Abstract
We measured rates of ammonium and phosphate uptake into 12 microatolls at One Tree reef lagoon, Australia, on 14 occasions over a range of seasonal and meteorological conditions from 1993 to 1995. Nine of the microatolls were spiked with ammonia and/or phosphate every low tide (2.5–4 h) during low- (15-month) and high- (13-month) loading periods. The remaining three microatolls that were not spiked with nutrient served as reference conditions. Ammonium concentrations were elevated from an average background of 0.7 to 11 µM NH4 during the low-loading period and 36 µM NH4 during the high-loading period, resulting in a loading to the benthos of 3.5 and 18 mmol NH4 m–2 low tide–1. Phosphate concentrations were elevated from an average background of 0.2 to 2.3 and 5.1 µM PO4 during low- and high-loading periods, respectively, resulting in a loading of 0.66 and 3.9 mmol PO4 m–2 low tide–1. Ammonium and phosphate concentrations decreased significantly over low-tides, and uptake rates were proportional to concentrations (first-order). The average uptake-rate constant, S (m s–1), for ammonium and phosphate did not differ between the two loading periods but was highly variable. Averaged over both loading periods, S for ammonium was 129±74×10–6 m s–1 and S for phosphate was 67±39×10–6 m s–1. At background nutrient concentrations, estimated nutrient-uptake rates were 7.8 mmol NH4 m–2 day–1 and 1.2 mmol PO4 m–2 day–1. Excretion rates—calculated from the mean difference in uptake rates measured in reference and nutrient-enriched microatolls—were estimated to be 4.3 mmol NH4 m–2 day–1 and 0.9 mmol PO4 m–2 day–1. We reason and suggest that nutrient uptake rates in these microatolls were close to mass-transfer limited rates. We conclude that nutrient uptake into coral reefs can be highly dynamic, varying 10-fold spatially and temporally.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uptake rates of inorganic nutrients (NH4, PO4, NO3, SiO3) into coral reef communities have been difficult to measure in situ. Earlier workers from the 1920s to 1980s hypothesized rapid net changes in nutrient concentrations across coral-reef flats, but discovered that nutrient concentrations remain nearly constant. This result led to suggestions of "tight" and "rapid" recycling of nutrients through symbiotic relationships (Johannes and Project Symbiosis Team 1972), as well as rapid recycling of nutrients between heterotrophs and autotrophs through the water column [Pilson and Betzer (1973); see Atkinson (1988) for a review].
During the 1980s, field and laboratory experiments revealed that maximal rates of phosphate uptake were relatively slow compared to advective fluxes; typical reefs flats could only remove very small percentages of nutrients flowing across them [<5%; Atkinson (1992)]. This fact indicated that recycling of nutrients through the water column could only occur on scales of hundreds of meters and certainly was not rapid. It became evident that nutrient concentrations do not change across most reefs, simply because reefs cannot remove those nutrients fast enough.
To observe nutrient uptake in the field at ambient concentrations, water must reside over a shallow (~1-m) reef community for at least several hours. The water must be shallow so that uptake results in measurable changes in nutrient concentrations within relatively short periods of time. It is unusual to find shallow, ponded water over typical high-biomass reef communities, so it has been difficult to make measurements of nutrient uptake under natural conditions.
In the 1990s, nutrient-uptake experiments were performed in flumes to determine the rate-limiting step for nutrient uptake into coral-reef benthos. Nutrient-uptake rates into experimental assemblages of coral-reef benthos are limited by diffusion through concentration-depleted boundary layers adjacent to these benthic surfaces; that is, nutrient uptake is mass-transfer limited (Atkinson and Bilger 1992; Baird and Atkinson 1997).
Nutrient-uptake rates for these experimental assemblages are positively correlated to nutrient concentration and water velocity, and can be quantified using a relatively simple equation:
m=St×Ub×[Cb−Co],
where m is the nutrient-uptake rate in mass per area per time, St is a dimensionless rate constant called the Stanton number, Ub is water velocity, and Cb is the concentration in the bulk water. Co is the idealized average nutrient concentration at the collective surfaces of the organisms; Co is assumed to be negligible under strict definition of mass-transfer limitation, but is probably in most cases some significant percentage of Cb (Bilger and Atkinson 1995).
