Abstract
Two key morphological traits, horizontal gape and eye diameter, were measured in a large representative group of coral reef fishes. These morphological traits were used concurrently to assess their utility in exploring abilities of coral reef fishes at an assemblage level. A total of 1,218 specimens from 181 species found on the Great Barrier Reef were examined. Cryptobenthic fishes were included to provide a broader representation of reef fish groups. In the analyses, a clear morphological distinction was found between nocturnal and diurnal fishes. Nocturnal fishes had larger relative horizontal gapes and relative eye diameters by factors of 1.6 and 1.5, respectively. A bivariate plot separated into quadrants was used to assess the implications of morphological variation. The morphological measures reflected distinct ecological traits in each quadrant. Whilst nocturnal fishes had large relative gapes and eye diameters, diurnal predators and detritivores had the same wide gapes, but small relative eye diameters. Highly selective, visual feeders such as the Chaetodontidae and Pseudochromidae had large eyes and small gapes, whilst non-selective feeders with low visual dependence such as the grazing herbivores (Acanthuridae, Siganidae, etc.) had both small eye diameters and gape sizes. The analysis proved to be robust enough to apply to a wide assemblage, but with enough subtlety to distinguish morphological differences within individual families. The methods used in this study may have broad applications to other fish assemblages, both fossil and extant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The fish assemblages found on modern day coral reefs display a huge degree of morphological diversity. The study of these morphologies in an ecological context has become a useful tool in determining how these differing shapes and structures bestow differing abilities. A broad range of morphological attributes of reef fishes have been studied and have revealed strong links between structure and performance in a range of functional systems, from locomotion (e.g., Wainwright et al. 2002; Collar et al. 2008) and prey acquisition (e.g., Ferry-Graham et al. 2001a, b; Wainwright and Bellwood 2002) to prey processing (Wainwright 1989; Choat et al. 2004). Whilst these studies have been of great use in furthering the understanding of reef fish ecology, the identification of reliable predictors is key to progressing in this field of ecological morphology. The goal of this study therefore, is to critically evaluate two key morphological traits. However, unlike previous studies that examine specific taxa, we will examine a broad representation of coral reef fishes, with multiple members from all major families found on the Great Barrier Reef.
The oral jaws of reef fishes are arguably one of the most diverse morphological systems among vertebrates. The variety of forms, from wide crushing beaks to greatly protrusible structures may be facilitated in many fishes by the functional decoupling of the oral and pharyngeal jaws, which has allowed fishes to diversify their feeding modes and exploit new niches (Liem 1973; Hulsey et al. 2006). In investigating the links between morphological and dietary diversity, gape measurements are one of the most frequently used metrics (e.g., Kotrschal 1988; Wainwright and Richard 1995; Persson et al. 1996; Truemper and Lauer 2005). Of all the potential measurements that could be made of fish jaws, the horizontal gape is arguably one of the most relevant and easily measured morphological traits. The size of a fish’s mouth is likely to determine the maximum size prey item that it can ingest and across fishes there is a common ontogenetic trend of increasing prey size with increasing gape (Scharf et al. 2000; Wainwright and Bellwood 2002). Horizontal gape may therefore represent a broad measure of potential prey size.
After the oral jaws, the sensory system is the most important component of prey procurement. Of all senses, vision is the primary sensory modality in almost all fishes (Guthrie and Muntz 1993; Myrberg and Fuiman 2002). This is true at all developmental stages. Even very small larval fishes can have surprising visual abilities and are often able to detect prey at the low light intensities found at water depths in excess of several hundreds of metres (Job and Bellwood 2000). When investigating these visual capabilities, or those of any organism, there are two important components to consider, sensitivity and acuity. Visual sensitivity relates to the ability of an organism to respond to visual stimuli in low light conditions, whereas visual acuity relates to the ability to resolve detail (McFarland 1991; Job and Shand 2001; Warrant 2004). Although these two components are fundamentally different, both can benefit from an increase in overall eye diameter. Larger eyes contain larger retinae and lenses, allowing more light to be captured and this, in turn benefits both sensitivity and acuity, depending on which component the eye is optimised for (Shand 1997). Larger eyes do, however, come at a price. They are energetically costly to develop and maintain (Protas et al. 2007), they take up space in the head, which could be used for other purposes such as jaw musculature and they provide an obvious target for predators (Gagliano 2008). A trade off appears to exist between the benefits of increased visual abilities and the cost of larger eyes.
