported from high-latitude reefs in the Southern Pacific and Indian Oceans; specifically, the Solitary Islands in 2005–2007 (Dalton and Carroll 2011), Rottnest Island (32°S) in 2011 and 2015–2016 (Thomson et al. 2011; Le Nohaïc et al. 2017), Houtman Abrolhos Islands (28–29°S) in 2011 (Abdo et al. 2012; Bridge et al. 2014), and Lord Howe Island (31°S) in 2011 (Harrison et al. 2011). Since 2016, coral bleaching has been recorded at 14 high-latitude reefs in New South Wales and SE Queensland (Hughes et al. 2018). However, while absolute thresholds for bleaching are likely lower, the extent of anomalous temperature that they can tolerate remains untested. Furthermore, the physiology and bacterial profiles underpinning high-latitude coral bleaching and recovery are still to be described.
Sydney Harbour is situated within the Hawkesbury Shelf marine bioregion (33–35°S) where temperate waters are inundated by warm tropical and subtropical waters supplied by the East Australia Current (EAC) ca. 50% of the time (Pollard et al. 1997). Thus, this region alternates between subtropical and temperate extremes (Breen 2007) with large temperature fluctuations ranging from 16 °C to 24 °C (see Fig. 2). Sydney Harbour (33°S) scleractinian coral communities are dominated by two species, the geographically cosmopolitan Plesiastrea versipora, with one of the largest latitudinal distribution known for any Indo-Pacific coral species (Veron 2000), and the southerly restricted temperate species, Coscinaraea mcneilli. Both species predominantly grow as veneering coral colonies (Madsen et al. 2014) and are thus non-reef forming on high-latitude reefs (Mizerek et al. 2016). While Plesiastrea versipora acclimates to increasing temperatures of up to 21 °C with increasing metabolic rates through enhanced autotrophic activity (Howe and Marshall 2001, 2002), it remains unknown to what extent their physiology adjusts as colonies experience a full seasonal range of temperatures. Similarly, how metabolic processes respond once pushed past normal physiological thresholds (i.e. thermal stress) remains largely unexplored. Plesiastrea versipora in Western Australia was recently shown to bleach during a sustained cold spell where temperatures were > 2 °C colder than the 10-yr monthly averages (Tuckett et al. 2017). Thus, a scleractinian coral species with a broad ecological niche (Sommer et al. 2014) appears susceptible to acute temperature stress, but how P. versipora responds to anomalously high temperatures is undocumented.
Whether and how heat wave-induced bleaching processes for temperate corals (both cosmopolitan and geographically restricted taxa) follow those commonly documented for tropical corals is unexplored. Therefore, during the Sydney Harbour 2015–2016 El Niño thermal anomaly, we examined two species, P. versipora and C. mcneilli, both in situ as well as tank-maintained populations in aquaria supplied with flowing seawater from the Harbour. We specifically quantified a number of key traits that have been shown to influence coral bleaching susceptibility and recovery in tropical corals including the photosynthetic physiology (Scheufen et al. 2017), microbiome composition (Röthig et al. 2017; Ainsworth et al. 2015; Bourne et al. 2008) and host metabolic rates (Grottoli et al. 2006; Bessell-Browne et al. 2014; Tremblay et al. 2016) before, during and after (recovery) heat stress from both coral populations in situ and held in aquaria. Intriguingly, of the two species, only the geographical generalist P. versipora exhibited widespread bleaching (but subsequent high recovery, whereas the southerly restricted species was unaffected during the heat wave event). We discuss how these different susceptibilities reflect differences in their microbiomes and physiologies.
Materials and methods
Site characterisation
As part of a long-term assessment programme, coral populations were periodically examined from February to August 2016 at two sites within Sydney Harbour, Fairlight Beach (33°48′3″S, 151°16′30″E) and Middle Head (33°49′29″S, 151°15′47″E) for physiological and microbiological metrics. Benthic video transects at both Middle head (n = 4, length 50 m at depths 2–4 and 4–6 m) and Fairlight (n = 4, 30 m at depths 3–5 and 5–7 m) were initially conducted in February, prior to the peak in SSTs and on-set of observed bleaching. Benthic transects followed the site substrate contours and video footage (GoPro Hero3) was taken ~ 30 cm above the substrate. P. versipora forms high-density patch communities generally clustered within each site. Therefore, to ensure adequate habitat representation, transects began at a P. versipora colony and continued to include surrounding habitat and capture habitat variance. Video transects were visually analysed following a continuous line intercept method (Gardner et al. 2019) to calculate percentage cover of coral. For each transect, the total number of colonies were counted (bleached and healthy colonies). The total number of colonies intercepted is expressed as the amount of coral intercepted relative to the total distance. Repeat transects were performed in April during the bleaching event and again in August during recovery. Coral bleaching is defined in this manuscript as atypical colouration where “bleached corals” include corals undergoing bleaching which are paler than those seen during seasonal norms (see S1).
In situ sample collections
Coral fragments (n = 5) were subsampled from each site (~ 3 to 5 m depth at Middle Head, 4–6 m at Fairlight) by SCUBA using a hammer and chisel (2 cm2) (Fig. 1). Healthy P. versipora and C. mcneilli fragments were sampled in February 2016 (pre-bleaching) and June (recovery), while bleached (P. versipora only) and unbleached colonies were sampled in April (during bleaching). Fragments were removed from the edges of the colonies and were held in falcon tubes filled with seawater. On the surface, water was removed from the tubes and samples were snap frozen immediately in liquid nitrogen and stored at − 80° in the laboratory until DNA extraction. 2 × 2 L of seawater was collected from around the coral for 16S rDNA analysis and kept on ice until processing.
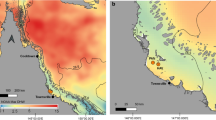
Overview of sampling locations within Sydney Harbour represented by letters a (Fairlight) and b (Middle Head). All other locations represented on the map (green circles) are sites with coral populations as observed by the authors. Inserts show specific sampling sites within locations. The base data for the map were collected from map tiles at www.openstreetmap.org (OpenStreetMap contributors, https://www.openstreetmap.org/copyright) under the Creative Commons Attribution-ShareAlike 2.0 licence (http://creativecommons.org/licenses/bysa/2.0/), and customised in Adobe Illustrator (version 16)
16S rDNA amplicon sequencing and analysis
DNA Extraction: Tissue was removed from coral fragments (n = 4, bleached and unbleached corals) using an air gun and 1× phosphate buffer saline (PBS) and further homogenised with a sterile syringe. Samples were centrifuged for eight mins at 3000 × g to pellet the coral tissue and mucus. Pellets were resuspended in 2 mL of PBS and centrifuged as above and the PBS supernatant was removed. A PowerPlant® Pro DNA isolation kit (cat # 13400) (Qiagen, USA) was used following manufacturer’s protocol. The optional phenolic separation solution was also used to combat the high polyphenolic compound content of corals, as per the manufacturer’s protocol. Mechanical tissue lysis was performed using a Qiagen TissueLyser LT (Hilden, Germany) at 50 Hz for 3 min. Equal volume of phenol: chloroform: isoamyl alcohol (pH 8) was added to the supernatant and then centrifuged for 13,000 × g for 5 min. The supernatant was then extracted with an equal volume of chloroform/isoamyl alcohol and centrifuged again as above. These additional DNA extraction steps were found to improve DNA yield and quality and were used on all samples. Genomic DNA concentrations were assessed using the Qubit® High-sensitivity dsDNA assay kit (Life Technologies, NSW, Australia). DNA template was screened for PCR efficiency using the barcoded primer pair 27F and 519R (Tout et al. 2015; Prazeres et al. 2017).
Bioinformatics & data analysis: Bacterial 16S extracted from the coral holobiont was sequenced using the Illumina MiSeq v3 platform (Ramaciotti Centre for Genomics). The universal Eubacterial primers 27 F (5′-AGAGTTTGATCMTGGCTCAG) and 519R (5′GWATTACCG CGGCKGCTG)) were used for PCR amplification, targeting the highly variable V1-V3 regions of the 16S rRNA. Reads were processed as outlined in Kahlke (2018) (https://github.com/timkahlke/ampli-tool). Briefly, sequences were joined using FLASh (Magoc and Salzberg 2011) and subsequently filtered and trimmed using mothur (PARAMETERS: maxhomop = 6, maxambig = 0, minlength = 469, maxlength = 503). Fragments were clustered into operational taxonomic units (OTUs) at a 97% identity threshold and chimeric sequences were removed using vsearch (Rognes et al. 2016) and the Silva v128 database. Taxonomies were assigned to the OTUs using QIIME (Caporaso et al. 2010) and the BLAST algorithm against the Silva v128 database. Negative kit controls and seawater controls were used to identify any laboratory or seawater contamination and these OTUs were removed from the analysis. Coral host mitochondrial sequences and 16S sequences identified as mitochondria or chloroplasts were also removed from the analysis. Data were rarefied to 3000 reads per sample, representing the lowest number of reads among all samples.
