Abstract
Many groups of tropical cnidarians including scleractinian corals, octocorals, corallimorphs, and anemones contain the tertiary sulfonium compound dimethylsulfoniopropionate (DMSP). It is not known if the compound is synthesized by the animals, their microalgal symbionts, or derived through their diet. We determined the source of the DMSP in several species of tropical and temperate anemones using three approaches: (1) conducting comparative measurements of DMSP in aposymbiotic and zooxanthellate anemones of three species that harbor zooxanthellae, and similar measurements in one species that can harbor both zooxanthellae and zoochlorellae, (2) manipulating the presence or absence of zooxanthellae by inoculating juvenile aposymbiotic anemones (Aiptasia pallida) with their symbiont, Symbiodinium bermudense, and (3) manipulating the numbers of S. bermudense by growing aposymbiotic and zooxanthellate A. pallida in the light and the dark. DMSP was present in zooxanthellate anemones in concentrations of 3.4–15 μmol g−1 fresh mass (FM). In aposymbiotic Aiptasia spp. and Anthopleura elegantissima that lacked large numbers of zooxanthellae, concentrations ranged from being undetectable to 0.43 μmol g−1 FM. When aposymbiotic A. pallida were inoculated with zooxanthellae, concentrations of DMSP were an average of 4.24 μmol g−1 FM after 5 weeks; DMSP was undetectable in uninoculated control animals. Aposymbiotic anemones maintained in the light or the dark for 6 weeks contained no DMSP or zooxanthellae. Zooxanthellate anemones in the light contained five times as many zooxanthellae and approximately 7.5 times as much DMSP as zooxanthellate anemones maintained in the dark. Taken together, these data show that the zooxanthellae are the sole source of DMSP in A. pallida. The trends in DMSP concentrations in other species of zooxanthellate anemones suggest that this phenomenon is not limited to A. pallida but may be more generally true for other anemones or even other cnidarians hosting species of Symbiodinium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The sulfonium compound dimethylsulfoniopropionate (DMSP) plays important roles in marine ecosystems because of its involvement in global sulfur cycling and local climate regulation in the open oceans (Malin et al. 1992; Liss et al. 1994; Malin 1996, 1997; Malin and Kirst 1997; Simó 2001; Yoch 2002). One of the by-products of DMSP cleavage (Cantoni and Anderson 1956) and metabolism (Todd et al. 2007) is the volatile compound dimethylsulfide (DMS). The movement of DMS from the oceans to the atmosphere is a major transport mechanism for oceanic sulfur (Lovelock et al. 1972; Malin 1996; Kettle and Andreae 2000; Yoch 2002; Van Alstyne 2008). Atmospheric DMS can also be oxidized into a variety of sulfur compounds that may be significant sources of cloud condensation nuclei (CCN) over the open oceans (Charlson et al. 1987; Malin et al. 1992; Brasseur et al. 1999) and near extensive coral reefs (Jones et al. 1994; Jones and Trevena 2005). These CCN are thought to regulate cloud formation in these areas, which can result in increases in albedo and decreases in local water temperatures and light levels (Bates et al. 1987; Charlson et al. 1987; Malin et al. 1992; Malin 1996, 1997; Malin and Kirst 1997; Yoch 2002).
DMSP is found in diverse marine organisms that include phytoplankton, zooplankton, macroalgae, vascular plants, macroinvertebrates, and fishes (Malin and Kirst 1997; Kiene et al. 1996; Otte et al. 2004; Van Alstyne 2008). Although each of these groups has species that contain DMSP, each also has species in which DMSP is not detectable; thus, the compound is very patchily distributed across broad taxonomic scales. In marine macroalgae, the distribution of DMSP often parallels taxonomic relationships at ordinal or lower levels (Van Alstyne and Puglisi 2007). In animals, the presence of large quantities of DMSP is often associated with the presence of endosymbiotic algae in the genus Symbiodinium (Hill et al. 1995; Broadbent et al. 2002; Van Alstyne et al. 2006; Van Alstyne and Puglisi 2007).
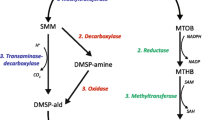
DMSP is synthesized via at least three different pathways from methionine (reviewed by Stefels 2000; Van Alstyne 2008). In photosynthetic organisms, methionine is synthesized from aspartate in the plastids (Wirtz and Droux 2005), and it is generally considered to be an essential amino acid that must be obtained by most animals from their diets (Buchanan et al. 2000). Thus, although plants and algae may be able to synthesize the methionine precursor needed to produce DMSP, animals may have to obtain DMSP from another source, either from their diets or from phototrophic symbionts, such as zooxanthellae (dinophytes in the genus Symbiodinium).
DMSP is present in zooxanthellae isolated from corals (Jones et al. 1994; Hill et al. 1995; Broadbent et al. 2002); however, the amounts of DMSP in zooxanthellae from the stony corals Montipora verrucosa and Pocillopora damicornis grown in laboratory cultures were only 90% and 41%, respectively, on a cell basis of the amounts reported from field-collected corals (Hill et al. 1995). DMSP in three tropical Pacific cnidarians, the corallimorph Discosoma nummiforme and the soft corals Sarcophyton trocheliophorum and Sinularia polydactyla, was positively correlated with zooxanthellae density (Van Alstyne et al. 2006). However, in Aiptasia pulchella, DMSP and zooxanthella density were not correlated (Van Alstyne et al. 2006). These results suggest that DMSP content may be affected by light or other environmental factors and is not always related to numbers of zooxanthellae in a simple proportional manner.