The aim of this study is to use data from these two key morphological traits simultaneously in order to assess their utility in exploring the abilities of coral reef fishes at an assemblage level. Where previous studies have focussed on complex morphological traits in a single or limited number of taxa, this study will consider two of the most functionally informative and robust morphological traits, eye diameter and horizontal gape, from a large representative group of fishes from one of the most complex ecosystems, a coral reef. In order to ensure complete coverage, this study will include cryptobenthic taxa. These fishes, with adults typically under 50 mm standard length (sensu Depczynski and Bellwood 2003) may be of great importance to coral reef ecology in terms of abundance (Ackerman and Bellwood 2000) and energy flows (Depczynski et al. 2007), but have typically been neglected in previous studies (Ackerman and Bellwood 2000). The inclusion of these fishes in this study will be one of the first occasions where the morphologies of representatives from an entire fish assemblage, including cryptobenthic taxa, have been studied. By examining such a broad range of species it is hoped to be able to explore the full expression of morphological complexity in the two key traits in an exceptionally diverse fish assemblage.
Materials and methods
A total of 1,218 specimens from 181 species in 57 families were examined. Of these, 141 species were represented by a mean of 8.4 replicate individuals whilst the remaining 40 were represented by single specimens. All were from the Great Barrier Reef, primarily from Orpheus Island and Lizard Island. The measurements taken from specimens were standard length (SL), horizontal gape, eye diameter and mass. SL was chosen as a measure of fish length as it accounts for any specimens that had highly modified or damaged caudal fins. Horizontal gape was measured as the largest internal horizontal distance in the oral jaws that could be measured without visible distortion of the fish’s mouth. Finally, eye diameter was measured as the maximum external width of the eyeball along the anterior–posterior axis. Measurements of large (>40 mm SL) fishes were made using digital or dial callipers accurate to at least 0.05 mm. For small cryptobenthic species (<40 mm SL) horizontal gape and eye diameter were measured using a binocular microscope with a calibrated eyepiece graticule. All specimens were formalin fixed; no corrections for shrinkage were undertaken, eye diameters might thus be slightly underestimated (Job and Bellwood 1996). The mass of whole fish specimens was measured on a balance accurate to 0.01 g.
The relative sizes of the key traits considered in this study were calculated against the SL of the fishes. Initially, the cubed root of the mass of fishes was used as a shape independent measure of somatic size instead of SL. This was used to reduce the effect of fish shape in the analysis. However, due to the similarity of the results from the two indices it was decided to use SL, as it is more accessible and more easily visualised (for cubed root of mass data and analysis see Electronic supplemental material).
Fishes in the dataset were classified as diurnal or nocturnal based on published observations in Randall et al. (1997), supplemented by Froese and Pauly (2008). Trophic categories likewise follow Randall et al. (1997). To analyse the data, the mean relative sizes of the key traits were considered. Regressions of the standardised data were tested against standard length to explore general allometric trends in the key traits. Following this, analyses of covariances (ANCOVAs; Type I and type III SS) were used to compare the slopes between nocturnal and diurnal species.
Results
Analyses of the 181 fish species revealed a slight negative allometry in the relative horizontal gape (slope = −0.0007), but with a high variance at all sizes (r 2 = 0.0003). There was no significant relationship between relative gape and absolute size (excluding nocturnal elongate species). Two distinct regressions were produced by separating the species into diurnal and nocturnal categories (Fig. 1a, ANCOVA, F 1,178 = 46.38, P < 0.0001). On average, nocturnal fishes had larger relative horizontal gapes than diurnal fishes by a factor of 1.6. If nocturnal elongate species are included, the differences between the two categories are even more marked.
The relationships between the two morphological traits a the mean horizontal gape and b the mean eye diameter, with the mean standard length of the fish species studied. Solid circles represent nocturnal species whilst open circles represent diurnal species. The upper regression line shows the trend in the data for nocturnal species (excluding elongate species) and the lower line shows the trends in the data for diurnal species. Each symbol represents one species based on the mean value of approximately eight specimens. The regression equations for relative horizontal gape were y = −0.006x + 11.262 and y = −0.002x + 6.421 for nocturnal and diurnal fishes, respectively. For relative eye diameter the regression equations were y = −0.019x + 13.750 and y = −0.0134x + 9.266 for nocturnal and diurnal fishes, respectively
The relative eye diameters of fishes again showed negative allometry (slope = −0.016) with high variance (r 2 = 0.20), although this time there is a significant relationship (P < 0.0001), however, the use of residual data changed little in the overall analysis (see Supplemental material). Splitting data into two categories once again produced two distinct regressions and on average, nocturnal fishes displayed relative eye diameters approximately 1.5 times larger than their diurnal counterparts (Fig. 1b, ANCOVA, Log10 (x + 1) transformed, F 1,178 = 30.48, P < 0.0001).