PRIMER + PERMANOVA (version 6.1; UK) was used to statistically analyse the data. Relative abundances were square root transformed and clustered with a Bray–Curtis resemblance matrix. Permutational multivariate analysis of variance (PERMANOVA) with pairwise comparisons identified the effect time and species have on microbiome composition. Similarity percentages analysis (SIMPER) uncovered dissimilarities based on OTUs over time and between species.
The core microbiome was defined as OTUs that were found in a minimum of 3 out of 4 replicates with a minimum relative abundance of 0.001%, identified using QIIME. Venn diagrams depicting the core microbiome were constructed with the online software from Bioinformatics and Evolutionary Genomics (http://bioinformatics.psb.ugent.be/software/details/Venn-Diagrams). To determine the differential abundance of OTUs between the two coral species the Statistical Analysis of Metagenomic Profiles (STAMP) software package was used for univariate analysis (Parks and Beiko 2010). P-values were calculated using the Kruskal–Wallis test with Storey’s False Discovery Rate (FDR) multiple test correction method and a p value < 0.05. The P-values and FDR values were visualised as extended error bar plots (CI = DP: Bootstrap 95%) which display the relative abundance of each bacterial taxa, specified for each sample grouping as bars, with the difference in proportions with 95% confidence interval error bars displayed for each bacterial taxon.
Genetic identifications of the resident endosymbiont
Colonies of P. versipora from Fairlight (n = 15) and Middle Head (n = 15 and colonies of C. mcneilli from Fairlight (n = 5) were sampled in June 2015 and samples processed for DNA analyses to determine the identity of the microalgal endosymbiont common to each species. Whole coral tissue DNA extractions were performed as described by LaJeunesse et al. (2003), consisting of a 2 min bead-beating step (0.4–0.6 mm glass beads) and a modified and abbreviated DNA wizard extraction protocol (Promega). Nuclear large-subunit ribosomal DNA (LSU) and chloroplast large-subunit rDNA (cp23S) were amplified and sequenced (LaJeunesse et al. 2012). Conditions for amplifying the LSU are provided in (Zardoya et al. 1995), and conditions for amplifying cp23S are provided by (Zhang et al. 2005).
To amplify DNA, reactions were performed in 25 µL volumes containing 2.5 µL of 2.5 mM dNTPs, 2.5 µL of 25 mM MgCl2, 2.5 µL standard Taq Buffer (New England Biolabs, Ipswich, MA, USA), 0.13 µL of 5 U µL−1Taq DNA Polymerase (New England Biolabs), 1 µL of each forward and reverse primer at 10 µM, and 1 µL of 5–100 ng DNA template. Products were cleaned with ExoSAP-IT (Affymetrix, Santa Clara, CA, USA) and directly sequenced on an Applied Biosciences sequencer (Applied Biosciences, Foster City, CA, USA) at the Pennsylvania State University Genomics Core Facility. Sequence electropherograms were each examined manually and nucleotide sequences aligned by eye. Raw sequences and alignments for each gene are available from the Dryad Digital Repository. Phylogenetic analyses using maximum parsimony were then performed on aligned data sets in PAUP* v.4.0d151 (Swofford 2014) with indels in rDNA included as a 5th character state. Bootstrap support was calculated based upon 1000 replicates.
Coral metabolism
Colonies of P. versipora and C. mcneilli were collected from Fairlight Beach (4–6 m depth) in September 2015 and allowed to acclimate for 6 weeks prior to long-term experimentation in aquaria at Manly SeaLife Sanctuary. Three replicate colonies of each species were fragmented to yield a total of n = 20 fragments per species, which were then distributed randomly across aquaria. The experimental setup consisted of four tanks on a continuous flow-through system supplied by fresh Sydney Harbour water (passing through two rapid sand filters and a sedimentation tank). Flow rate was maintained at ~ 2.5 L min−1 and light intensity (µmol photons m−2 s−1), measured with a Li-250A with 4π sensor (µmol Li-Cor, Lincoln, Nebraska, USA), was changed seasonally as determined by modelling the underwater light field adjusted for 7 m depth based on the diffuse attenuation coefficient (as per Hennige et al. 2010).
Rates of net photosynthesis (PG), dark respiration (R) and light calcification (G) were obtained by respirometry for five fragments of P. versipora and C. mcneilli at each timepoint as per (Camp et al. 2015). Timepoints t0, t1 and t2 represent the pre-bleaching months December, January and February, respectively, t3 is during bleaching in April and t4 is recovery in August. Importantly, both tank and in situ corals had bleached by April; however, metabolism measurements of bleached corals in aquaria were not made until June. Thus, the rates measured and reported here reflect metabolic responses during a long-term (> 2 months) bleaching event. Each colony was individually incubated for three hours in a 500 mL gas tight chamber submerged in a flow-through experimental tank acting as a water bath and stirred manually every 5 min (as well as two control chambers containing only seawater to correct for any biological activity of the seawater). Rates of PN and R (µmol O2 cm−2 h−1) were quantified for each fragment simultaneously at the start and end of each incubation using a FireSting optical oxygen meter with a needle-type microsensor (Pyroscience, Germany). Dark incubations were conducted 30 min after the light incubations by covering the experimental tanks with blackout material. PG was calculated as PN + R (Camp et al. 2017). Calcification rates (µmol CaCO3 cm−2 h−1), determined as changes in TA, and O2 for each chamber during each 3-h incubation was corrected for any changes in TA or O2 from the seawater controls (n = 3) (as per Camp et al. 2017). Surface area was determined for each fragment at the end of the experiment using the advanced geometric technique (Naumann et al. 2013) and were used to normalise metabolic rates for each fragment. Following a normality check using the Shapiro test, one-way ANOVAs and Tukey’s post hoc tests were performed for C. mcneilli, on each metabolic variable (PG, R and G), to test for differences between time points for each species. Data for P. versipora was not normally distributed therefore non-parametric Kruskal–Wallis tests with pairwise comparisons were used.
Abiotic measurements were made in triplicate for each experimental tank bi-monthly (WTW Multiprobe 3630 and sensors, calibrated for each sensor before each use). Total alkalinity was measured using an autotitrator (Metrohm 916 Ti-Touch Autotitrator) with accuracy and precision of less than or equal to 2 µmol kg−1 as verified with Dickson standards and pH (total) was measured with a Metrohm iUnitrode electrode and calibrated with tris buffers (precision ca. ± 0.0005 pH units). Aragonite saturation state and dissolved inorganic carbon were calculated using CO2SYS software (Lewis et al. 1998). Temperature and light intensity of each experimental tank was also continuously measured using HOBO pendent loggers (Microdaq, USA) set at a 10-min interval, calibrated before each use and values given in LUX were converted to PAR using the daylight coefficient (Camp et al. 2015).
Common measurements in situ and aquaria
A number of common metrics were used to ensure data sets between in situ and aquaria experiments were comparable.
Environmental variables Water temperature data were recorded semi-continuously at both sites from July 2015–August 2016 using temperature/light loggers (HOBO Pendant, Microdaq, USA) set to measure at 30-min intervals and are reported here as monthly averages. Monthly composite SST data were obtained from the MODIS (MODerate Resolution Imaging Spectroradiometer) platform using the Giovanni online data system and used to calculate temperature anomalies from 2010 to 2017 and average SST from 2015 to 2017 (Fig. 2). Degree Heating Weeks (DHW) were obtained from the National Environmental Satellite Data and Information Service (NESDIS) of the U.S. National Oceanic and Atmospheric Administration (NOAA) (50 km). Water temperatures were strongly correlated between the experimental system and in situ temperatures as determined with a Pearson correlation coefficient analysis (r2 = 0.924, not shown). All other environmental parameters were measured bi-monthly at both sites as explained above. Triplicate water samples were taken at each site using a nutrient analyser (Quikchem QC8500 Automated Ion Analyser) following manufactures protocol and quality control procedures (LACHAT Instruments, USA). Data were interpreted using Omnion version 3 software (LACHAT Instruments, USA).