Although zooxanthellae are clearly one source of DMSP in cnidarians, it is possible that the cnidarians themselves may be responsible for part of its synthesis. Both zooxanthellate scleractinian corals (Montastraea faveolata, Acropora cervicornis and Porites porites) and corals that do not harbor zooxanthellae (Tubastrea coccinea and Astrangia poculata) have been reported to synthesize methionine (Fitzgerald and Szmant 1997). The pathway by which methionine is synthesized in these animals has not been investigated, and it is not known if it is the same pathway used by photosynthetic organisms. However, because some cnidarians are reported to synthesize methionine, it is possible that they can also produce DMSP since they contain the amino acid precursor needed for its synthesis.
The purpose of this study was to test the hypothesis that zooxanthellae (S. bermudense) are the sole source of DMSP in a cnidarian, the tropical sea anemone Aiptasia pallida. Although DMSP has not been reported from this species, it has been found in high quantities in a congener, A. pulchella, as well as in other symbiotic cnidarians including other species of anemones, corallimorphs, soft corals, and stony corals (Hill et al. 1995; Broadbent et al. 2002; Broadbent and Jones 2004; Van Alstyne et al. 2006).
Methods
Study organisms
Sea anemones in the genus Aiptasia have been used for experimental studies of algal symbiosis since the 1970s. These anemones reproduce asexually by pedal laceration, resulting in large numbers of clonal individuals. They are maintained readily in both zooxanthellate and aposymbiotic (=algae-free) states, are hardy and easy to maintain under laboratory conditions, and their zooxanthellae are readily isolated and cultured. In nature, these anemones are found in shallow tropical and subtropical waters, in both shaded and sunlit habitats (Muller-Parker 1987; Cook et al. 1988).
Individual zooxanthellate and aposymbiotic A. pallida (Verrill) were obtained from a clonal culture maintained under laboratory conditions. The original zooxanthellate anemone was collected from Walsingham Pond, Bermuda (see Cook et al. 1988); this clone has been used in multiple studies of the symbiosis between anemones and zooxanthellae (e.g., Muller-Parker et al. 1996). The anemone contains Symbiodinium bermudense, in clade B (McNally et al. 1994). Stock cultures were maintained in covered crystallizing dishes (100 × 50 mm) at 23°C in seawater (FSW) that was filtered through a 5-μm filter (30 psu, 150–200 ml in each dish) and fed weekly with freshly hatched brine shrimp (Artemia franciscana) nauplii (Argentemia brand). Zooxanthellate individuals were maintained under irradiance levels ranging from 15 to 30 μmol photons m−2 s−1 under a 12:12 L:D cycle. Aposymbiotic individuals were maintained under the same conditions, except in complete darkness. Seawater was replaced weekly, within 10 h after feeding the stock cultures.
Two other species of Aiptasia were maintained under the same conditions as the stock cultures of A. pallida. A. pulchella (Carlgren), originally from Kaneohe Bay, Oahu Island Hawaii, has been maintained in continuous culture since 1978. Its zooxanthellate symbiont, Symbiodinium pulchrorum (Trench, unpublished in Banaszak et al. 1993), is also in clade B and is closely related to S. bermudense (McNally et al. 1994). Aiptasia californica from Mexico has been maintained in culture at the Shannon Point Marine Center (SPMC) in Anacortes, Washington, USA, since the 1990s. Its symbiont has not been characterized.
DMSP concentrations in symbiotic and aposymbiotic anemones
Concentrations of DMSP in zooxanthellate and aposymbiotic anemones were measured in three species of Aiptasia, A. californica, A. pallida, and A. pulchella, from the clonal cultures maintained at SPMC. We also measured DMSP in freshly collected individuals of Anthopleura elegantissima, an intertidal temperate anemone that is symbiotic with zooxanthellae (Symbiodinium muscatinei, LaJeunesse and Trench 2000) and zoochlorellae (Trebouxiophyceae, Chlorophyta, Lewis and Muller-Parker 2004) and that also occurs with no or few symbionts in dark crevices. These anemones were collected from local populations in Anacortes, Washington, in late July 2001 and immediately were taken to the SPMC where measurements of DMSP concentrations in their tentacles were made from the fresh material. For the purposes of this study, A. elegantissima that harbor primarily zooxanthellae (S. muscatinei) are designated as “brown”; A. elegantissima that harbor primarily zoochlorellae are designated as “green”; and A. elegantissima that harbor no or very few zooxanthellae or zoochlorellae are designated as “white” to reflect the overall coloration of the field-collected animals. Anemones that are known to contain only zooxanthellae are referred to as “zooxanthellate”; however, if they can contain multiple types of symbionts or if the symbiont type is unspecified or unknown, they are referred to as “symbiotic.”