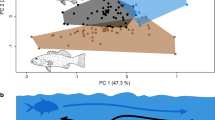
Although the diurnal and nocturnal fishes were seen to show differing morphologies, a relatively large number of overlapping data points were found in both the relative horizontal gape and relative eye diameter data (Fig. 1a, b). However, this overlap was greatly reduced when a biplot of both the average relative horizontal gape and eye diameter of each species was constructed. If the mean values of the two traits are drawn onto the biplot, effectively splitting it into four quadrants, each of the quadrants exhibits distinctive morphological and ecological characteristics (Fig. 2, values available in the electronic supplemental material).
The mean relative horizontal gape of fish species plotted against the mean relative eye diameter (as a percentage of standard length). The lines separating the biplot show the mean value of that morphological trait for all fishes studied. Filled circles represent nocturnal species and open circles represent diurnal species. Each point represents one species based on the mean value of approximately five specimens
The upper right quadrant is comprised of fishes with relative gapes and eye diameters larger than average. Most of the nocturnal species (80%) were found to occupy this quadrant and these species differed significantly from their diurnal counterparts (ANCOVA, F 1,177 = 13.13, P < 0.001, slope equations y = 0.12x + 6.98 and y = 0.79x + 3.47 for diurnal and nocturnal fishes, respectively), of which just 11.2% of species occupied the same quadrant. The diurnal fishes in this quadrant were primarily planktivorous species from the Pomacentridae along with members of the Tetraodontidae, Scorpaenidae, Gobiidae and Ephippidae. The bivariate mean position of the nocturnal fishes was found in the upper right hand quadrant with a relative gape of 10.4 ± 0.5% SL and a relative eye diameter of 11.7 ± 0.7% SL (mean ± SE). Diurnal fishes were found in all other quadrants, but the bivariate mean of diurnal fishes occupied the lower left hand quadrant with a relative gape of 6.6 ± 0.2% SL and a relative eye diameter of 7.8 ± 0.2% SL (mean ± SE). The occupants of the lower right hand quadrant, characterised by having relatively small eye diameters and large gapes, were primarily from the Blenniidae, Haemulidae, Mugilidae, Synanceiidae and Synodontidae.
The two quadrants to the left of the biplot are characterised by small relative horizontal gapes. Those in the upper quadrant (the Chaetodontidae, Monacanthidae, Zanclidae, several Pomacentridae and numerous other taxa), have relatively large eye diameters. Meanwhile, the majority of diurnal fish taxa studied (including the Acanthuridae, Labridae and Siganidae along with most of the Pomacanthidae, Gobiidae, Kyphosidae, Siganidae, etc.) were found to occupy the lower of these quadrants with small relative eye diameters. The upper quadrant was found to host one nocturnal taxon, the Sargocentron (Holocentridae) whilst the morphology of the other member of this family; the Myripristis (Holocentridae) placed them with the nocturnal fishes. A small cluster of both diurnal and nocturnal fishes was found to sit away from the other clusters of fishes in the lower left hand quadrant. These were all found to be elongate species including diurnal syngnathids and fistulariids and nocturnal syngnathids, bythitids and muraenids.
The cryptobenthic fishes, like all others were predominantly found in the lower left quadrant of the biplot but were represented in all other quadrants. The Blenniidae occupied the lower right hand quadrant of the biplot along with several species of Gobiidae from the genera Callogobius, Signigobius, Valenciennea and Gobiodon. The upper left hand quadrant hosted the Callionymidae and Tripterygiidae along with most of the Pseudochromidae, Assessor macneilli (Plesiopidae) and Eviota sp. G. (Gobiidae). Several cryptobenthic species were found in the upper right quadrant including Paragobiodon echinocephalus, Amblygobius phalaena, Bathygobius fuscus (Gobiidae), Entomacrodus decussatus (Blenniidae) and Plesiops coeruleolineatus (Plesiopidae).
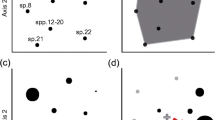
To better visualise the results of the biplot, a representative group of individual coral reef fish families were plotted onto the biplot structure (Fig. 3). This allowed a better understanding of the patterns seen in the biplot and some further trends to be elucidated which were missed when using the results table. The Siganidae and Acanthuridae both occupy a similar region of the biplot, with most species occupying the lower left quadrant. Most of the Chaetodontidae exist in a highly constrained morphospace, with the exception of two species, Chelmon rostratus, which has a slightly smaller relative eye diameter than the other chaetodontids and a considerably narrower relative horizontal gape and Chaetodon lunulatus with a larger relative horizontal gape than the other chaetodontids.