Satellite-derived data characterising a Sea Surface Temperature (SST) anomalies during the period 2010–2017 with the study period highlighted in red, b 10-yr average SST (open circles), observed SST (Fairlight) (blue squares), and bars of degree heating weeks (DHWs) from June 2015 (winter)-June 2017. Monthly composite SST data were obtained from the MODIS (MODerate Resolution Imaging Spectroradiometer) platform and DHWs (50-km) data from the National Environmental Satellite Data and Information Service (NESDIS) of the U.S. National Oceanic and Atmospheric Administration (NOAA). Alert level bars correspond to satellite bleaching alert levels based on current 50-km Hotspot data
Symbiodinium Cell density coral Tissue (~ 1 cm2) was removed from fragments (additional fragments to those used for physiology measurements) using a waterpik and GF/F-filtered seawater. The resulting tissue slurry was homogenised, and an aliquot taken for cell quantification using a haemocytometer (Neubauer Haemocytometer, Fisher Scientific, Loughborough, UK) with eight replicate quadrats counted for each sample.
Photophysiology A diving-PAM underwater fluorometer (Waltz, Germany) was used to obtain maximal quantum yields (Fv/Fm) of chlorophyll a fluorescence of colonies in situ (measurements taken early morning prior to direct sunlight exposure) and ex situ following 20 min of low light acclimation (settings: measuring light intensity = 8, saturation pulse intensity = 11, saturating width = 0.8, damp = 2, gain = 4) (e.g. Suggett et al. 2012). Triplicate measurements were made for each colony with a total of eight colonies measured at each timepoint. All measurements were made at a constant distance from the coral (3 mm), standardised using a fibre optic adaptor and taken from the middle of the colony.
Results
Environmental conditions
Satellite-observed SST from February to August 2016 consistently exceeded long-term (10 yr) monthly averages, peaking at 3.7 °C above the average in February, resulting in DHWs of 5.4 °C-weeks and bleaching alert level 1 (bleaching likely) (NOAA Coral Reef Watch. 2000, updated twice-weekly). In March, DHWs reached 12.8 °C-weeks and bleaching Alert level 2. SSTs continued to exceed monthly averages until August 2016, resulting in a Bleaching Watch status over this time period. Sydney is subjected to warm and cold thermal anomalies yearly. However, warm SST anomalies were greater in 2015 and 2016 than the previous 6 yrs coinciding with the study period of interest (Fig. 2a). As expected, most physicochemical conditions within the aquaria closely matched the in situ conditions (Table S1) since the aquaria were supplied by continuously flowing seawater from Sydney Harbour. Notably temperature ranged from 17.2 to 25.9 °C in situ and 17.8 to 25.9 °C in aquaria and pH ranged from 7.96 to 8.11 in situ and 7.76 to 7.97 in aquaria. However, phosphate levels were consistently higher in the tank experiment (by a factor of ca. 2.4–14.8).
Bleaching and endosymbiont dynamics
Plesiastrea versipora account for 40.0 ± 17.0% (range 11.0–88.0%) and 33.0 ± 15.0% (range 0–62.0%) of total benthic cover at sites targeted within Fairlight and Middle Head, respectively. In April, up to 60 ± 8.1% of colonies displayed signs of bleaching undergoing bleaching or completely white) across the two harbour sites (Fig. 3). By July, corals had begun to regain normal colour (Fig. 3) with bleached corals making up only 25 ± 1.2% of colonies. Healthy coral cover increased from 40.4 ± 8.1% of colonies during April to 77.4 ± 1.4% in August.
a—Symbiodinium cell density (cells/cm2 surface area) of C. mcneilli (circles) in situ and ex situ, P. versipora (squares) (bleached, red) and P. versipora (healthy, blue) (in situ) expressed as mean ± SEM (P. versipora n = 8, C. mcneilli n = 4, C. mcneilli was sampled from Fairlight only). b—Maximum quantum yield of PSII (Fv/Fm) expressed as mean ± SEM of healthy (in situ), bleached (ex situ), bleached (in situ) colonies of P. versipora and C. mcneilli (ex situ) (P. versipora n = 8, C. mcneilli n = 4, C. mcneilli was sampled from Fairlight only). In situ measurements are of eight colonies in total, four colonies from each harbour site and ex situ measurements are of eight coral fragments maintained in aquaria. ANOVA with Tukey’s post hoc tests were used for statistical analysis with numbers indicating significant differences (p < 0.05). Measurements were taken in February (pre-bleaching), April (during bleaching), June and August (recovery period) 2016. Image of bleached (April) and non-bleached (July, recovery) colony of P. versipora at Middle Head (depth 4 m)
Changes in maximum quantum yield of PSII (Fv/Fm) values are commonly used to describe coral responses to heat stress (e.g. Suggett and Smith 2011), and values for both the aquarium housed and in situ colonies were equal at the beginning of the experiment (0.65 ± 0.02, mean ± SE, and 0.63 ± 0.02 respectively, Fig. 3). Healthy colonies of P. versipora in situ maintained Fv/Fm values greater than 0.6 for the duration of the study. In April, mean values for Fv/Fm for in situ and tank housed fragments were reduced to 0.24 ± 0.03 and 0.35 ± 0.03, respectively. These values remained significantly lower than the Fv/Fm of healthy colonies until the end of the experiment. Fv/Fm values returned to within pre-bleaching values for in situ corals, whereas tank corals only increased to 0.45 ± 0.04, with a slower overall recovery.
Changes of in situ Symbiodinium cell densities followed the loss and recovery trends in Fv/Fm during heat stress. Symbiodinium cell densities were significantly reduced for bleached colonies of P. versipora in April (during bleaching) (Fig. 3) (3.55 × 106 ± 7.83 × 104 to 3.77 × 105 ± 1.49 × 104 cells/cm−2) for bleached colonies before returning to within pre-bleaching values by June.
In contrast to P. versipora, C. mcneilli, accounting for < 10% of total benthic cover at sites targeted within Fairlight did not show any visual or physiological signs of bleaching in either tank experiment or in situ, where values of Fv/Fm (ca. 0.65–0.76)) and symbiont cell densities (3.6 × 106–3.85 × 106 cells cm2) remained generally constant throughout (Fig. 3).
Identity of endosymbiotic microalgae
Sampling from a variety of colonies across all sites revealed that P. versipora and C. mcneilli contained a Symbiodinium sp. whose LSU rDNA were identical, indicating the presence of a single undescribed species of Breviolum (= formerly Symbiodinium clade B), provisionally referred to as type B18a. However, phylogenetic analyses of the cp23S marker exhibited a fixed difference that distinguished symbionts in P. versipora from C. mcneilli. In addition, the identity of Symbiodinium was determined for P. versipora colonies pre-bleaching and during bleaching (Fujise 2018, unpublished), showing no difference in the composition of Symbiodinium clades using DNA metabarcoding based on the cp23S primer set between the two health states.
Holobiont bacterial communities
Potential shifts in the holobiont microbiomes of P. versipora and C. mcneilli were determined using 16S rRNA gene sequencing. After quality control, a total of 5,414,105 reads were matched to reference sequences within the Silva 128 database with an average of 30 000 reads for each sample (non-rarefied). Seawater controls samples contained significantly distinct microbial communities compared to coral samples across the study period (PERMANOVA; p < 0.05, t = 1.65), indicating low-levels of sample contamination with non-holobiont DNA. At the class taxonomic level, Gammaproteobacteria and Actinobacteria dominate the microbiomes of both P. versipora and C. mcneilli (Figure S4, S5).
In February (t2), prior to bleaching, P. versipora and C. mcneilli had significantly distinct microbiomes (Table 1) driven primarily (as shown with SIMPER and supported by STAMP analysis, Figure S6, S7) Flavobacteriaceae (2.67%, STAMP 13.33%, p = 0.004) Oceanospirralles (3.59%, STAMP 5.8% p = 0.003) and Photobacterium (2.68%, STAMP 4.68% p = 0.002) associated with C. mcneilli, and Xanthomonadales (2.41%, STAMP 5.73% p < 0.001) and Geothermobacter (2%, STAMP 4.69%, p = 0.0003) associated with P. versipora. Core taxa that distinguished the host microbiomes were unclassified (UC) Flavobacteriaceae and UC Oceanospirillales for C. mcneilli and UC Xanthomonadales, and Geothermobacter for P. versipora (Fig. 4). The core microbiome of C. mcneilli was richer than that for P. versipora (5 core OTUs in contrast to 2 core OTUs, respectively), yet the Shannon index indicates similar overall diversity (3.8 for P. versipora and 4.0 for C. mcneilli). Escherichia/Shigella were core taxa common to both hosts [10 ± 4% SE (here on in denoted as % ± SE) and 5 ± 4% for P. versipora and C. mcneilli, respectively, Fig. 4]. Pseudomonas was also a core taxon for both coral species but consistently represented in abundances < 1% for the duration of the experiment. Chlorobiaceae was a dominant taxon present across both species at this time (Table 1).