DMSP concentrations and symbiont densities were measured using methods similar to those described in Van Alstyne et al. (2006). Briefly, anemones (Aiptasia spp.) or clipped tentacles (A. elegantissima) were blotted with Kimwipes to remove surface water, weighed to 0.001 g, and sealed in gastight vials containing 4 N NaOH. Zooxanthellate A. californica (mean mass ± 1 SD: 0.0581 ± 0.0220 g), aposymbiotic A. pallida (mean mass ± 1 SD: 0.0086 ± 0.0047 g), and zooxanthellate and aposymbiotic A. pulchella (mean masses ± 1 SD: 0.1749 ± 0.0263 g and 0.0817 ± 0.0347 g, respectively), and brown (mean mass ± 1 SD: 0.0158 ± 0.0097 g), green mean mass ± 1 SD: 0.0133 ± 0.0034 g), and white tentacles (mean mass ± 1 SD: 0.0220 ± 0.0135 g) from five different A. elegantissima of each type, were placed in 10 ml gastight vials containing 2 ml of 4 N NaOH. Because of their larger size, zooxanthellate A. pallida (mean mass ± 1 SD: 0.4930 ± 0.2320 g) were placed in 30 ml gastight vials containing 4 ml of 4 N NaOH. The next day, DMSP was measured as DMS from the headspaces of the vials by direct injection into an SRI gas chromatograph (Chromosil 330 column, flame photometric detector; lower detection limit: 81 pmol DMS). DMSP standard additions using commercially obtained DMSP (Center for Analysis, Spectroscopy and Synthesis, University of Groningen; purity >98%) added to equal volumes of NaOH were used to generate standard curves. Separate standard curves were made for standards from the 10- and 30-ml vials. After the DMSP measurements were made, the vials containing tissues were shaken vigorously to further dissolve any intact tissues and the numbers of zooxanthellae and zoochlorellae, which were still intact, were estimated with a hemocytometer.
DMSP concentrations and algal densities were compared among the tentacle samples of brown, green, and white A. elegantissima, and among zooxanthellate Aiptasia spp. with a one-way analysis of variance (ANOVA, SPSS 15.0). We were unable to compare DMSP concentrations between zooxanthellate and aposymbiotic Aiptasia spp. statistically because the concentrations in aposymbiotic anemones had means and standard deviations of zero.
Inoculation experiment
To determine whether zooxanthellae or anemones were the source of the DMSP, we began an experiment on 22 February 2007 in which six small (recent pedal lacerates) aposymbiotic A. pallida were inoculated with freshly isolated S. bermudense. Six uninoculated aposymbiotic A. pallida served as controls. The 12 small anemones (~1 mm diameter) were placed individually in small crystallizing dishes (50 × 35 mm) in ~30 ml of filtered seawater. The anemones were maintained under standard culture conditions (average irradiance of 42 μmol photons m−2 s−1) and were starved for 1 week before zooxanthellae were provided to the experimental group. The experimental anemones were inoculated with S. bermudense obtained from a large (~1 cm diameter) zooxanthellate A. pallida. To obtain the inoculum, the anemone was emulsified in ~10 ml of FSW with a manual tissue grinder. The homogenate was centrifuged in a table top swinging bucket centrifuge at ~1,600g; the animal supernatant was discarded and the algal pellet re-suspended in FSW. To clean the algae, this process of centrifuging and re-suspension was repeated three times and the final algal suspension was filtered through a 25-μm Nitex screen to remove residual animal tissue. The filtered algal sample was pelleted to concentrate the cells and was mixed with a brine shrimp homogenate before pipetting small amounts of the mixture on the oral disks of experimental anemones.
All anemones were returned to the incubator (23°C). Their seawater was changed after 48 h. The anemones were fed and cleaned twice weekly. After 5 weeks, each anemone was digitally photographed with a Photometrics Cool Snap camera mounted on a dissecting microscope at 64 or 100× to obtain an estimate of its size. The number of tentacles per anemone and oral disk diameter were quantified with Image Pro image analysis software from the National Institutes of Health (http://rsb.info.nih.gov/nih-image/). Four days later, anemones were individually placed in 400 μl of 4 N NaOH in gastight 2 ml vials. The vials were kept in the dark overnight. The following day, DMSP was measured as headspace DMS by injecting a 100-μl sample of headspace gas from the vial into an SRI gas chromatograph as described above and the numbers of zooxanthellae in each sample were quantified with a hemocytometer.
Anemone biomass (milligram dry weight) was estimated with the equation Y = 0.18Z 1.45, where Y is milligram dry mass and Z is oral disk diameter in millimeter (Clayton and Lasker 1985). The dry masses were then converted to fresh masses using regression equations obtained by weighing an additional eight zooxanthellate (0.23–11.5 mg) and eight aposymbiotic (0.12–20.8 mg) anemones, drying them at 50°C for 66 h in an oven, and then reweighing them. Separate equations were calculated for zooxanthellate (fresh mass (mg) = 0.00099 + 5.24 × dry mass (mg); R 2 = 0.991, P < 0.001) and aposymbiotic (fresh mass (mg) = 0.00166 + 6.10 × dry mass (mg); R 2 = 0.986, P < 0.001) anemones.