Discussion
Relative eye size and gape revealed a fundamental division in reef fish morphology between diurnal and nocturnal fishes. Nocturnal fishes have both larger eyes and horizontal gapes than most diurnal fishes, resulting in them sitting in the top right hand quadrant of the biplot. Diurnal fishes occupied all quadrants, but clear differences in the morphological and ecological characteristics of these fishes can be found which characterise the occupants of each separate quadrant. The outcomes of the simple morphometric analyses performed in this study build upon foundations put forward in works such as Kotrschal’s (1988) study of jaw morphology across reef fishes and Myrberg and Fuiman’s (2002) considerations of eye morphology between diurnal and nocturnal reef fishes. However, the application of key morphological indicators to a broad assemblage of fishes has provided a new perspective on the utility of reef fish morphology.
Fishes with large eyes and large mouths
The large eye diameters and horizontal gapes seen in the nocturnal fishes may be related. Large eyes increase overall visual performance in fishes (Johns and Easter 1977; Hairston et al. 1982; Fernald 1991; Miller et al. 1993) although fishes adapted to life in low light environments are likely to have eyes optimised for sensitivity, thus sacrificing acuity (Warrant 2004). Evidence of reduced acuity has been shown for some nocturnal fishes. Although the reaction distances of Apogon annularis (Apogonidae) are light dependent, the visual sensitivity of this species is high and the fishes are unable to detect planktonic organisms below 0.9 mm in length at any light level due to reduced visual acuity (Holzman and Genin 2005). This concept helps to highlight a possible reason for the relatively large horizontal gapes seen in nocturnal fishes. The size of a fish’s gape does not provide a definite indication of the size of prey it ingests; however, it does provide an indication of the maximum potential size of prey (Scharf et al. 2000). Nocturnal fishes may not be as capable as diurnal fishes at detecting small prey items due to potential visual acuity-sensitivity trade-offs, thus are likely to be restricted to targeting relatively large prey items. Larger gapes will, therefore, maximise the proportion of items detected, which can be acquired as prey. This is consistent with Marnane and Bellwood (2002) in that nocturnal Apogonidae were found to have larger prey than their diurnal plankton feeding counterparts.
Of the diurnal taxa to inhabit this quadrant, many were small planktivores, particularly from the Pomacentridae. These fishes, which select individual prey from the water column, are highly visually dependent as their prey is small and often transparent (Johnsen 2001). Large mouth diameters in these small fishes are expected to maximise the range of prey available whilst large eye diameters can allow increased visual acuity, in turn increasing prey detection and acquisition (Hairston et al. 1982; McFarland 1991).
Fishes with small eyes and large mouths
The lower right hand quadrant is characterised by large relative gapes and small relative eye diameters and is populated by many diurnal raptorial predators. These fishes from the Serranidae, Synanceiidae, Synodontidae and a few other taxa generally feed in the relatively high light environment found during the day on coral reefs (Shpigel and Fishelson 1989; Randall et al. 1997). The large gapes of these fishes may, as considered for the nocturnal taxa, facilitate the ingestion of larger prey items. The detection of these large items in high light conditions would not demand great visual performance, allowing the relatively small eye diameters. The small eye diameters may also reflect a negative allometry in eye size and the large somatic size of most diurnal raptorial predators (Strauss 1984). The use of residuals (which allow for changes in body size), however, did not change the pattern, suggesting that allometry played only a small role in eye size distributions.
With wide gapes, the detritivorous Blenniidae (Depczynski and Bellwood 2003) and Mugilidae (Blaber 1976) also occupied this quadrant indicating that this morphology may be of benefit for fishes that depend on benthic detritus. The difference between the predatory and detritivorous fishes in this quadrant is likely to be driven by the vertical gape, which is expected to be larger in the predators and may identify two discrete functional modes within this morphospace.
Fishes with large eyes and small mouths
All fishes in the left half of the biplot are characterised by having small horizontal gapes. In previous studies, these fishes have been placed in the same morphospace, as only jaw morphologies were considered (e.g., Kotrschal 1988; Bellwood 2003). The addition of a gradient of eye diameter helps to separate the data and highlights differences between taxa with small gapes. Here, reliance on vision in prey acquisition is a potential functional character dividing this group. The large relative eye diameters in the upper left quadrant are seen in fishes that target both mobile and sedentary prey items, but most are highly selective. Some diurnal planktivorous fishes, such as Abudefduf sexfasciatus, Acanthochromis polyacanthus and Chromis atripectoralis (Pomacentridae), occupied this quadrant. As discussed above, these fishes will benefit from increased visual acuity afforded by large eye diameters. However, their small gapes suggest a more precise feeding mode than their counterparts in the upper right quadrant.