Venn diagram of the core microbiomes of healthy P. versipora and C. mcneilli colonies pre-bleaching. The number of OTUs for each species core and the overall shared core are shown. Only core taxa with > 1% abundance are shown. Each OTU is presented to genus level where possible. UC: Unclassified. Escherichia/Shigella is unclassified (UC) at species level
In April (t3) during the bleaching event, despite certain colonies of P. versipora showing visual and photophysiological signs of stress, overall microbial composition did not differ significantly between bleached and healthy P. versipora colonies or across species (Table 1). Escherichia/Shigella increased in abundance from 10 ± 4% to 16 ± 7% for P. versipora and 5 ± 4% to 26 ± 3% for C. mcneilli and remained as shared core bacterial taxa from February. This taxon was also in extremely high abundance in bleached colonies of P. versipora (14 ± 6%) together explaining much of the similarity in microbial community composition across species and between bleached and healthy colonies of P. versipora. Geothermobacter, no longer a core taxon for P. versipora, and Chlorobiaceae were overrepresented in bleached P. versipora colonies (6 ± 4% and 9 ± 4%, respectively), these taxa thought to be key drivers between healthy and bleached coral in our study (Randall et al. 2016; Cai et al. 2015). Overall, Shannon diversity indices are the same between unbleached and bleached colonies (2.8).
Convergence of bacterial communities in April was also attributed largely to Anaerolineaceae, which increased in unbleached P. versipora and decreased in C. mcneilli. Flavobacteriaceae decreased in C. mcneilli without parallel increases in P. versipora (Table 1). Such changes reflect shifts towards an increased microbiome similarity between the two-host species suggesting that the thermal anomaly exerted general selection pressure on the structure of these bacterial communities regardless of host identity. C. mcneilli, however, contained a higher overall diversity (Shannon: 3.78 vs 2.82 for P. versipora, Table 1).
In August (t4) as Harbour waters cooled, the two-host species were again characterised by distinct bacterial communities (Table 1) with shifts in the microbiome potentially indicative of seasonal changes. This was driven by Escherichia/Shigella which explained 5% of the dissimilarity according to SIMPER, and which no longer represented a shared core taxon, only a core for P. versipora. Anaerolineaceae showed decreasing average relative abundance for both species and Geothermobacter and Flavobacteriaceae decreased for P. versipora, while Flavobacteriaceae and Chlorobiaceae disappeared entirely from C. mcneilli (Table 1). Marinicella became part of the core microbiome for C. mcneilli, but was absent in P. versipora.
A number of taxa appeared (< 1% relative abundances) for the first time associating only with C. mcneilli further driving the dissimilarity between the two-host species. Of these, Porphyromonadaceae (2.13% of the dissimilarity in SIMPER and 8.86% p = 0.002 in STAMP) accounted for a large proportion (~ 19% ± 5%) of relative abundance and Blastopirellula (3.16% of the dissimilarity in SIMPER and 2.2% p < 0.01 in STAMP) accounting for 4 ± 1.8% relative abundance. Increases in Flavobacteriaceae for C. mcneilli explained 2.42% (0.4%, p = 0.007 in STAMP) of the dissimilarity, respectively. During recovery, P. versipora had a greater diversity of taxa than C. mcneilli (Shannon = 5.25 and 3.99, respectively) and a lower diversity of unique core OTUs (1 OTUs compared to 3 OTUs for C. mcneilli).
In summary, Escherichia/Shigella was a dominant taxon for both coral species in February and increased during the thermal stress event in April, suggesting the importance of this taxon in bleaching resistance (abundances were higher in unbleached P. versipora and C. mcneilli than bleached P. versipora). Escherichia/Shigella remained dominant in P. versipora with the recovery of this coral in June, while disappearing entirely from the comparatively bleaching- resistant C. mcneilli. Overall, changes in the microbiome of P. versipora are characterised by shifting abundances of a few key taxa, indicating a highly conserved microbiome throughout the thermal anomaly. In contrast, these key taxa also found in C. mcneilli were lost by June and a number of new taxa, such as Marinicella, appeared indicating a less conserved microbiome for this coral species, and potentially seasonal shifts in the bacterial community as waters cooled.
Holobiont physiology
December, January and February (pre-bleaching) net rates of Respiration (R) and light driven calcification (GL) and gross photosynthesis (PG) were higher for P. versipora than C. mcneilli (Table S2). The photosynthesis to respiration ratio (P: R) values for P. versipora and C. mcneilli remained above 1 during this period ranging from 1.5 to 2.2 and 2.1 to 2.3, respectively (Fig. 5). In June (during bleaching), P. versipora exhibited reduced photosynthesis (53–56% reduction in PG) that corresponded to a similar decrease in symbiont cell density (85% decrease) relative to pre-bleaching levels (Figure S2). Symbiodinium cell normalised gross photosynthesis rates (PG cell−1) were enhanced for the remaining Symbiodinium cells (PG cell−1 was 4.85 × 10−7 pre-bleaching and 1.71 × 10−6 PG/cell during bleaching, Figure S3). These trends were accompanied by a decrease in GL by 51–58% (T0 and T2) from pre-bleaching levels, and active calcification appeared to largely cease. Values of P: R decreased during this time (25–48% decrease to 1.16 ± 0.08) via a reduction in PG (53–56% reduction), while respiration remained relatively unchanged (Fig. 5).
Metabolic comparisons of photosynthesis and respiration (P: R) and calcification (G) rates (µmol cm−2 h−1) (± SEM) over the experimental period (December- August) for aP. versipora and bC. mcneilli. Dashed lines connect metabolic shifts through time. The bleaching timepoint is indicated with a red data point and recovery with a blue data point. All other timepoints are pre-bleaching
In contrast to P. versipora, C. mcneilli upregulated PG and R during the bleaching period (24–31% and 6–11%, respectively, Table S2) and did not display signs of coral bleaching (no paling, no change in Symbiodinium density and no decrease in Fv/Fm; Fig. 2). As a result, P: R was increased by 21% during the bleaching period independent of Symbiodinium cell density. PG per cell increased during the bleaching period (4.01 × 10−7 pre-bleaching to 5.55 × 10−7 during the bleaching period) although this was not significant and was less than the rates of PG/cell for bleached P. versipora colonies (5.55 × 10−7 and 1.71 × 10−6 PG/cell, respectively, Table S2). GL for C. mcneilli was highly variable across the experiment with measurements for June (T3) 93–98% lower (resulting in net dissolution) than the other time points.
In August during recovery (T4), PG increased back to pre-bleaching levels for P. versipora (with a 74% increase in Symbiodinium density) (Table S2). P: R increased during recovery (74% increase to 4.4 ± 0.2) largely from a decrease in respiration (Fig. 5), which was significantly different to all other time points. For C. mcneilli, GL increased, while R significantly decreased, leading to only a 26% increase in P: R (with Symbiodinium cell density remaining stable) (Fig. 5).
Discussion
Tropical coral communities have been increasingly subjected to heat wave events that induce mass bleaching (Hughes et al. 2018). Consequently, there has been renewed focus on understanding why and how tropical corals are sensitive to thermal stress. In 2016, the greatest heat wave to date in Australia extended to temperate systems where we witnessed the first documented temperate coral bleaching event. In tropical systems, heat stress sensitivity is influenced by the host (Grottoli et al. 2006; Bessell-Browne et al. 2014; Tremblay et al. 2016), symbiont (Scheufen et al. 2017; Sampayo et al. 2008; Silverstein et al. 2017) and/or bacterial (Röthig et al. 2017; Ainsworth et al. 2015; Bourne et al. 2008) responses. Our data examining how metabolism versus microbial dynamics suggests that heat sensitivity of temperate taxa may similarly reflect a complex interplay of factors.