The Stanton number, St, is a somewhat confusing constant for non-engineers; it represents a dimensionless ratio of uptake into a benthic surface compared to the rate of advection of a substance past the surface. St is typically a very small number—around 10–4 for shallow coral reefs—and quantifies the mass transfer between the benthos and the overlying water flow. St is strongly a function of the form-drag and skin-friction of the benthic community, and can be parameterized by a friction factor (cf), which is a measure of the energy dissipated by benthic surface friction under steady flows (Bilger and Atkinson 1992; Kays and Crawford 1993). Thus, "rough" benthic surfaces with highly branched, rigid organisms, such as coral, dissipate more energy than "smooth" surfaces, giving higher St and higher uptake rates (Baird and Atkinson 1997; Thomas and Atkinson 1997).
The product of St and Ub is expressed as the uptake-rate constant, S, which can either be derived from engineering literature on heat and mass transfer, or measured directly. S for flume studies on coral-reef assemblages with moderate water velocities (4–39 cm s–1) range approximately an order of magnitude, between 20 and 200×10–6 m s–1 or between ~2 and 20 m day–1 (Bilger and Atkinson 1995).
There are, however, no published data sets of S derived from in situ measurements of nutrient uptake [except for a respiration-normalized constant of Atkinson (1987)]. Two obvious questions arise: (1) are uptake rate constants, S, for field studies similar to flume studies, and (2) are these uptake constants variable, as mass-transfer limitation would imply?
The ENCORE (Enrichment of Nutrients on a Coral Reef Experiment) project included measurements of nutrient uptake into 12 coral microatolls within One Tree reef lagoon, Australia, providing a comprehensive database to determine nutrient-uptake rates into natural coral-reef communities. This paper is the first study to report values of S for nutrient uptake in the field, and compare those constants with laboratory and flume experiments. Measurements of physical parameters to calculate mass-transfer limited rates, such as water velocity and surface friction, were not part of the ENCORE study; thus we cannot in this paper prove whether the microatolls are mass-transfer limited.
Methods
Experimental design
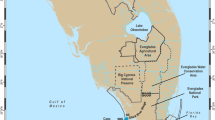
The detailed design of ENCORE is given in Larkum and Steven (1994). Briefly, 12 microatolls of similar size, volume, and benthic composition were used as natural, replicated subsystems (Fig. 1 and Table 1). The microatolls are all located in the northern end of the One Tree reef lagoon (23°30′S, 152°06′E) and are grouped in three clusters, demarcated by the surrounding lagoon morphology. During low tide, the perimeter of coral isolates a shallow pool (<1 m) inside each microatoll for 2.5–4 h from the surrounding lagoon, thus forming clearly defined boundaries. Twice daily, during low tide, each of the 12 microatolls received one of four nutrient treatments: either the addition of NH4 (+N), PO4 (+P), NH4 and PO4 combined (+N+P), or no nutrient addition (reference, –N–P). The nine nutrient-enriched microatolls were fertilized using automated "nutrient-dispensing units" that discharged concentrated nutrients through multiple pipe outlets distributed through the microatolls (Koop et al. 2001). Thus, organisms growing naturally, or transplanted into the microatoll basins, were maintained in natural environmental conditions, but subjected to nutrient-enriched waters during low-tide periods.
Oblique aerial photographs showing overall morphology of the 12 microatolls and ordered vertically by nutrient treatment. Refer to Table 1 for dimensions
The 28-month experiment was divided into a low-loading period (LL, September 1993–December 1994), followed by a high-loading period (HL, January 1995–February 1996). Throughout the low-loading period, concentrated NH4 (as NH4Cl) and PO4 (as KH2PO4) were added every low tide as a single pulse to the water body within the microatoll basin to achieve initial concentrations of 10 µM NH4 and 2 µM PO4. During the high-loading period, nutrients were added three times every low tide (~37 min apart) to sustain elevated concentrations of 20 µM NH4 and 4 µM PO4.