Visual prey detection may also be important in the Chaetodontidae, which in almost all cases feed on small benthic prey. These fishes can accurately select small prey items from complex substrata (Motta 1989; Ferry-Graham et al. 2001a) and are often highly dependent on very specific prey types (Motta 1989; Ferry-Graham et al. 2001a; Pratchett et al. 2006; Berumen and Pratchett 2008). Whilst the importance of vision in finding food patches for the Chaetodontidae is understood (Reese 1989), the large relative eye diameters observed in this family imply that vision is also highly important for finding individual small prey items in highly topographically complex reef environments.
Fishes with small eyes and small mouths
The fishes in the lower quadrant have smaller eyes and it may be hypothesised that they are less reliant on vision. In a broad study of the skeletal morphology of both fossilised and recent fish jaws, Bellwood (2003) identified an “herbivorous morphospace” defined by fishes having small forceful jaws. Of the taxa found to exist in this morphospace, the majority were found to occupy the lower left hand quadrant of the biplot created in the present study. However, the concept of an exclusively “herbivorous morphospace” is not fully supported in the modern reef assemblage studied herein, as many of the Gobiidae, Labridae and Pomacanthidae were found to inhabit the same morphospace as the herbivorous taxa. These additional taxa are not obligate herbivores, although some degree of herbivory is known from many species within each of the families (Hourigan et al. 1989; Bellwood and Choat 1990; Ryan 1991; Depczynski and Bellwood 2003; MacArthur and Hyndes 2007). Considering all occupants of the lower left hand quadrant, there is a suggestion that rather than an “herbivorous morphospace” there is a morphospace into which many of the reef herbivores (sensu Bellwood 2003) fit, which may be more accurately identified as a region of morphospace dominated by less visually dependent fishes that feed in a precise manner on small or relatively immobile prey.
Exceptions to the general patterns
The most obvious exceptions to the general patterns seen in the biplot were found in the lower left quadrant. Whether diurnal or nocturnal, all elongate fishes were found in a cluster close to the origin of the biplot. Elongate morphology constrains relative eye diameters and horizontal gape independent of diel activity and the extreme length may distort the relative data to such a degree that the data must cluster. Furthermore, as data approach the origin of the biplot there is less two-dimensional mathematical space available to occupy, thus the data will again be forced to cluster. This factor may not be of great consequence as these species do share a similar morphology and can be seen to occupy a unique area of morphospace.
The other exceptions to the general pattern show the ability of the biplot to distinguish morphological variations within families. The positioning of Sargocentron (Holocentridae) in the upper left quadrant away from all other nocturnal fishes including the other holocentrid taxon, Myripristis, is strange. However, these morphological differences within the Holocentridae are reflected in the diet, Sargocentron feed mainly on benthic crustaceans, whereas Myripristis prey on planktonic organisms (Randall 1967; Randall et al. 1997; Holzman et al. 2005).
Another example of within-family variation can be seen in the Chaetodontidae, with two species sitting slightly away from the tight grouping formed by the other chaetodontids. The long snout of Chelmon rostratus may have an effect on the relative sizes of the morphological traits by essentially elongating the fish. However, the functional morphology of this species differs from many other members of the Chaetodontidae (Ferry-Graham et al. 2001b), as does the diet, which includes free-living, non-coralline invertebrates (Allen et al. 1998; Pratchett 2005). The procurement of these may be aided by the prognathus morphology and narrow gape, allowing small prey items to be plucked from reef crevices (Allen et al. 1998). Chaetodon lunulatus was found to have a wider gape than the other chaetodontids. This wide gape has been previously documented (Motta 1988) and corresponds with the relatively novel feeding mode of this chaetodontid, which involves scraping a number of polyps at a time from the surface of a coral colony, rather than picking at individual polyps. Among chaetodontids, grazing in this fashion is known only from this species and one other, C. ornatissimus (Motta 1988) both of which have wide gapes enabling the grazing action. C. lunulatus, although considered an obligate generalist corallivore, is known to be selective of which coral taxa it feeds upon (Berumen et al. 2005; Graham 2007), thus, it’s relatively large eye diameter and potentially high visual acuity, similar to the other chaetodontids, will still be of use in identifying prey.
Cryptobenthic species
Cryptobenthic species were expected to have larger relative gapes than large fishes, to maximise potential prey availability. Furthermore, allometric studies of fish ontogeny consistently emphasise the large eyes of small fishes and the negative allometry of this trait with growth (Strauss 1984; Job and Bellwood 2000), as found herein. One would therefore expect all cryptobenthic fishes to lie in the upper right hand quadrant of the biplot. However, this was not seen. Cryptobenthic fishes may be small, but they still conform to the basic trends displayed by larger reef fishes. Cryptobenthic fishes have varying diets (Depczynski and Bellwood 2003) and the morphologies were seen to vary accordingly and follow similar patterns to the larger taxa. The Blenniidae had wide gapes and were one of a range of detritivorous taxa in the lower right hand quadrant. This pattern was not constrained to cryptobenthic taxa as the detritivorous Mugilidae also displayed wide gapes. Callogobius and a number of other gobies also occupied this morphospace. A wider understanding of cryptobenthic trophic ecology will elucidate whether this is due to similarities to the carnivorous taxa or the detritivorous taxa in this quadrant.