Coral bleaching dynamics of corals in temperate regions
Tropical corals typically show a reduction in Fv/Fm, symbiont densities and net photosynthesis of Symbiodinium in response to thermal stress (e.g. Scheufen et al. 2017; Gardner et al. 2017). Such suppression of holobiont photosynthesis via coral bleaching is similarly characteristic for the coral P. versipora in temperate regions, which bleached in this study. Rates of gross photosynthesis per cell have been shown to decrease during thermal stress for some tropical corals (i.e. M. cavernosa; Scheufen et al. 2017) and increase for others (i.e. O. faveolata, Scheufen et al. 2017; P. damicornis, Wangpraseurt et al. 2017). In our study, rates of PG cell−1 for P. versipora increased with coral bleaching (Figure S2), indicating that the remaining symbionts retained cell functionality and are likely critical in maintaining metabolic capacity (Sampayo et al. 2008). Whether, that with a loss of symbiont cells, carbon limitation is relieved (or there is more carbon available per Symbiodinium cell) for the fraction of cells that remain (that have not been impacted by heat stress) is unclear (Levas et al. 2016; Suggett et al. 2013). However, P: R ratios decreased for this coral species at this time suggesting a transition to heterotrophic carbon acquisition.
Bleaching susceptibility and recovery, while not well resolved into a single model, is strongly dictated by the photosynthetic physiology (Scheufen et al. 2017) and species of microalgal endosymbiont harboured within the coral. It is known from extensive research on tropical corals that associations with thermally tolerant endosymbionts (Sampayo et al. 2008; Silverstein et al. 2017) influence bleaching susceptibility and post-bleaching mortality. In Western Australia, P. versipora is known to associate with clade B18 (Silverstein et al. 2011). In Sydney, P. versipora and C. mcneilli maintain specific associations with a particular undescribed species of Breviolum spp., provisionally referred to as type B18a. This symbiont remained dominant throughout the thermal stress event (Fujise 2018, unpublished). However, DNA sequence analyses revealed fixed differences in the cp23S gene that differentiated symbiont populations associated with each species. Breviolum spp. appears relatively rare in the south east Pacific (https://sites.google.com/site/geosymbio/home) and thus clearly warrants further study given the ability to rapidly recover from stress by increasing cell productivity (PG/Cell) that we observed here.
An intriguing outcome was that the widely geographically distributed species P. versipora exhibited greater signs of stress, whereas the southerly restricted species C. mcneilli appeared unaffected. These differential responses were observed both in the field and in the aquaria exposed to the same temperatures and light levels. We initially considered that due to the cryptic nature of C. mcneilli, which typically grows under boulders and overhangs or in deeper water, this taxon was spared the synergistic impact of high temperature and irradiance, well reported as a driver of coral bleaching (Mumby et al. 2001; Wooldridge 2009). Plesiastrea versipora colonies were most abundant on the upwards facing section of boulders, exposed to increased solar radiation, which reaches summer levels of ~ 300 µmol photons m−2 s−1. However, given that C. mcneilli did not bleach ex situ, under the same irradiance levels as P. versipora, this taxon therefore appears to have attributes that provide greater thermal tolerance.
Coral bacterial communities
We identified a number of dominant bacterial taxa present during the thermal anomaly and temporal dynamics, consistent with the notion that highly stable microbiomes may confer bleaching resilience (Ziegler et al. 2017; Grottoli et al. 2018), and that bleaching sensitivity is also paralleled by the emergence of opportunistic bacteria species. Bacterial taxa Anaerolineaceae and Flavobacteriaceae were dominant in the coral C. mcneilli in February (summer), but rare in P. versipora. Surprisingly, these taxa exhibit increased abundance at anthropogenically impacted sites (Ziegler et al. 2016) and are found commonly in diseased tissues (Ainsworth et al. 2007; Roder et al. 2014). This pattern is commonly observed for corals under thermal stress (Bourne et al. 2008; Thurber et al. 2009), but clearly in our study the physiological functioning of C. mcneilli did not seem to be impacted by these potential pathogen-associated bacteria. The convergence of P. versipora and C. mcneilli microbiomes in April (during the bleaching period) was driven by increases in Anaerolineaceae and Flavobacteriaceae in P. versipora and their corresponding decrease in C. mcneilli (in August). Therefore, the true nature of these bacterial groups remains to be determined. Overall, colonies of C. mcneilli did not exhibit significant shifts in their microbiome between February (summer) and April (autumn- bleaching period), whereas P. versipora had significant shifts in their microbiome over this period (driven by changes in the relative abundances of dominant taxa with decreasing diversity). Thus, the more stable microbiomes in C. mcneilli provide further and independent evidence that this taxon experienced less stress than P. versipora.
Gammaproteobacteria Escherichia/Shigella represent a shared core taxon between the two species over time until August (recovery) where it disappears from C. mcneilli entirely. From February to April this bacterium increased in average relative abundance from 10 to 16% in colonies of P. versipora and 14 to 26% for C. mcneilli. With a shift to greater heterotrophy in colonies of P. versipora with diminished symbionts cell densities, the complementary increase in abundance of Escherichia/Shigella sp. may enhance nutrient acquisition by supplementing host feeding (Röthig et al. 2017), although this would need to be proven with isotopic analysis. Escherichia spp. have been previously shown to associate with healthy corals at high temperatures (Kimes et al. 2013; Littman et al. 2011); however, increased abundance can generally be attributed to human impact, specifically faecal contamination and are considered as potentially pathogenic (Kegler et al. 2017). Sydney Harbour is an urban centre therefore it is perhaps not surprising that Escherichia/Shigella sp. are found associating with these corals. Interestingly, Escherichia/Shigella sp. was not detected in seawater samples and thus, the functional role of this bacterium to coral health warrants further investigation.
A shift in metabolic pathways from autotrophy to heterotrophy under thermal stress was also seen in a metagenomic study by Yang et al. (2016), showing increases in bacterial genes responsible for the metabolism of proteins, simple carbohydrates, phosphorous and sulphur. On comparing bleached vs unbleached colonies of P. versipora, there is no difference in the relative abundances of Escherichia/Shigella sp. (14% and 16% respectively) and the complete absence of Escherichia/Shigella sp. in C. mcneilli in August (no thermal stress) together could indicate that this taxon is only abundant in times of stress aiding thermal tolerance, and further consistent with the restoration of normal physiological functioning by August for C. mcneilli.
In addition, Chlorobiaceae and Geothermobacter were consistently abundant in P. versipora and became more abundant in April associated with bleached colonies. Chlorobiaceae, a phylum of bacteria Chlorobi, are well-known green sulphur bacteria with the ability to supply nutrients through nitrogen fixation and are capable of anoxygenic photosynthesis (Randall et al. 2016). Geothermobacter is likely a thermophilic of the family Geobacteraceae and has been previously found associating with both healthy and diseased tropical corals (Hernández-Zulueta et al. 2016; Kashefi et al. 2003) although little is known about the function of this bacterium. It has been proposed that this bacterium is a predominant Fe(III)-reducing microorganism in many environments and plays an important role in nitrogen cycling (Vega Thurber et al. 2014). With the increased abundance of these taxa associating with bleached colonies, increases in nitrogen fixation may increase the N:P ratio in corals, altering coral physiology and resulting in increased susceptibility to bleaching and disease (McDevitt-Irwin et al. 2017).
Marinicella (Gammaproteobacteria) was a dominant taxon associated only with P. versipora in February (4% average abundance) that was lost entirely in bleached colonies and did not return in August during recovery. Gammaproteobacteria has been associated with enriched functional profiles of corals subjected to heat stress (Ziegler et al. 2017) and the disappearance of Marinicella from bleached P. versipora may increase the bleaching susceptibility of this coral. It has been shown that this class of bacteria are strongly associated with healthy corals and dominate corals recovering from bleaching (Bourne et al. 2008), which may explain the abundance of Marinicella in C. mcneilli in August (7% average abundance). It is likely that the services provided by the microbiome, in particular Escherichia/Shigella sp. enabled the rapid and widespread recovery of P. versipora. In addition, this species had a highly diverse microbiome (Shannon 5.3 ± 1, Table 1) during the recovery period consistent with previous evidence of higher microbial diversity associated with healthy corals compared to bleached corals (Bourne et al. 2008; Castillo and Helmuth 2005), a seemingly important metric dictating coral health state.