Microatoll characteristics
The basins of the microatolls vary from oblong to elliptical, are often convoluted, and have volumes of 27–323 m3 at low tide (Table 1, Fig. 1). The southeast walls of the microatolls are well developed (1–2 m wide), while the northerly walls can be thin and uneven. The height of the inside wall of the microatolls varies from 0.5–0.9 m, with the deeper microatolls occurring in the northwest sector of One Tree reef lagoon (6, 11, 12). The planar surface area of the microatoll basins are 107–829 m2 and the volume-to-area ratios of the microatolls vary from 0.30–0.64 (Table 1).
Most of the coral and algal biota are distributed along the inside walls. Mean cover of scleractinian corals ranges from 6–26%, with greater biomass occurring in the deeper microatolls. The most abundant coral colonies are encrusting (Porites lichen, P. murrayensis, Goniopora tenuidens, Favites abdita, Platygyra sinensis, and Goniastrea retiformis) and small-branching (Acropora bushyensis, A. palifera, Pocillopora damicornis, Stylophora pistillata, and Seriatopora hystrix) species. Stands of staghorn-branching corals (A. aspera, A. nobilis, and A. longiciathus) are common in the deeper microatolls where they proliferate along the southern walls. Coralline algae (Lithophyllum spp. and Porolithon spp.) cover up to 12% of the walls. Macroalgae, mainly Laurencia spp., Chlorodesmis fastigiata, Turbinaria ornata, and Caulerpa spp., are seasonal, but low in cover (~2%). The epilithic algae community covers all other substrate.
The bottoms of the microatolls are predominantly sand (40–60%), with small outcrops of dead coral covered in biota. Live coral cover is 5–18% and is comprised mainly of stands of branching corals such as Acropora grandis and A. pulchra. Also distributed on the bottom of the microatolls during the experiment were plastic racks holding a variety of coral, soft coral, and algae species transplanted from adjacent areas [see Larkum and Steven (1994) for project details].
Wind is the primary force controlling water motion within the microatolls, once they become isolated from the lagoon. Winds, originating from the southeast and northwest varied from 0.5–7.2 m s–1 during the study (Table 2).
Sampling nutrient concentrations
To measure rates of nutrient depletion, microatoll waters were sampled 4–11 times during each low-tide period (Table 2). Replicate water samples were taken from three locations within each microatoll by drawing water along PVC pipes (14 mm i.d.) using a bilge pump. The inlets of the pipes were mounted on poles ~0.2 m above the substratum to minimize sediment resuspension. To ensure a representative sample, 5–10 L was flushed through each pipe before retaining a final volume of 5 L. Sub-samples for nutrient analysis were taken with a 100-mL syringe, and filtered through 0.45 µm minisart filters into bottles. Bottles were rinsed with filtrate, before retaining 50 mL for analysis. Wind direction and speed (m s–1), cloud cover (oktas), and sea surface temperature (°C) were recorded at each sampling period.
On 9 days during the low-loading period, nutrient samples in each of the 12 microatolls were collected immediately after the addition of nutrients and at hourly intervals thereafter, until the tide rose and inundated the microatolls with lagoonal water (see Table 2 for sampling times and conditions). Samples for nutrient analysis were stored on ice and returned to the laboratory where they were analyzed immediately for NH4 and PO4 using standard spectrophotometric techniques.
On 5 days during high loading (Table 2), nutrient samples were collected at ~15-min intervals, prior to and following the three nutrient additions. With increased sampling frequency, it was logistically possible to only sample four to six microatolls on the same low tide. Samples were analyzed within 2 weeks for NH4, NO3, and PO4 on a segmented flow autoanalyzer at the Queensland Department of Health, Brisbane.
Calculations
For each sampling period, the mean (n=3) concentration of NH4 and PO4 in each microatoll was plotted against time, t. The uptake-rate constant, S, was calculated as the slope of ln N or P vs. t, multiplied by the microatoll volume (V) to area (A) ratio. This approach assumes the excretion rate during the uptake period is constant. Nutrient loading (L) per low tide was estimated as the amount of nutrient added to the background nutrient concentration, minus the final nutrient concentration at the end of low tide, multiplied by the V to A ratio (Table 3). We did not use initial concentrations in the above nutrient-loading calculation because the error due to initial patchiness is variable and difficult to estimate.