In contrast to the large raptorial predators in the lower right hand quadrant, many of which were ambush predators, the known cryptobenthic predators may have different feeding modes. The Pseudochromidae, Assessor macneilli (Plesiopidae) and all Trimma spp. (Gobiidae) were found in the upper left quadrant. These fishes all inhabit crevices on reefs (Randall et al. 1997; Depczynski and Bellwood 2004) and therefore exist in a relatively low light environment. The ability of these fishes to detect mobile prey items in this low light environment may be increased by their relatively large eye diameters. Also, it must be considered that many of these predatory cryptobenthic fishes are essentially planktivorous and clearly correspond with the concept of the upper left quadrant being populated by highly visual dependent species.
A generalised functional morphospace
The biplot appears to reflect fundamental functional attributes of reef fishes. Visual abilities separate taxa along the y-axis whilst the x-axis follows feeding modes. The latter appears to mark a division between narrow, accurate jaws used for biting and manipulating on the left hand side of the biplot to wide gaping mouths designed for ram or suction feeding on the right hand side of the quadrant. This reflects the underlying division in fish feeding mechanics between manipulation and ram-suction feeding modes (Wainwright and Bellwood 2002).
In coral reef environments, the morphological biplot was useful in explaining some discrepancies found in previous studies (e.g., Kotrschal 1988; Bellwood 2003) and may, by providing a robust but sensitive measure of abilities, be useful in studies of other aquatic systems. Although the method may not work well in highly turbid or temperate systems due to constant low light environments, one potential application could be in clear water environments such as African Rift Lake fish assemblages, which have comparable diversity to those found on coral reefs (Keenleyside 1991; Streelman and Danley 2003). There is also a potential that the biplot could be used as a predictive tool. Fish species with unknown ecologies could be plotted in the biplot and the quadrant occupied could help identify potential abilities of that fish. One such application may be in inferring the abilities of extinct reef fish species from fossilised reef assemblages such as those of Monte Bolca (Bellwood 1996). The use of the biplot as a tool may thus allow a greater understanding of past reef fishes and the development of modern reef fish assemblages.
Overall, for the first time we have examined key morphological traits across a wide range of reef fish families. Fundamental differences in the morphologies of reef fish groups were observed, particularly between nocturnal and diurnal taxa. Yet, the method was sensitive enough to divide superficially similar taxa (e.g., species within the Holocentridae and Chaetodontidae). Investigating two morphological traits simultaneously allows a greater separation of data and is a more powerful tool for analysis than considerations of single morphological traits. Simple morphological traits examined across a broad assemblage revealed an unseen and unexpected subtlety in the variation in feeding modes. Further axes of variation in other key morphological traits (e.g., locomotion or visceral morphology) promise even greater resolution and new insights into the functional abilities that underpin the spectacular diversity seen on coral reefs.