Holobiont metabolic rate-responses to heat stress
Photosynthesis to respiration ratios (P: R) of P. versipora and C. mcneilli ranged from 1.5–2.2 and 2.1–2.3, respectively, over 20–26 °C (December–February), indicating persistent net autotrophy for both species throughout. P: R values closer to 2 suggest that these species are largely autotrophic at these temperatures, likely explaining the success of P. versipora over such a wide geographical range (Howe and Marshall 2001). Intriguingly, rates of photosynthesis, respiration and calcification (normalised to surface area) in P. versipora and C. mcneilli did not show significant variation with increasing temperature, which may indicate efficient thermal compensation as a way to sustain the symbiotic relationship over a wide temperature range (Anthony et al. 2009; Gibbin et al. 2018). Rates of PG for P. versipora and C. mcneilli in our study were comparable to those previously reported by (Howe and Marshall 2001) at ~ 19 °C and 21 °C and are within the range of most reef corals (Camp et al. 2017) despite the lower temperature and light intensities. Respiration rates of both P. versipora and C. mcneilli obtained in this study were similar to or higher than many reef corals (Camp et al. 2017; Hoogenboom et al. 2010) potentially due to the slow calcification rate of these corals (as also shown by Howe and Marshall 2002), and likely driving the enhanced autotrophic capabilities seen.
In our study during coral bleaching, the decrease in P: R to 1.2 for P. versipora was largely driven by a significant decrease in PG (53–56%) and R remained relatively unchanged. The reduction in PG could be attributed to a decrease in cell density. Here, P. versipora went from a highly autotrophic carbon acquisition mode to increased reliance on heterotrophy between February and June 2016 when this taxon bleached. This metabolic shift is widely reported in for tropical corals under heat stress (Anthony and Fabricius 2000; Grottoli et al. 2006; Tremblay et al. 2016) as well as corals in extreme environments characterised by high-sediment/reduced light conditions (Camp et al. 2017). Enhanced heterotrophic feeding yields additional nutrients (i.e. nitrogen and phosphorus), especially in highly turbid and sediment-enriched environments (e.g. Sydney Harbour). In doing so, these corals from temperate regions may be able to increase in symbiont cell numbers to maximise light capture and restore a stable symbiosis (Rädecker et al. 2015).
For both C. mcneilli and P. versipora, metabolic re-adjustments appear to occur at the expense of calcification under thermal stress. For P. versipora, this could be a direct consequence of the inability to maintain photosynthesis, while respiration remained unchanged (Camp et al. 2017) or it may be an indication that by June 2016 (winter), stored energy reserves had been depleted, and a loss of symbionts resulted (Schoepf et al. 2015). In contrast, C. mcneilli upregulated PG and R during bleaching and as a result P: R was increased by ~ 20% and was > 2, maintaining high autotrophic rates during thermal stress. This was not accompanied with an increase in symbiont density and may suggest that the symbiont has reduced translocation to the host, suppressing potential growth, forcing the host to utilise energy stores or require shifts in the bacterial community to regulate energy transfer within the holobiont (Sorek and Levy 2012). Alternatively, C. mcneilli may have rapidly decreasing calcification rates and redirected the products of photosynthesis as a strategy to cope with stress. Previous studies have in fact shown that coral species compensate for temperature changes in their natural environment by exchanging one set of rate-determining reactions for another (Howe and Marshall 2001; Houlbrèque and Ferrier-Pagès 2009).
P. versipora had regained its colour by August (winter), recovering photosynthetic capacity rapidly even though Symbiodinium cell density was still ~ 50% less than in February (pre-bleaching). It is plausible that heterotrophy helped to restore and maintain the nutritional exchange between host and symbiont by promoting symbiont growth and density, which in turn would allow for increased carbon translocation and lipid storage with an eventual return to autotrophy, ultimately preventing coral mortality as shown previously by Tremblay et al. (2016). For C. mcneilli, “recovery” (even through this coral did not bleach) showed increasing P: R. Together, the decrease in respiration during this period for both species suggests that these corals increased their metabolic efficiency through decreasing respiration as a means to conserve energy stores (Rodrigues and Grottoli 2007), which were most likely depleted during bleaching. The marked return to active calcification could be attributed to a return to normal levels of aragonite saturation or heterotrophy, through the supply of organic molecules and energy or as a direct response to increasing photosynthesis for P. verispora (Tremblay et al. 2016). High adult survivorship has recently been shown as particularly important for the persistence of P. versipora populations in Sydney Harbour (Precoda et al. 2018). Thus, while the physiological patterns seen in this study are highly consistent with those shown for tropical corals under heat stress, clearly a more targeted investigation is needed to resolve the exact nature of the processes driving these metabolic patterns throughout bleaching and recovery for corals on temperate rocky reefs which are acclimatised to extremes.
In summary, by examining the metabolic rates and microbial community properties of high-latitude corals, we observed strong consistencies with bleaching dynamics commonly described for tropical corals, suggesting that there is a common set of general constraints on the physiologies of scleractinian corals under thermal stress irrespective of latitude. Intriguingly, the southerly restricted species (C. mcneilli) proved to be the most tolerant to heat stress and was able to upregulate autotrophic capability during thermal stress. In contrast, the more widely distributed species (P. versipora) metabolic patterns were similar to those observed in tropical corals with coral bleaching, but exhibiting restored photosynthetic capacity rapidly when anomalous temperatures subsided. Our results suggest that as thermal anomaly events grow in frequency and intensity, “tropicalisation” (Vergés et al. 2014, 2016) will likely occur through the simultaneous arrival of subtropical coral species and the loss of temperate coral fitness perhaps for some species only, highlighting the variability and complexity of responses to thermal anomaly events.
Change history
16 April 2019
Co-author name was misspelt and should read as Todd C. LaJeunesse.
References
Abdo DA, Bellchambers LM, Evans SN (2012) Turning up the heat: increasing temperature and coral bleaching at the high latitude coral reefs of the Houtman Abrolhos Islands. PLoS One 7(8):e43878
Ainsworth TD, Krause L, Bridge T, Torda G, Raina JB, Zakrzewski M, Gates RD, Padilla-Gamiño JL, Spalding HL, Smith C, Woolsey ES (2015) The coral core microbiome identifies rare bacterial taxa as ubiquitous endosymbionts. The ISME Journal 9(10):2261
Ainsworth TD, Kvennefors EC, Blackall LL, Fine M, Hoegh-Guldberg O (2007) Disease and cell death in white syndrome of Acroporid corals on the Great Barrier Reef. Marine Biol 151(1):19–29
Anthony KR, Fabricius KE (2000) Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. Journal of experimental marine biology and ecology 252(2):221–253
Anthony KR, Hoogenboom MO, Maynard JA, Grottoli AG, Middlebrook R (2009) Energetics approach to predicting mortality risk from environmental stress: a case study of coral bleaching. Functional ecology 23(3):539–550
Beger M, Sommer B, Harrison PL, Smith SD, Pandolfi JM (2014) Conserving potential coral reef refuges at high latitudes. Diversity and distributions 20(3):245–257
Bessell-Browne P, Stat M, Thomson D, Clode PL (2014) Coscinaraea marshae corals that have survived prolonged bleaching exhibit signs of increased heterotrophic feeding. Coral Reefs 33(3):795–804
Bourne D, Iida Y, Uthicke S, Smith-Keune C (2008) Changes in coral-associated microbial communities during a bleaching event. The ISME journal 2(4):350
Bridge TC, Ferrari R, Bryson M, Hovey R, Figueira WF, Williams SB, Pizarro O, Harborne AR, Byrne M (2014) Variable responses of benthic communities to anomalously warm sea temperatures on a high-latitude coral reef. PloS one 9(11):e113079