Results
Ammonium
Ammonium loading into reference (–N–P) microatolls during the 2.5- to 4-h low-tide periods averaged (±SD) 0.18±0.06 mmol N m–2. In N-enriched (+N, +N+P) microatolls, NH4 loading averaged 3.6±0.55 mmol N m–2 in low loading, and 17.6 ± 3.2 mmol N m–2 in high loading (Table 3). NH4 concentrations in –N–P microatolls significantly decreased (F 1,80=31, p<0.0001) from 1.1±0.2 to 0.4±0.4 μM N and averaged 0.7±0.7 µM N (Table 3, Fig. 2A). NH4 concentrations in the N-enriched microatolls during low loading significantly decreased (F 1,92=246, p<0.0001) from 10.7±5.0 to 0.9±0.8 μM N and averaged 4.4 μM N (Table 3, Fig. 2B); similarly, during the high-loading period, NH4 also significantly decreased (F 1,31=7.3, p=0.011) from 36.2±21.9 to 11.3±10.2 µM N and averaged 18.6 μM N (Table 3, Fig. 2C). In all cases NH4 was removed from the water column and taken up by the benthos. Ammonium uptake-rate constants (SN) averaged (±SD) 60±29×10–6 m s–1 for –N–P microatolls, 131±69×10–6 m s–1 for low-loading conditions, and 125±88×10–6 m s–1 for high-loading conditions (Table 3). SN of individual microatolls did not vary significantly between low and high loading (t-tests, p=0.1702–0.9183), giving an overall mean SN of 129×10–6 m s–1. Thus, NH4 uptake, m, calculated at background concentrations (0.7 μM N) was 7.8 mmol m–2 day–1.
NH4 concentrations measured in –N–P (A) and N-enriched microatolls during low (B) and high (C) loading. Data are the composite of nine sampling dates in low loading and five in high loading (Table 2). The mean (±SE) of these data are plotted as diamonds. During low loading, nutrients were added every low tide as a single pulse to the water body within the microatoll basin; during high loading, nutrients were added thrice at ~37-min intervals
Phosphate
Phosphate loading into microatolls over low tide averaged (±SD) 0.017±0.009 mmol m–2 in –N–P, 0.66±0.20 mmol m–2 in low loading, and 3.9±0.90 mmol m–2 in high loading. PO4 concentrations in –N–P microatolls showed no consistent trends and averaged 0.20±0.1 µM P (Table 3, Fig. 3A). Phosphate concentrations in P-enriched (+P, +N+P) microatolls significantly decreased (F 1,88=98, p<0.0001) during the low-load period from 2.3±1.0 to 0.5±0.3 μM (Table 3, Fig. 3B), whereas during high loading, phosphate concentrations rose with each successive nutrient addition, reaching an average maximum concentration of 5.1±2.8 µM P, and subsequently decreasing significantly (F 1,81=65, p<0.0001) to an average of 2.4±1.6 µM P (Table 3, Fig. 3C). Phosphate uptake-rate constants (SP) averaged 13±5×10–6 m s–1 for –N–P microatolls, and for P-enriched microatolls, 63±36×10–6 m s–1 for low loading and 80±46×10–6 m s–1 for high loading (Table 3). SP of individual microatolls did not vary significantly between low and high loading (t-tests, p=0.2985–0.9977), and combined had a mean SP of 67×10–6 m s–1. The average phosphate uptake at background concentrations (0.2 μM P) was 1.2 mmol m–2 day–1.
PO4 concentrations measured in –N–P (A) and P-enriched microatolls during low (B) and high (C) loading. Data are the composite of nine sampling dates in low loading and five in high loading (Table 2). The mean (± SE) of these data are plotted as diamonds. During low loading, nutrients were added every low tide as a single pulse to the water body within the microatoll basin; during high loading, nutrients were added thrice at ~37-min intervals
Discussion
These results clearly show that nutrients were removed from the water in the microatolls. Other studies demonstrate that biological uptake and assimilation were the principal fate of these nutrients (Steven 2000). The reported uptake rate constants, S, for reference (–N–P) microatolls are much lower than S for low- and high-loading periods. Assuming the lowered net uptake is simply a result of uptake and excretion being closely balanced at low concentration, an excretion rate of the benthic communities in the microatolls can be calculated by equating net uptake rate in the –N–P patch-reefs to gross uptake less excretion:
where the subscripts R and L refer to data from reference and nutrient-loaded microatolls, respectively. For these estimates, we assume that the excretion rate is constant during the low-tide period; neither uptake kinetics nor internal nutrient pools are changing significantly during this brief period, so we believe this assumption is warranted and consistent with previous studies. Using data from Table 3, daily excretion rates are estimated to be 4.3 mmol NH4 m–2 day–1and 0.86 mmol PO4 m–2 day–1.