After conducting a Levene’s test for unequal variances, a multivariate analysis of variance (MANOVA; SPSS, version 14.0) was used to examine the effects of inoculation with S. bermudense on the following individual anemone measures: numbers of zooxanthellae, mass, number of tentacles, oral disk diameter, and DMSP concentration (in μmol g−1 FM anemone). DMSP and morphological measurements were not made on several of the uninoculated anemones, so data from these individuals were not included in the MANOVA analyses.
Light experiment
To determine if light affects DMSP production by A. pallida or S. bermudense, small (~1 mm diam) zooxanthellate and aposymbiotic anemones were grown for 6 weeks in the presence and absence of light (N = 6 per treatment). A total of 24 anemones were placed individually in small crystallizing dishes in ~30 ml FSW. Six zooxanthellate and six aposymbiotic anemones were maintained on a 12:12 light:dark cycle in the incubator (23°C, 42 μmol photons m−2 s−1). An additional six zooxanthellate and six aposymbiotic anemones were maintained in the same incubator but were covered with dark plastic to exclude light. The anemones were fed twice weekly. After 6 weeks, DMSP concentrations, zooxanthellae numbers, numbers of tentacles per anemone, and oral disk diameters were quantified as described above.
Results
DMSP concentrations in symbiotic and aposymbiotic anemones
In Aiptasia spp., DMSP was present in zooxanthellate individuals at mean concentrations ranging from 3.4 to 15 μmol g−1 fresh mass (FM); however, it was uniformly undetectable in aposymbiotic individuals (Table 1). Mean DMSP concentrations of zooxanthellate individuals differed significantly among Aiptasia species (one-way ANOVA: df = 2, F = 35.802, P < 0.001), with A. californica having significantly higher concentrations (post hoc Tukey’s test: P < 0.05) than A. pallida and A. pulchella, which were statistically indistinguishable (post hoc Tukey’s test: P > 0.05). Concentrations of DMSP per zooxanthella also differed significantly among Aiptasia species (one-way ANOVA: df = 2, F = 27.712, P = 0.001). The patterns for these data paralleled those for the amounts of DMSP per gram of animal, with zooxanthellae in A. californica having significantly higher concentrations (post hoc Tukey’s test: P < 0.05) than zooxanthellae in A. pallida and A. pulchella, which were again statistically indistinguishable (post hoc Tukey’s test: P > 0.05).
In A. elegantissima, densities of zooxanthellae and zoochlorellae differed significantly among the tentacles of field-collected brown, green, and white A. elegantissima (Table 1, zooxanthellae: one-way ANOVA: df = 2, F = 6.363, P = 0.013, zoochlorellae: one-way ANOVA: df = 2, F = 108.615, P < 0.001). DMSP was detectable (Table 1), but statistically different, among the three A. elegantissima types (one-way ANOVA: df = 2, F = 123.478, P < 0.001). It was at least 25 times higher in brown individuals (post hoc Tukey’s test: P < 0.05) than green and white individuals, which were statistically indistinguishable from each other (post hoc Tukey’s test: P > 0.05). Amounts of DMSP per zooxanthella were approximately 10 times higher in white than in brown anemones (one-way ANOVA: df = 2, F = 6.416, P = 0.022; post hoc Tukey’s test: P < 0.05) and approximately four times higher for zooxanthellae located in green anemones than for those in brown anemones, although this difference was not statistically significant (post hoc Tukey’s test: P < 0.05).
Inoculation experiment
Inoculating small aposymbiotic A. pallida with S. bermudense significantly affected the measured anemone characteristics after 5 weeks (Table 2, MANOVA: Pillai’s Trace = 0.996, F = 92.612, hypothesis df = 5, error df = 2, P = 0.011). The inoculated anemones were brown in color and had abundant zooxanthellae, whereas the uninoculated anemones were white-colored and had no zooxanthellae (Table 2; MANOVA inoculation effect: F = 10.291, df = 1, P = 0.018). DMSP was also present in inoculated anemones but was not detected in uninoculated anemones (Table 2; MANOVA inoculation effect: F = 27.626, df = 1, P = 0.002). Inoculating anemones with zooxanthellae had no effect on anemone mass (Table 2; MANOVA inoculation effect: F = 2.227, df = 1, P = 0.186), number of tentacles (Table 2; MANOVA inoculation effect: F = 0.034, df = 1, P = 0.860), and oral disk diameter (Table 2; MANOVA inoculation effect: F = 0.009, df = 1, P = 0.929).
There were no significant correlations among the number of zooxanthellae per anemone, anemone mass, number of tentacles per anemone, oral disk diameter, and DMSP concentration (Spearman’s ρ, P ≥ 0.257) with the exception of the following: DMSP concentration and zooxanthellae number were highly correlated if anemones lacking zooxanthellae were included (Spearman’s ρ = 0.931, P < 0.001, N = 9), but not if they were excluded (Spearman’s ρ = 0.772, P = 0.071, N = 6). The number of tentacles per anemone and oral disk diameter (Spearman’s ρ = 0.723, P = 0.043, N = 8) were also correlated.