Breen DA (2007) Systematic conservation assessments for marine protected areas in New South Wales, Australia. PhD Thesis, James Cook University, Australia
Cai W, Wang G, Santoso A, McPhaden MJ, Wu L, Jin FF, Timmermann A, Collins M, Vecchi G, Lengaigne M, England MH (2015) Increased frequency of extreme La Niña events under greenhouse warming. Nature Climate Change 5(2):132
Camp EF, Krause SL, Santos LM, Naumann MS, Kikuchi RK, Smith DJ, Wild C, Suggett DJ (2015) The “Flexi-Chamber”: A novel cost-effective in situ respirometry chamber for coral physiological measurements. PloS one 10(10):e0138800
Camp EF, Nitschke MR, Rodolfo-Metalpa R, Houlbreque F, Gardner SG, Smith DJ, Zampighi M, Suggett DJ (2017) Reef-building corals thrive within hot-acidified and deoxygenated waters. Scientific reports 7(1):2434
Camp EF, Schoepf V, Mumby PJ, Hardtke LA, Rodolfo-Metalpa R, Smith DJ, Suggett DJ (2018) The future of coral reefs subject to rapid climate change: lessons from natural extreme environments. Frontiers in Marine Science 5:4
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA (2010) QIIME allows analysis of high-throughput community sequencing data. Nature methods 7(5):335
Castillo KD, Helmuth BST (2005) Influence of thermal history on the response of Montastraea annularis to short-term temperature exposure. Marine Biology 148(2):261–270
Dalton SJ, Carroll AG (2011) Monitoring coral health to determine coral bleaching response at high latitude eastern Australian reefs: an applied model for a changing climate. Diversity 3(4):592–610
Fujise R (2018) Distribution, abundance and life cycle of free-living Symbiodinium (Doctoral dissertation)
Gardner SG, Raina JB, Ralph PJ, Petrou K (2017) Reactive oxygen species (ROS) and dimethylated sulphur compounds in coral explants under acute thermal stress. Journal of Experimental Biology 220(10):1787–1791
Gardner SG, Camp EF, Smith DJ, Kahlke T, Osman EO, Gendron G, Hume BCC, Pogoreutz C, Voolstra CR, Suggett DJ (2019) Coral microbiome diversity reflects mass coral bleaching susceptibility during the 2016 El Niño heat wave. Ecology and evolution 9(3):938–956
Gibbin EM, Krueger T, Putnam HM, Barott KL, Bodin J, Gates RD, Meibom A (2018) Short-Term Thermal Acclimation Modifies the Metabolic Condition of the Coral Holobiont. Frontiers in Marine Science 5:10
Grottoli AG, Rodrigues LJ, Palardy JE (2006) Heterotrophic plasticity and resilience in bleached corals. Nature 440(7088):1186
Grottoli AG, Martins PD, Wilkins MJ, Johnston MD, Warner ME, Cai WJ, Melman TF, Hoadley KD, Pettay DT, Levas S, Schoepf V (2018) Coral physiology and microbiome dynamics under combined warming and ocean acidification. PloS one 13(1):e0191156
Harrison PL, Dalton SJ, Carroll AG (2011) Extensive coral bleaching on the world’s southernmost coral reef at Lord Howe Island. Australia. Coral Reefs 30(3):775
Hennige SJ, Smith DJ, Walsh SJ, McGinley MP, Warner ME, Suggett DJ (2010) Acclimation and adaptation of scleractinian coral communities along environmental gradients within an Indonesian reef system. Journal of Experimental Marine biology and ecology 391(1–2):143–152
Hernández-Zulueta J, Araya R, Vargas-Ponce O, Díaz-Pérez L, Rodríguez-Troncoso AP, Ceh J, Ríos-Jara E, Rodríguez-Zaragoza FA (2016) First deep screening of bacterial assemblages associated with corals of the Tropical Eastern Pacific. FEMS microbiology ecology 92(12):fiw196
Heron SF, Maynard JA, Van Hooidonk R, Eakin CM (2016) Warming trends and bleaching stress of the world’s coral reefs 1985–2012. Scientific reports 6:38402
Hoogenboom M, Rodolfo-Metalpa R, Ferrier-Pagès C (2010) Co-variation between autotrophy and heterotrophy in the Mediterranean coral Cladocora caespitosa. Journal of Experimental Biology 213(14):2399–2409
Houlbrèque F, Ferrier-Pagès C (2009) Heterotrophy in tropical scleractinian corals. Biological Reviews 84(1):1–17
Howe SA, Marshall AT (2001) Thermal compensation of metabolism in the temperate coral, Plesiastrea versipora (Lamarck, 1816). Journal of experimental marine biology and ecology 259(2):231–248
Howe SA, Marshall AT (2002) Temperature effects on calcification rate and skeletal deposition in the temperate coral, Plesiastrea versipora (Lamarck). Journal of experimental marine biology and ecology 275(1):63–81
Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R, Bridge TC (2017) Global warming and recurrent mass bleaching of corals. Nature 543(7645):373
Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Claar DC (2018) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359(6371):80–83
Kahlke T (2018) Ampli-Tools (Version 1.0). Zenodo. https://doi.org/10.5281/zenodo.1137872
Kashefi K, Holmes DE, Baross JA, Lovley DR (2003) Thermophily in the Geobacteraceae: Geothermobacter ehrlichii gen. nov., sp. nov., a novel thermophilic member of the Geobacteraceae from the “Bag City” hydrothermal vent. Applied and environmental microbiology 69(5):2985–2993
Kegler HF, Lukman M, Teichberg M, Plass-Johnson J, Hassenrück C, Wild C, Gärdes A (2017) Bacterial community composition and potential driving factors in different reef habitats of the Spermonde Archipelago. Indonesia. Frontiers in microbiology 8:662
Kimes NE, Johnson WR, Torralba M, Nelson KE, Weil E, Morris PJ (2013) The Montastraea faveolata microbiome: ecological and temporal influences on a Caribbean reef-building coral in decline. Environmental microbiology 15(7):2082–2094
Kleypas JA, Buddemeier RW, Archer D, Gattuso JP, Langdon C, Opdyke BN (1999) Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 284(5411):118–120
LaJeunesse TC, Loh WK, Van Woesik R, Hoegh-Guldberg O, Schmidt GW, Fitt WK (2003) Low symbiont diversity in southern Great Barrier Reef corals, relative to those of the Caribbean. Limnology and Oceanography 48(5):2046–2054
LaJeunesse TC, Parkinson JE, Reimer JD (2012) A genetics‐based description of Symbiodinium minutum sp. nov. and S. psygmophilum sp. nov.(Dinophyceae), two dinoflagellates symbiotic with cnidaria. Journal of Phycology 48(6):1380–1391
Le Nohaïc M, Ross CL, Cornwall CE, Comeau S, Lowe R, McCulloch MT, Schoepf V (2017) Marine heatwave causes unprecedented regional mass bleaching of thermally resistant corals in northwestern Australia. Sci Rep 7(1):14999
Levas S et al (2016) Can heterotrophic uptake of dissolved organic carbon and zooplankton mitigate carbon budget deficits in annually bleached corals? Coral Reefs 35(2):495–506
Lewis E, Wallace D, Allison LJ (1998) Program developed for CO2 system calculations. No. ORNL/CDIAC-105. Brookhaven National Lab., Dept. of Applied Science, Upton, NY (United States); Oak Ridge National Lab., Carbon Dioxide Information Analysis Center, TN (United States)
Littman R, Willis BL, Bourne DG (2011) Metagenomic analysis of the coral holobiont during a natural bleaching event on the Great Barrier Reef. Environmental Microbiology Reports 3(6):651–660
Madsen A et al (2014) The reproductive biology of the scleractinian coral Plesiastrea versipora in Sydney Harbour, Australia. Sexuality and Early Development in Aquatic Organisms 1(1):25–33
Magoč T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27(21):2957–2963
McDevitt-Irwin JM, Baum JK, Garren M, Vega Thurber RL (2017) Responses of coral-associated bacterial communities to local and global stressors. Frontiers in Marine Science 4:262
Mizerek TL, Baird AH, Beaumont LJ, Madin JS (2016) Environmental tolerance governs the presence of reef corals at latitudes beyond reef growth. Global ecology and biogeography 25(8):979–987
Mumby PJ, Chisholm JR, Edwards AJ, Andrefouet S, Jaubert J (2001) Cloudy weather may have saved Society Island reef corals during the 1998 ENSO event. Marine Ecology Progress Series 222:209–216
Naumann MS, Jantzen C, Haas AF, Iglesias-Prieto R, Wild C (2013) Benthic primary production budget of a Caribbean reef lagoon (Puerto Morelos, Mexico). PLoS One 8(12):e82923
Pandolfi JM, Connolly SR, Marshall DJ, Cohen AL (2011) Projecting coral reef futures under global warming and ocean acidification. Science 333(6041):418–422
Parks DH, Beiko RG (2010) Identifying biologically relevant differences between metagenomic communities. Bioinformatics 26(6):715–721
Prazeres M, Ainsworth T, Roberts TE, Pandolfi JM, Leggat W (2017) Symbiosis and microbiome flexibility in calcifying benthic foraminifera of the Great Barrier Reef. Microbiome 5(1):38
