Abstract
To date around 140 genetic alleles have been identified as being responsible for mouse cataract pathology, including Crya, Cryb, Cryg, Maf, Pax6, Pitx3, Sox, Connexins, MIP, and Lim-2. We obtained a dominant cataract mouse model from a spontaneous mutation in the F1 hybrids of outbred strain ICR mice crossed to the inbred strain BALB/cJ mice. Heterozygous and homozygous mutants expressed a nuclear cataract in both eyes. In 8-day-old mice, histological analysis showed that polygon epithelial cells were in the equatorial region and cortex underneath, and vacuole and sponge-like degeneration were in the cortical area underneath the posterior lens capsule. The nucleus of the lens was a deeply stained pink, with the shorter fibers losing their normal arrangement. For the entire eye, there was a blank zone in the equatorial region in 8-day-old mice; however, there was a certain degree of atrophy in cornea tension and retina in the lens in 3-month-old mice. The lens had been serious damaged in the homozygous mutants. For mutation mapping, heterozygous carriers were mated to wild-type C3H/HeJ mice, and offspring (F1 generation) with cataracts were backcrossed to the wild-type C3H/HeJ mice again. N2 mice with cataracts were used for genotyping. Using genome-wide linkage analysis, the mutation was mapped to chromosome 1 and the Cryg gene cluster between two markers was confirmed as the candidate gene. After direct sequencing the cDNA of the Cryg gene cluster, a 1-bp deletion was found in exon 3 of the Crygc gene, leading to a stop codon at the 76th amino acid of exon 3 which results in production of a truncated protein in mutant mice (Leu160Stop). Bioinformatic analysis of the mutant γC-crystallin reveals that the COOH-terminal of the mutant protein deletes a β-sheet, which affects the function of the lens proteins and leads to the development of cataracts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A cataract manifested as a lens opacity is a common disease that causes blindness (Liu et al. 2006; Kang et al. 2008). A great variety of mouse cataract mutants affecting ocular development were acquired mainly through spontaneous mutation, chemical mutagenesis, radiation, and knockout or transgenic mice (Graw 2004). Typically, whereas cataract mutations are often induced by radiation or ethylnitrosourea (ENU) treatment (Brown and Balling 2001) and occasionally found in knockout or transgenic mice (e.g., Shi et al. 2009), spontaneous mutations usually originated from large-scale animal breeding (Omi et al. 2008).
To date, about 140 cataract mutations have been identified according to the Mouse Genome Database (Mouse Genome Informatics, The Jackson Laboratory, Bar Harbor, ME, http://www.informatics.jax.org) and the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/), and 24 of them were mapped to the chromosome 1. Most frequently hit the six highly related genes (Cryga → Crygf) of the family of Cryg genes (Zhao et al. 2010). In detail, the Cryg genes belong to the superfamily of β- and γ-crystallin-encoding genes. Conservatively in all mammals, the Cryg genes consist of three exons: exon 1 codes for three amino acids and the other two for two Greek key motifs. All three exons of each Cryg gene encode for 21-kDa proteins (Graw 1997; Slingsby and Clout 1999).
In this study we report the characterization of a novel spontaneous mutation, which causes nuclear cataracts, that was initially found in crossing ICR, an outbred mouse strain, with inbred strain BALB/cJ (Zhao et al. 2009). To identify the gene, we carried out a genome-wide linkage analysis and mapped the disease gene to mouse chromosome 1 within the interval that includes Cryg. Finally, cDNA sequence analysis was performed to find a 1-bp deletion in exon 3 of the Crygc gene, leading to a stop codon.
Materials and methods
Animals
In 2006, two spontaneous mutant cataract mice, in the F1 hybrids of ICR outbred strain crossed to inbred strain BALB/cJ, were found in Shanghai Institute of Experimental Animal Research Center, Chinese Academy of Sciences. The disease allele maintained a stable autosomal dominant inheritance pattern in the further backcross to BALB/cJ mice. Cataracts were identified at weaning through direct observation. Homozygous mutants were obtained by brother × sister mating. For control, wild-type C3H/HeJ and BALB/cJ mice were used. All mice were maintained under the Specific Pathogen Free (SPF) according to The People's Republic of China Laboratory Animal Regulations.