Light experiment
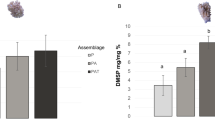
Both the presence of zooxanthellae (Fig. 1, two-way MANOVA: Pillai’s Trace = 0.999, F = 519.29, hypothesis df = 5, error df = 7, P < 0.001) and maintenance of anemones in the light versus the dark (Fig. 1, two-way MANOVA: Pillai’s Trace = 0.925, F = 22.31, hypothesis df = 5, error df = 7, P < 0.001) had significant effects on the measured characteristics after 6 weeks. There was a significant zooxanthella × light interaction effect (two-way MANOVA: Pillai’s Trace = 0.921, F = 20.917, hypothesis df = 5, error df = 7, P < 0.001). A Levene’s test for the equality of error variances indicated significant (P < 0.05) error variances in numbers of zooxanthellae and tentacles per animal, and DMSP concentration. Subsequent attempts to transform the data to reduce the error variance were unsuccessful; however, Pillai’s V statistic is robust to moderate levels of heterogeneity of variances as long as the design is balanced or nearly so (Johnson and Field 1993).
Aiptasia pallida. Characteristics of zooxanthellate (dark bars) and aposymbiotic (open bars) anemones after maintenance in light and dark conditions for 6 weeks. a Number of zooxanthellae anemone−1. b DMSP concentration (μmol g−1 FM anemone). c DMSP concentration zooxanthella−1. d Estimated anemone fresh mass (mg). e Number of tentacles anemone−1. f Anemone oral disk diameter (mm). Data are means ± 1 SE (N = 4–6). Statistical analyses are presented in Table 3. ND not detected, N/A not applicable
After 6 weeks, aposymbiotic anemones did not contain zooxanthellae or DMSP, whereas zooxanthellae (Fig. 1a) and DMSP (Fig. 1b) were present in all the zooxanthellate anemones (Table 3, P < 0.001). Zooxanthellate anemones maintained in the dark contained about 78% fewer zooxanthellae and 83% less DMSP than animals maintained in the light (Table 3; P < 0.001). There was no significant difference in the amount of DMSP per zooxanthella (Fig. 1c) in anemones grown in light versus dark conditions for 6 weeks (Student’s t-test: t = 1.149, df = 6, P = 0.294).
Aposymbiotic anemones were about 30% larger than zooxanthellate anemones (Fig. 1d, P = 0.001), and there was a non-significant tendency (P = 0.055) for them to be larger when grown in the light. Zooxanthellate anemones did not significantly differ in numbers of tentacles (Fig. 1e) or oral disk diameter (Fig. 1f) from aposymbiotic anemones (Table 3, P ≥ 0.258). Anemones grown in the dark had 4% more tentacles than anemones grown in the light (Fig. 1e, Table 3, P = 0.043). On average, anemones grown in the dark weighed 16% more (Fig. 1d) and had 10% larger oral disk diameters (Fig. 1f), although these differences were not statistically significant (Table 3, P = 0.081 and 0.083, respectively). There were no zooxanthella × light treatment interaction effects for anemone mass, tentacle number, or oral disk diameter (Table 3, P ≥ 0.196).
The amount of DMSP per gram FM of anemone and the number of zooxanthellae were highly correlated, even when the anemones that did not contain zooxanthellae were excluded from the analysis (Spearman’s ρ = 0.895, P = 0.001, N = 6). With the exception of this result, there were no other significant correlations among anemone mass, number of tentacles, oral disk diameter, number of zooxanthellae per anemone, and DMSP concentrations (Spearman’s ρ: P > 0.05).
Discussion
The results of this research indicate that S. bermudense is the source of DMSP in A. pallida. Detectable amounts of DMSP were never found in aposymbiotic anemones, regardless of whether they were grown in light or dark conditions. DMSP was present in anemones that were inoculated with zooxanthellae but could not be detected in uninoculated anemones, even though both were grown under identical conditions in a lighted incubator. Zooxanthellate anemones that were grown in the dark for 6 weeks had fewer zooxanthellae than anemones that were grown in the light and contained correspondingly lower amounts of DMSP.
Our measurements of DMSP concentrations in several species of anemones suggest that this result may be applicable to symbiont-harboring anemones other than A. pallida. In all three species of Aiptasia that we examined, DMSP was present in high concentrations in zooxanthellate individuals and could not be detected in aposymbiotic individuals of two of these species (A. pallida and A. pulchella). A similar pattern was found in A. elegantissima (Family Actiniidae), which is in a different family than Aiptasia (Family Aiptasiidae), suggesting that these patterns occur across broader taxonomic groups.