Precoda K, Baird AH, Madsen A, Mizerek T, Sommer B, Su SN, Madin JS (2018) How does a widespread reef coral maintain a population in an isolated environment? Marine Ecol Prog Ser 594:85–94
Pollard D, Ortiz E, Pethebridge R (1997) New South Wales marine and coastal bioregionalisation study: towards the development of a representative system of marine and estuarine protected areas for New South Wales. Coasts and Clean Seas Program; Ocean Rescue 2000 Project Report Series
Rädecker N, Pogoreutz C, Voolstra CR, Wiedenmann J, Wild C (2015) Nitrogen cycling in corals: the key to understanding holobiont functioning? Trends in Microbiology 23(8):490–497
Randall CJ, Jordán-Garza AG, Muller EM, van Woesik R (2016) Does dark-spot syndrome experimentally transmit among Caribbean corals? PloS one 11(1):e0147493
Roder C, Arif C, Bayer T, Aranda M, Daniels C, Shibl A, Chavanich S, Voolstra CR (2014) Bacterial profiling of White Plague Disease in a comparative coral species framework. The ISME journal 8(1):31
Rodrigues LJ, Grottoli AG (2007) Energy reserves and metabolism as indicators of coral recovery from bleaching. Limnology and oceanography 52(5):1874–1882
Rognes T, Flouri T, Nichols B, Quince C, Mahé F (2016) VSEARCH: a versatile open source tool for metagenomics. PeerJ 4:e2584
Röthig T, Yum LK, Kremb SG, Roik A, Voolstra CR (2017) Microbial community composition of deep-sea corals from the Red Sea provides insight into functional adaption to a unique environment. Scientific Reports 7:44714
Sampayo EM, Ridgway T, Bongaerts P, Hoegh-Guldberg O (2008) Bleaching susceptibility and mortality of corals are determined by fine-scale differences in symbiont type. Proceedings of the National Academy of Sciences
Scheufen T, Iglesias-Prieto R, Enríquez S (2017) Changes in the number of symbionts and Symbiodinium cell pigmentation modulate differentially coral light absorption and photosynthetic performance. Frontiers in Marine Science 4:309
Schoepf V, Stat M, Falter JL, McCulloch MT (2015) Limits to the thermal tolerance of corals adapted to a highly fluctuating, naturally extreme temperature environment. Scientific reports 5:17639
Silverstein RN, Correa AM, LaJeunesse TC, Baker AC (2011) Novel algal symbiont (Symbiodinium spp.) diversity in reef corals of Western Australia. Marine Ecology Progress Series 422:63–75
Silverstein RN, Cunning R, Baker AC (2017) Tenacious D: Symbiodinium in clade D remain in reef corals at both high and low temperature extremes despite impairment. Journal of Experimental Biology 220(7):1192–1196
Sommer B et al (2014) Trait-mediated environmental filtering drives assembly at biogeographic transition zones. Ecology 95(4):1000–1009
Sommer B, Beger M, Harrison PL, Babcock RC, Pandolfi JM (2018) Differential response to abiotic stress controls species distributions at biogeographic transition zones. Ecography 41(3):478–490
Sorek M, Levy O (2012) Influence of the quantity and quality of light on photosynthetic periodicity in coral endosymbiotic algae. PLoS One 7(8):e43264
Suggett DJ, Smith DJ (2011) Interpreting the sign of coral bleaching as friend vs. foe. Global Change Biology 17(1):45–55
Suggett DJ, Hall-Spencer JM, Rodolfo-Metalpa R, Boatman TG, Payton R, Tye Pettay D, Johnson VR, Warner ME, Lawson T (2012) Sea anemones may thrive in a high CO2 world. Global Change Biology 18(10):3015–3025
Suggett DJ, Dong LF, Lawson T, Lawrenz E, Torres L, Smith DJ (2013) Light availability determines susceptibility of reef building corals to ocean acidification. Coral reefs 32(2):327–337
Swofford DL (2014) PAUP Phylogenetic Analysis Using Parsimony (and other methods). Sinauer Associates, Sunderland, Massachusetts
Thomson DP et al (2011) High latitude, deeper water coral bleaching at Rottnest Island, Western Australia. Coral Reefs 30(4):1107
Thurber RV, Willner-Hall D, Rodriguez-Mueller B, Desnues C, Edwards RA, Angly F, Dinsdale E, Kelly L, Rohwer F (2009) Metagenomic analysis of stressed coral holobionts. Environmental Microbiology 11(8):2148–2163
Tout J, Siboni N, Messer LF, Garren M, Stocker R, Webster NS, Ralph PJ, Seymour JR (2015) Increased seawater temperature increases the abundance and alters the structure of natural Vibrio populations associated with the coral Pocillopora damicornis. Frontiers in microbiology 6:432
Tremblay P, Gori A, Maguer JF, Hoogenboom M, Ferrier-Pagès C (2016) Heterotrophy promotes the re-establishment of photosynthate translocation in a symbiotic coral after heat stress. Scientific reports 6:38112
Tuckett CA, de Bettignies T, Fromont J, Wernberg T (2017) Expansion of corals on temperate reefs: direct and indirect effects of marine heatwaves. Coral Reefs 36(3):947–956
Tuckett CA, Wernberg T (2018) High Latitude Corals Tolerate Severe Cold Spell. Frontiers in Marine Science 5:14
Vega Thurber RL, Burkepile DE, Fuchs C, Shantz AA, McMinds R, Zaneveld JR (2014) Chronic nutrient enrichment increases prevalence and severity of coral disease and bleaching. Global change biology 20(2):544–554
Vergés A, Steinberg PD, Hay ME, Poore AG, Campbell AH, Ballesteros E, Heck KL Jr, Booth DJ, Coleman MA, Feary DA, Figueira W (2014) The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proc R Soc B 281(1789):20140846
Vergés A, Doropoulos C, Malcolm HA, Skye M, Garcia-Pizá M, Marzinelli EM, Campbell AH, Ballesteros E, Hoey AS, Vila-Concejo A, Bozec YM (2016) Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. Proceedings of the National Academy of Sciences 113(48):13791–13796
Veron JEN (2000) Corals of the World (No. C/593.6 V4)
Wangpraseurt D, Holm JB, Larkum AW, Pernice M, Ralph PJ, Suggett DJ, Kühl M (2017) In vivo microscale measurements of light and photosynthesis during coral bleaching: evidence for the optical feedback loop? Frontiers in microbiology 8:59
Warner M et al (2002) Seasonal fluctuations in the photosynthetic capacity of photosystem II in symbiotic dinoflagellates in the Caribbean reef-building coral Montastraea. Marine Biology 141(1):31–38
Wooldridge SA (2009) A new conceptual model for the warm-water breakdown of the coral–algae endosymbiosis. Marine and Freshwater Research 60(6):483–496
Yang SH, Lee ST, Huang CR, Tseng CH, Chiang PW, Chen CP, Chen HJ, Tang SL (2016) Prevalence of potential nitrogen-fixing, green sulfur bacteria in the skeleton of reef-building coral I sopora palifera. Limnology and Oceanography 61(3):1078–1086
Zardoya R, Costas E, López-Rodas V, Garrido-Pertierra A, Bautista JM (1995) Revised dinoflagellate phylogeny inferred from molecular analysis of large-subunit ribosomal RNA gene sequences. Journal of molecular evolution 41(5):637–645
Zhang H, Bhattacharya D, Lin S (2005) Phylogeny of dinoflagellates based on mitochondrial cytochrome b and nuclear small subunit rdna sequence comparisons 1. Journal of Phycology 41(2):411–420
Ziegler M et al (2017) Bacterial community dynamics are linked to patterns of coral heat tolerance. Nature Communications 8:14213
Ziegler M, Roik A, Porter A, Zubier K, Mudarris MS, Ormond R, Voolstra CR (2016) Coral microbial community dynamics in response to anthropogenic impacts near a major city in the central Red Sea. Marine pollution bulletin 105(2):629–640
Acknowledgements
AL was instrumental in experimental design and operation at Manly SeaLife Sanctuary. Fieldwork support was provided by MN, EC, LF and AL. Sample processing and data analysis support was provided by TL, EC, MN, TK and DS. Manly SeaLife Sanctuary provided the laboratory and technical support for aquaria experimentation. Fieldwork support was provided by Caitlin Lawson, Trent Haydon, Shannen Smith, Rochelle Johnson and Penny McCracken. Sample processing and data analysis support was provided by Nahshon Siboni and Leo Hardtke. This research was supported by an Australian Postgraduate Award, and research funding was provided by the Climate Change Cluster and the School of Life Sciences, University of Technology Sydney, Experiment.com crowdfunding and Bioplatforms Australia Marine Microbes. Contribution of DS and EC were under ARC Discovery Grant DP160100271. Corals were collected under permit number P15/0042-1. We thank the reviewers Claire Ross and Brigitte Sommer for their comments greatly improving the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Topic Editor Morgan S. Pratchett
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Goyen, S., Camp, E.F., Fujise, L. et al. Mass coral bleaching of P. versipora in Sydney Harbour driven by the 2015–2016 heatwave. Coral Reefs 38, 815–830 (2019). https://doi.org/10.1007/s00338-019-01797-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-019-01797-6