Measured nutrient concentrations and uptake-rate constants were highly variable, both between days and among microatolls (Fig. 4, Table 3). Variation among microatolls resulted from intrinsic differences in their physical dimensions (i.e., surface area, volume) and construction (i.e., "leakiness"), biological composition, and local hydrodynamic conditions. In particular, microatoll 11 had substantially higher SN and SP than other microatolls (Fig. 4).
On any one day, uptake rates were more similar among the 12 microatolls than rates measured in the same microatoll on different days. SN of N-enriched microatolls varied significantly between days (F 13,44=5.88, p<0.0001), but not among microatolls on the same day (F 4,45=0.98, p= 0.580). Similarly, SP of P-enriched microatolls varied more among days (F 13,40=1.89, p=0.067) than among microatolls on the same day (F 4,40=0.66, p= 0.780).
A principal source of this daily variation was, we believe, due to differences in water velocities and mixing characteristics within the microatolls; these conditions were driven primarily by the prevailing wind speed and direction. On very still days (<1.5 m s–1 wind speed), nutrient concentrations were initially patchy (i.e., high initial replicate variability), often exceeded desired concentrations, and had enhanced uptake rates. At wind speeds greater than 7 m s–1, surface water was sometimes driven over the northerly rim, or flushed through the porous matrix of the microatolls, resulting in low initial concentrations. However, on moderately windy days (1.5–6.5 m s–1 wind speed), nutrients mixed quickly within the water volume, resulting in excellent first-order regression fits of the data (note curvature in Fig. 2B,C and Fig. 3B,C; mean R 2 adj=0.92, n=209). Under these conditions, SN varied significantly with wind speed (Fig. 5A; R 2 adj=0.853, p<0.0001, n=31), but no such relationship could be established for SP (Fig. 5B; R 2 adj=0.058, p<0.1137, n=28). Measured SN significantly correlated (r=0.919, n=31, p<0.0001) with SN predicted from estimates of water velocity (2% of wind speed) and roughness [using previous published mass-transfer correlations, Thomas and Atkinson (1997)]. Measured and predicted SP was not correlated (r=0.3101, n=28, p<0.1083).
SN (A) and SP (B) of nutrient-enriched microatolls during low loading plotted against average wind speed. Data within the range of moderate wind speeds (1.5–6.5 m s-1) are shown in the shaded box with lines of best fit: SN=75.21+47.61X, R 2 adj=0.853, n=31, p<0.0001; SP=32.38+6.71X, R 2 adj=0.058, n=28, p<0.1137
S for experimental coral-reef communities in flumes range 26–147×10–6 m s–1 for NH4 and 5–60×10–6 m s–1 for PO4, over a range of water velocities from 4–39 cm s–1 (Bilger and Atkinson 1995). S for this study (Table 3) compares very well to flume S; SN ranges 26–352 and averages 129×10–6 m s–1, and SP ranges 9–214 and averages 67×10–6 m s–1.
In microatolls with simultaneous measurements of N and P uptake (+N+P), measured SN was also significantly correlated to SP (r=0.56, n=29, p=0.0016). The ratio of average SN: SP was 2.1±0.23 (n=29). If nutrient uptake is mass-transfer limited, then the NH4 uptake should be 2.2 times greater than PO4 uptake because NH4 ions diffuse faster than PO4 ions (Bilger and Atkinson 1992).
Limited data from the high-loading period also show that uptake rates following the final third nutrient addition were ~3 times slower than uptake rates following the first nutrient addition. This is consistent with S being near mass-transfer limited rates because nutrient loading decreases S (Bilger and Atkinson 1995). For example, Bilger and Atkinson (1995) showed that increases in nutrient loading to 25–50 mmol N m–2 day–1 decreased S about three-fold. In the present study, the high loading treatment was about 17.6 mmol N m–2 per loading period or about 35 mmol N m–2 day–1, consistent with the previous flume data.