The presence of high concentrations of DMSP in brown A. elegantissima, which harbors only S. muscatinei, and its occurrence in very low concentrations in green A. elegantissima, which harbor an unidentified Chlorophyte in the Class Trebouxiophyceae (Table 1), suggests that DMSP is not produced by zoochlorellae. This is further supported by the lack of a statistically significant difference in the amounts of DMSP produced per zooxanthella in brown and green A. elegantissima; all the DMSP in green A. elegantissima can be accounted for by the number of zooxanthellae present. Many other invertebrates, including giant clams (Tridacna spp.) and zooxanthellate cnidarians such as other anemones, corallimorphs, soft corals, and scleractinian corals, are known to contain relatively high quantities of DMSP (Jones et al. 1994; Hill et al. 1995; Hill et al. 2000; Broadbent et al. 2002; Broadbent and Jones 2004; Van Alstyne et al. 2006). The only other example that we know of in which a non-Symbiodinium algal symbiont is thought to produce DMSP in a marine animal is in the flatworm Convoluta roscoffensis (Van Bergeijk and Stal 2001). C. roscoffensis harbors a green algal symbiont, Tetraselmis sp. (Prasinophyceae) that contains DMSP in concentrations of about 45 fmol cell−1 (Van Bergeijk and Stal 2001) and free-living Tetraselmis subcordiformis is known to contain DMSP concentrations of 5–30 fmol cell−1 (Gröne and Kirst 1992).
The significant differences in the amounts of DMSP produced per zooxanthella between brown and white A. elegantissima (Table 1) suggests that there is plasticity in the amounts of DMSP produced by the zooxanthellae. The only zooxanthellate symbiont known from A. elegantissima in Washington is S. muscatinei (LaJeunesse and Trench 2000). Therefore, the differences in DMSP concentrations on a per cell basis cannot be accounted for by differences in the species composition of the symbionts of brown and white anemones, although it is possible that different taxa of Symbiodinium could be present in white and brown anemones and be producing constitutively different amounts of DMSP. However, it is more likely that the differences are due to the respective environments of white and brown anemones, which affect the rate of DMSP production and/or turnover. White anemones were collected from under rocks and experienced lower temperatures, lower light, less wave action, and less desiccation than the exposed brown and green anemones. Intraspecific differences in DMSP concentrations have been documented in both macroalgae and microalgae (e.g., Wolfe et al. 1997; Van Alstyne et al. 2007), and DMSP concentrations are known to be affected by a variety of environmental conditions including light, temperature, and salinity (reviewed by Liss et al. 1994; Malin and Kirst 1997; Stefels 2000; Yoch 2002; Van Alstyne 2008).
The relationship between DMSP content and biogeographical distributions of the zooxanthellae within hosts would be interesting to pursue to determine if DMSP production is characteristic of zooxanthellae in different clades. Symbiodinium spp. belonging to clade B (in A. pallida, A. pulchella, and in A. elegantissima) exhibited similar levels of DMSP. The cladal affiliation of the symbionts in A. californica that contained higher amounts of DMSP is unknown. Southern populations of A. elegantissima and A. xanthogrammica contain another species of Symbiodinium, S. californium, in addition to S. muscatinei (LaJeunesse and Trench 2000). Studies investigating DMSP in cnidarians hosting multiple types of zooxanthellae may help elucidate the function of DMSP in these symbioses.
The presence of zooxanthellae had no effect on anemone size (oral disk diameter) and on the development of tentacles, indicating that the twice weekly feeding schedule with Artemia nauplii was sufficient to support the growth and development of both zooxanthellate and aposymbiotic anemones. These results suggest the DMSP content of zooxanthellate anemones is independent of the nutritional status of the host anemone, although further studies examining fed and starved anemones might reveal differences in DMSP content of the zooxanthellae.
The function of DMSP in the anemone–algal symbiosis is not known. DMSP is produced by many other algal species and is a multifunctional compound. It can function as a compatible solute, a cryoprotectant, and a methyl donor (reviewed by Kiene et al. 1996; Stefels 2000; Otte et al. 2004; Van Alstyne 2008). DMSP can also be enzymatically cleaved (Challenger and Simpson 1948; Cantoni and Anderson 1956), and its cleavage products, DMS, acrylate, and acrylic acid may be involved in the removal of excess energy and sulfur, or have antioxidant, antimicrobial or anti-herbivore properties (reviewed by Kiene et al. 1996; Stefels 2000; Otte et al. 2004; Van Alstyne 2008). In tropical sea anemones, such as A. pallida, the most likely functions of DMSP and/or its products are as antioxidants, anti-predator compounds, or compatible solutes, although there is currently no data available from studies with anemones to support any of these functions.
Regardless of why zooxanthellae produce DMSP, its production may have ecological consequences for the animals that contain the symbionts or for co-occurring plants and animals. For example, corals, which contain high concentrations of DMSP (Jones et al. 1994; Hill et al. 1995; Broadbent et al. 2002; Broadbent and Jones 2004; Van Alstyne et al. 2006), release large amounts of DMSP and DMS into the surrounding seawater (Broadbent and Jones 2004; Jones and Trevena 2005). DMSP is used as a foraging cue by planktivorous reef fishes (DeBose et al. 2008) and is a feeding attractant to herbivorous urchins (Van Alstyne et al. 2001). When DMS diffuses into the atmosphere, it can attract foraging procellariiform seabirds (Nevitt et al. 1995; Nevitt 1999; Nevitt and Bonadonna 2005). Thus, the presence of DMSP in animals that harbor zooxanthellae may make them or co-occurring species more likely to be preyed upon. For example, DMSP in brown A. elegantissima may explain why the tidepool sculpin Clinocottus globiceps preferentially feeds on the tentacles of brown A. elegantissima over those of green anemones (Augustine and Muller-Parker 1998).