References
Ackerman JL, Bellwood DR (2000) Reef fish assemblages: a re-evaluation using enclosed rotenone stations. Mar Ecol Prog Ser 206:227–237
Allen GR, Steene R, Allen M (1998) A guide to angelfishes and butterflyfishes. Odyssey Publishing, Perth, p 250
Bellwood DR (1996) The Eocene fishes of Monte Bolca: the earliest coral reef fish assemblage. Coral Reefs 15:11–19
Bellwood DR (2003) Origins and escalation of herbivory in fishes: a functional perspective. Paleobiology 29:71–83
Bellwood DR, Choat JH (1990) A functional analysis of grazing in parrotfishes (family Scaridae): the ecological implications. Env Biol Fish 28:189–214
Berumen ML, Pratchett MS (2008) Trade-offs associated with dietary specialization in corallivorous butterflyfishes (Chaetodontidae: Chaetodon). Behav Ecol Sociobiol 62:989–994
Berumen ML, Pratchett MS, McCormick MI (2005) Within-reef differences in diet and body condition of coral-feeding butterflyfishes (Chaetodontidae). Mar Ecol Prog Ser 287:217–227
Blaber SJM (1976) The food and feeding ecology of Mugilidae in the St. Lucia lake system. Biol J Linn Soc 8:267–277
Choat JH, Robbins WD, Clements KD (2004) The trophic status of herbivorous fishes on coral reefs. II. Food processing modes and trophodynamics. Mar Biol 145:445–454
Collar DC, Wainwright PC, Alfaro ME (2008) Integrated diversification of locomotion and feeding in labrid fishes. Biol Lett 4:84–86
Depczynski M, Bellwood DR (2003) The role of cryptobenthic reef fishes in coral reef trophodynamics. Mar Ecol Prog Ser 256:183–191
Depczynski M, Bellwood DR (2004) Microhabitat utilisation patterns in cryptobenthic coral reef fish communities. Mar Biol 145:455–463
Depczynski M, Fulton CJ, Marnane MJ, Bellwood DR (2007) Life history patterns shape energy allocation among fishes on coral reefs. Oecologia 153:111–120
Fernald RD (1991) Teleost vision: seeing while growing. J Exp Zool S 5:167–180
Ferry-Graham LA, Wainwright PC, Bellwood DR (2001a) Prey capture in long-jawed butterflyfishes (Chaetodontidae): the functional basis of novel feeding habits. J Exp Mar Biol Ecol 256:167–184
Ferry-Graham LA, Wainwright PC, Hulsey CD, Bellwood DR (2001b) Evolution and mechanics of long jaws in butterflyfishes (family Chaetodontidae). J Morphol 248:120–143
Froese R, Pauly D (2008) FishBase. www.fishbase.org, version (04/2008)
Gagliano M (2008) On the spot: the absence of predators reveals eyespot plasticity in a marine fish. Behav Ecol [doi: 10.1093/beheco/arn013]
Graham NAJ (2007) Ecological versatility and the decline of coral feeding fishes following climate driven coral mortality. Mar Biol 153:119–127
Guthrie DM, Muntz WRA (1993) Role of vision in fish behaviour. In: Pitcher TJ (ed) The behaviour of teleost fishes, 2nd edn. Chapman and Hall, London, pp 89–128
Hairston NG, Li KT, Easter SS (1982) Fish vision and the detection of planktonic prey. Science 218:1240–1242
Holzman R, Genin A (2005) Mechanisms of selectivity in a nocturnal fish: a lack of active prey choice. Oecologia 146:329–336
Holzman R, Reidenbach MA, Monismith SG, Koseff JR, Genin A (2005) Near-bottom depletion of zooplankton over a coral reef II: relationships with zooplankton swimming ability. Coral Reefs 24:87–94
Hourigan TF, Stanton FG, Motta PJ, Kelley CD, Carlson B (1989) The feeding ecology of three species of Caribbean angelfishes (family Pomacanthidae). Env Biol Fish 24:105–116
Hulsey CD, García de León FJ, Rodiles-Hernández R (2006) Micro- and macroevolutionary decoupling of cichlid jaws: a test of Liem’s key innovation hypothesis. Evolution 60:2096–2109
Job SD, Bellwood DR (1996) Visual acuity and feeding in larval Premnas biaculeatus. J Fish Biol 48:952–963
Job SD, Bellwood DR (2000) Light sensitivity in larval fishes: implications for vertical zonation in the pelagic zone. Limnol Oceanogr 45:362–371
Job SD, Shand J (2001) Spectral sensitivity of larval and juvenile coral reef fishes: implications for feeding in a variable light environment. Mar Ecol Prog Ser 214:267–277
Johns PR, Easter SS (1977) Growth of the adult goldfish eye. II. Increase in retinal cell number. J Comp Neur 176:331–342
Johnsen S (2001) Hidden in plain sight: the ecology and physiology of organismal transparency. Biol Bull 201:301–318
Keenleyside MHA (1991) Cichlid fishes behaviour, ecology and evolution. Chapman and Hall, London
Kotrschal K (1988) Evolutionary patterns in tropical marine reef fish feeding. Z Zool Syst Evol 26:51–64
Liem KF (1973) Evolutionary strategies and morphological innovations: cichlid pharyngeal jaws. Syst Zool 22:425–441
MacArthur LD, Hyndes GA (2007) Varying foraging strategies of Labridae in seagrass habitats: herbivory in temperate seagrass meadows? J Exp Mar Biol Ecol 340:247–258