We suggest that the rates of nutrient uptake in the microatolls may be close to mass-transfer limitation for the following reasons: (1) nutrient-uptake rates, m, are proportional to concentration (first-order); (2) S, the first-order uptake-rate constant, is positively correlated to wind speed (which is positively correlated to water motion); (3) S is similar in magnitude to S in flume studies, in which uptake rates were shown to be mass-transfer limited; (4) SN are ~ twice SP, confirming the different diffusivities of NH4 and PO4; and (5) in the high-loading period sequential additions over the course of a low tide decreased both SN and SP. Thus, we suggest that the reported order of magnitude variability in the uptake rate constants, S, for both NH4 and PO4, may be directly related to changes in the physical environments of the microatolls. Differences in water velocity, rather than biological or physical differences, appear to be the dominant source of this variation in S. We conclude that nutrient input, one of the basic factors limiting the quantity and quality of organic production, is highly variable spatially and temporally, maintaining a range of habitats for the diversity of coral reef autotrophs.
References
Atkinson MJ (1987) Rates of phosphate uptake by coral reef flat communities. Limnol Oceanogr 32:426–435
Atkinson MJ (1988) Are coral-reefs nutrient limited? Proc 6th Int Coral Reef Symp 1:157–166
Atkinson MJ (1992) Productivity of Enewetak Atoll reef flats predicted from mass transfer relationships. Cont Shelf Res 12:799–807
Atkinson MJ, Bilger RW (1992) Effect of water velocity on phosphate uptake in coral reef-flat communities. Limnol Oceanogr 37:273–279
Baird ME, Atkinson MJ (1997) Measurement and prediction of mass transfer to experimental coral reef communities. Limnol Oceanogr 42:1685–1693
Bilger RW, Atkinson MJ (1992) Anomalous mass transfer of phosphate on coral reef flats. Limnol Oceanogr 37:261–272
Bilger RW, Atkinson MJ (1995) Effects of nutrient loading on mass-transfer rates to a coral-reef community. Limnol Oceanogr 40:279–289
Kays MW, Crawford ME (1993) Convective heat and mass transfer, 3rd edn. McGraw Hill, New York
Koop K, Steven ADL, McGill RM, Drew E, McDonald B (2001) Use of a telemetered dispensing system in controlling nutrient enrichment in the ENCORE study at One Tree Island, Great Barrier Reef, Australia. Mar Pollut Bull 42:121–126
Johannes RE, Project Symbiosis Team (1972) The metabolism of some coral reef communities: a team study of nutrient and energy flux at Eniwetok. Bioscience 22:541–572
Larkum AWD, Steven ADL (1994) ENCORE: the effect of nutrient enrichment on coral reefs.1. Experimental design and research programme. Mar Pollut Bull 29:112–120
Pilson ME, Betzer FB (1973) Phosphorus flux across a coral reef. Ecology 54:581–588
Steven ADL (2000) Effects of inorganic nitrogen and phosphorus enrichment on the calcification and growth of hermatypic corals and coral-reef communities. PhD Thesis, James Cook University of North Queensland, Townsville, 210 pp
Thomas FIM, Atkinson MJ (1997) Ammonium uptake by coral reefs: effects of water velocity and surface roughness on mass transfer. Limnol Oceanogr 42:81–88
Acknowledgements
We thank Drs. Klaus Koop and Andrew Broadbent for field assistance. Funding for this research was provided by the Great Barrier Reef Marine Park Authority and the University of Hawaii NOAA SeaGrant Award no. NA36RG0507 Yr 31 R/EL-1 to M.J.A. This is Hawaii Institute of Marine Biology (HIMB) contribution 1156 and School of Ocean and Earth Science and Technology (SOEST) contribution 6154.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Steven, A.D.L., Atkinson, M.J. Nutrient uptake by coral-reef microatolls. Coral Reefs 22, 197–204 (2003). https://doi.org/10.1007/s00338-003-0303-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-003-0303-8