In summary, our experiments have shown that the DMSP in the A. pallida–S. bermudense symbiosis is produced by the zooxanthellae. Based on measurements from other anemone species, we speculate that symbiont production of DMSP is probably the rule in anemone–zooxanthella symbiosis, and possibly in other cnidarian–zooxanthella interactions, most notably those involving corals. The functions and consequences of DMSP production by zooxanthellae that are symbiotic with cnidarian hosts have not yet been determined; however, potential functions include providing protection from reactive oxygen species, microbes, and predators, although DMSP may also attract predators as well. Thus, the loss of zooxanthellae, such as that which occurs during coral bleaching events, could affect hosts both physiologically and ecologically.
References
Augustine L, Muller-Parker G (1998) Selective predation by the mosshead sculpin Clinocottus globiceps on the sea anemone Anthopleura elegantissima and its two algal symbionts. Limnol Oceanogr 43:711–715
Banaszak A, Iglesias-Prieto R, Trench R (1993) Scrippsiella velellae sp. nov. (Peridiniales) and Gloeodinium viscum sp. nov. (Phytodiniales), dinoflagellate symbionts of two hydrozoans (Cnidaria). J Phycol 29:517–528
Bates TS, Charlson RJ, Gammon RH (1987) Evidence for climatic role of marine biogenic sulphur. Nature 329:319–321
Brasseur G, Orlando J, Tyndall G (1999) Atmospheric chemistry and global change. Oxford, New York
Broadbent A, Jones G (2004) DMS and DMSP in mucus ropes, coral mucus, surface films and sediment pore waters from coral reefs in the Great Barrier Reef. Mar Freshwater Res 55:849–855
Broadbent A, Jones G, Jones R (2002) DMSP in corals and benthic algae from the Great Barrier Reef. Estuar Coast Shelf Sci 55:547–555
Buchanan BB, Gruissem W, Jones RL (2000) Biochemistry and molecular biology of plants. Wiley, Somerset
Cantoni G, Anderson D (1956) Enzymatic cleavage of dimethylpropiothetin by Polysiphonia lanosa. J Biol Chem 222:171–177
Challenger F, Simpson M (1948) Studies on biological methylation. Part 12. A precursor of the dimethyl sulphide evolved by Polysiphonia fastigiata. Dimethyl-2-carboxyethylsulphonium hydroxide and its salts. J Chem Soc 3:1591–1597
Charlson RJ, Lovelock JE, Andreae MO, Warren SG (1987) Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate. Nature 326:655–661
Clayton WJ, Lasker H (1985) Individual and population growth in the asexually reproducing anemone Aiptasia pallida Verrill. J Exp Mar Biol Ecol 90:249–258
Cook C, D’Elia C, Muller-Parker G (1988) Host feeding and nutrient sufficiency for zooxanthellae in the sea anemone Aiptasia pallida. Mar Biol 98:253–262
DeBose J, Lema S, Nevitt G (2008) Dimethylsulfoniopropionate as a foraging cue for reef fishes. Science 319:1356
Fitzgerald L, Szmant A (1997) Biosynthesis of “essential” amino acids by scleractinian corals. Biochem J 322:213–221
Gröne T, Kirst GO (1992) The effects of nutrient deficiency, methionine, and inhibitors of methionine metabolism on the DMSP contents of Tetraselmis subcordiformis (Stein). Mar Biol 112:497–503
Hill RW, Dacey JWH, Krupp DA (1995) Dimethylsulfoniopropionate in reef corals. Bull Mar Sci 57:489–494
Hill RW, Dacey JWH, Edward A (2000) Dimethylsulfoniopropionate in giant clams (Tridacnidae). Biol Bull 199:108–115
Johnson C, Field C (1993) Using fixed-effects model multivariate analysis of variance in marine biology and ecology. Oceanogr Mar Biol Ann Rev 31:177–221
Jones G, Trevena A (2005) The influence of coral reefs on atmospheric dimethylsulphide over the Great Barrier Reef, Coral Sea, Gulf of Papua and Solomon and Bismark Seas. Mar Freshwater Res 56:85–93
Jones G, Curran M, Broadbent A (1994) Dimethylsulphide in the South Pacific. 6th Pacific Congr Mar Sci Technol:183–190
Kettle A, Andreae MO (2000) Flux of dimethylsulfide from the oceans: a comparison of updated data sets and flux models. J Geophys Res 26:26793–26808
Kiene RP, Visscher P, Keller M, Kirst GO (1996) Biological and environmental chemistry of DMSP and related sulfonium compounds. Plenum Press, New York
LaJeunesse T, Trench R (2000) The biogeography of two species of Symbiodinium (Freudenthal) inhabiting the intertidal anemone, Anthopleura elegantissima (Brandt). Biol Bull 199:126–134
Lewis L, Muller-Parker G (2004) Phylogenetic placement of “zoochlorellae” (Chlorophyta), algal symbiont of the temperate sea anemone Anthopleura elegantissima. Biol Bull 207:87–92
Liss PS, Malin G, Turner SM, Holligan PM (1994) Dimethyl sulphide and Phaeocystis: a review. J Mar Syst 5:41–53