Marnane MJ, Bellwood DR (2002) Diet and nocturnal foraging in cardinalfishes (Apogonidae) at One Tree Reef, Great Barrier Reef, Australia. Mar Ecol Prog Ser 231:261–268
McFarland WN (1991) The visual world of coral reef fishes. In: Sale PF (ed) The ecology of fishes on coral reefs. Academic Press, San Diego, pp 16–38
Miller TJ, Crowder LB, Rice JA (1993) Ontogenetic changes in behavioural and histological measures of visual acuity in three species of fish. Env Biol Fish 37:1–8
Motta PJ (1988) Functional morphology of the feeding apparatus of ten species of Pacific butterflyfishes (Perciformes, Chaetodontidae): an ecomorphological approach. Env Biol Fish 22:39–67
Motta PJ (1989) Dentition patterns among Pacific and Western Atlantic butterflyfishes (Perciformes, Chaetodontidae): relationship to feeding ecology and evolutionary history. Env Biol Fish 25:159–170
Myrberg AA, Fuiman LA (2002) The sensory world of coral reef fishes. In: Sale PF (ed) Coral reef fishes: dynamics and diversity in a complex ecosystem. Academic Press, San Diego, pp 123–148
Persson L, Andersson J, Wahlström E, Eklöv P (1996) Size-specific interactions in lake systems: predator gape limitation and prey growth rate and mortality. Ecology 77:900–911
Pratchett MS (2005) Dietary overlap among coral-feeding butterflyfishes (Chaetodontidae) at Lizard Island, northern Great Barrier Reef. Mar Biol 148:373–382
Pratchett MS, Wilson SK, Baird AH (2006) Declines in the abundance of Chaetodon butterflyfishes following extensive coral depletion. J Fish Biol 69:1269–1280
Protas M, Conrad M, Gross JB, Tabin C, Borowsky R (2007) Regressive evolution in the Mexican cave tetra, Astyanax mexicanus. Curr Biol 17:452–454
Randall JE (1967) Food habits of reef fishes of the West Indies. Stud Trop Oceanogr 5:655–847
Randall JE, Allen GR, Steene RC (1997) Fishes of the Great Barrier Reef and Coral Sea, 2nd edn. University of Hawai’i Press, Honolulu
Reese ES (1989) Orientation behavior of butterflyfishes (family Chaetodontidae) on coral reefs: spatial learning of route specific landmarks and cognitive maps. Env Biol Fish 25:79–86
Ryan PA (1991) The success of the Gobiidae in tropical Pacific insular streams. New Zeal J Zool 18:25–30
Scharf FS, Juanes F, Rountree RA (2000) Predator size–prey size relationships of marine fish predators: interspecific variation and effects of ontogeny and body size on trophic-niche breadth. Mar Ecol Prog Ser 208:229–248
Shand J (1997) Ontogenetic changes in retinal structure and visual acuity: a comparative study of coral-reef teleosts with differing post-settlement lifestyles. Env Biol Fish 49:307–322
Shpigel M, Fishelson L (1989) Habitat partitioning between species of the genus Cephalopholis (Pisces: Serranidae) across the fringing reef of the Gulf of Aqaba (Red Sea). Mar Ecol Prog Ser 58:17–22
Strauss RE (1984) Allometry and functional feeding morphology in haplochromine cichlids. In: Echelle AA, Kornfield I (eds) Evolution of Fish Species Flocks. Univ Maine Press, Maine, pp 217–230
Streelman JT, Danley PD (2003) The stages of vertebrate evolutionary radiation. Trends Ecol Evol 18:126–131
Truemper HA, Lauer TE (2005) Gape limitation and piscine prey size-selection by yellow perch in the extreme southern area of Lake Michigan, with emphasis on two exotic prey items. J Fish Biol 66:135–149
Wainwright PC (1989) Prey processing in haemulid fishes: patterns of variation in pharyngeal jaw muscle activity. J Exp Biol 141:359–375
Wainwright PC, Bellwood DR (2002) Ecomorphology of feeding in coral reef fishes. In: Sale PF (ed) Coral reef fishes. Dynamics and diversity in a complex ecosystem. Academic Press, San Diego, pp 33–55
Wainwright PC, Richard BA (1995) Predicting patterns of prey use from morphology of fishes. Env Biol Fish 44:97–113
Wainwright PC, Bellwood DR, Westneat MW (2002) Ecomorphology of locomotion in labrid fishes. Env Biol Fish 65:47–62
Warrant E (2004) Vision in the dimmest habitats on Earth. J Comp Physiol A 190:765–789
Acknowledgements
We thank A. Hoey, C. Syms, P. Wainwright, S. Wismer and an anonymous reviewer for helpful comments, the staff of Lizard Island and Orpheus Island Research Stations for field support and R. Bonaldo, M. Depczynski, M. Gagliano, A. Gonzalez-Cabello, T. Holmes and K. Johnston, for access to fish specimens. This work was supported by the Australian Research Council (DRB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Biology Editor Dr. Clay Cook
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Goatley, C.H.R., Bellwood, D.R. Morphological structure in a reef fish assemblage. Coral Reefs 28, 449–457 (2009). https://doi.org/10.1007/s00338-009-0477-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-009-0477-9