Lovelock JE, Maggs C, Rasmussen R (1972) Atmospheric dimethyl sulfide and the natural sulfur cycle. Nature 237:452–453
Malin G (1996) The role of DMSP and DMS in the global sulfur cycle and climate regulation. In: Kiene RP, Visscher P, Keller M, Kirst GO (eds) Biological and environmental chemistry of DMSP and related sulfonium compounds. Plenum Press, New York, pp 177–189
Malin G (1997) Sulphur, climate and the microbial maze. Nature 387:857–859
Malin G, Kirst GO (1997) Algal production of dimethyl sulfide and its atmospheric role. J Phycol 33:889–896
Malin G, Turner SM, Liss PS (1992) Sulfur: the plankton/climate connection. J Phycol 28:590–597
McNally K, Govind N, Thomé P, Trench R (1994) Small-subunit ribosomal DNA sequence analyses and a reconstruction of the inferred phylogeny among symbiotic dinoflagellates (Pyrrophyta). J Phycol 30:316–329
Muller-Parker G (1987) Seasonal variation in light-shade adaptation of natural populations of the symbiotic sea anemone Aiptasia pulchella (Carlgren, 1943) in Hawaii. J Exp Mar Biol Ecol 112:165–183
Muller-Parker G, Lee K, Cook C (1996) Changes in the ultrastructure of symbiotic zooxanthellae (Symbiodinium sp., Dinophyceae) in fed and starved sea anemones maintained under high and low light. J Phycol 32:987–994
Nevitt G (1999) Foraging by seabirds on an olfactory landscape. Amer Scient 87:46–53
Nevitt G, Bonadonna F (2005) Sensitivity to dimethyl sulphide suggests a mechanism for olfactory navigation by seabirds. Biol Lett 1:303–305
Nevitt GA, Veit RR, Kareiva P (1995) Dimethyl sulphide as a foraging cue for Antarctic procellariiform seabirds. Nature 376:680–682
Otte ML, Wilson G, Morris J, Moran B (2004) Dimethylsulphoniopropionate (DMSP) and related compounds in higher plants. J Exp Bot 55:1919–1925
Simó R (2001) Production of atmospheric sulfur by oceanic plankton: biochemical, ecological, and evolutionary links. Trends Ecol Evol 16:287–294
Stefels J (2000) Physiological aspects of the production and conversion of DMSP in marine algae and higher plants. J Sea Res 43:183–197
Todd J, Rogers R, Li Y, Wexler M, Bond P, Sun L, Curson A, Malin G, Steinke M, Johnson A (2007) Structural and regulatory genes required to make the gas dimethyl sulfide in bacteria. Science 315:666–669
Van Alstyne KL (2008) Ecological and physiological roles of dimethylsulfoniopropionate (DMSP) and its products in marine macroalgae. In: Amsler CD (ed) Algal chemical ecology. Springer, Heidelberg, pp 173–194
Van Alstyne KL, Puglisi MP (2007) DMSP in marine macroalgae and macroinvertebrates: distribution, function, and ecological impacts. Aquat Sci 69:394–402
Van Alstyne KL, Wolfe GV, Freidenburg TL, Neill A, Hicken C (2001) Activated defense systems in marine macroalgae: evidence for an ecological role for DMSP cleavage. Mar Ecol Prog Ser 213:53–65
Van Alstyne KL, Schupp P, Slattery M (2006) The distribution of dimethylsulfoniopropionate (DMSP) in tropical Pacific coral reef invertebrates. Coral Reefs 25:321–327
Van Alstyne KL, Koellermeier L, Nelson T (2007) Spatial variation in dimethylsulfoniopropionate (DMSP) production in Ulva lactuca (Chlorophyta) from the Northeast Pacific. Mar Biol 150:1127–1135
Van Bergeijk SA, Stal LJ (2001) Dimethylsulfoniopropionate and dimethylsulfide in the marine flatworm Convoluta roscoffensis and its algal symbiont. Mar Biol 138:209–216
Wirtz M, Droux M (2005) Synthesis of the sulfur amino acids: cysteine and methionine. Photosynth Res 86:345–362
Wolfe GV, Steinke M, Kirst GO (1997) Grazing-activated chemical defence in a unicellular marine alga. Nature 387:894–897
Yoch DC (2002) Dimethylsulfoniopropionate: its sources, role in the marine food web, and biological degradation to dimethylsulfide. Appl Environ Microbiol 68:5804–5815
Acknowledgments
We thank A. Gehman for assistance with laboratory analyses and two anonymous reviewers for their comments, which improved this manuscript. This work was funded by National Science Foundation grants to K. Van Alstyne (OCE 0526644) and the Shannon Point Marine Center (OCE 0228618).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Biology Editor Dr. Michael Lesser
Rights and permissions
About this article
Cite this article
Van Alstyne, K.L., Dominique, V.J. & Muller-Parker, G. Is dimethylsulfoniopropionate (DMSP) produced by the symbionts or the host in an anemone–zooxanthella symbiosis?. Coral Reefs 28, 167–176 (2009). https://doi.org/10.1007/s00338-008-0443-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-008-0443-y