Morphologic analysis
For gross documentation, lenses from 8-day-old and 3-month-old mice were enucleated and photographed. For detailed histological analysis, eyes from 8-day-old and 3-month-old mice were fixed for 24 h in Carnoy’s solution, dehydrated, and embedded in paraffin medium according to the manufacturer’s instructions. Sectioning was performed with an ultramicrotome (RM 2016, Leica, Bensheim, Germany). Serial transverse 2-μm sections were cut with a dry glass knife and collected in water drops on glass slides. After drying, the sections were stained with methylene blue and basic fuchsin. The sections were evaluated by inverted fluorescence microscopy (IX 71, Olympus, Tokyo, Japan). Images were acquired by means of an image-processing program (Image-Pro Express, Olympus).
Genome-wide linkage analysis
Prior to genetic analysis, the cataract gene was transferred into BALB/cJ genomic background by repeated backcrossing for seven generations. After seven generations of backcross, the mutant mice were mated to wild-type C3H/HeJ mice; offspring (second generation) with cataracts were backcrossed to the wild-type C3H/HeJ mice. DNA was prepared from the tail tips of 83 cataractous offspring of the third generation (G3) using a mouse genome DNA isolation kit according to standard procedure. In genome-wide linkage analysis, 43 microsatellite (Supplementary Table 1) markers were used for 19 autosomes. In order to fine map the cataract locus, another three microsatellite markers, D1Mit236 (25.7 cM), D1Mit19 (36.9 cM), and D1Mit49 (54.5 cM) were used. All data concerning the linkage of genes or markers were taken from the Mouse Genome Informatics database (http://www.informatics.jax.org).
RT-PCR, sequencing, and genotyping
For mutation analysis, RNA was isolated from lenses of 4-week-old wild-type BALB/cJ mice, wild-type ICR mice, and heterozygous and homozygous mutant mice using the Qiagen RNeasy mini kit (Qiagen, Hilden, Germany). For expression studies, RNA was isolated from wild-type BALB/cJ mice and heterozygous or homozygous mutant mice embryos at embryonic days 11.5, 12.5, and 13.5 (head), embryonic days 14.5 and 15.5 (eye), and postnatal days 1, 14, or 21 (lens), respectively (Sandilands et al. 2002), using the Qiagen RNeasy mini kit. The age of the embryos was timed from the morning of detection of the vaginal plug; that was considered embryonic day 0.5. A total of 10 μl total RNA was used in RT–PCR to generate cDNA with the Promega RT–PCR kit (Promega, Madison, WI, USA). For amplification of the coding region of Crygc, we used the primer pair 5′-ACGGGTCAGCCAGCCATG-3′ (for the left side) and 5′-TGCCAACAATACAGACTAAA-3′ (for the right side) (Klopp et al. 1998). Using an annealing temperature of 55°C, a 606-bp fragment was amplified.
Three individuals of wild-type BALB/cJ mice, wild-type ICR mice, and heterozygous and homozygous mutant mice were sequenced. Sequencing was carried out using an ABI 3100 sequencer (Applied Biosystems, Foster City, CA, USA). Contigs were assembled by the programs phred and Phrap, and possible mutations were examined with the aid of the BioEdit programs.
To confirm the deletion mutations from Crygc (exon 3) causing cataracts, we used a new pair of primers: left-side primer (5′-FAM-CAGAATGCGGCTGTATGAGA-3′) and right-side primer (5′-GAGCCCGCCTTAGCATCTAC-3′), based on the reference sequence of the C57BL/6 J strain. Using an annealing temperature of 57°C, a 233/234-bp fragment was amplified to distinguish heterozygous and homozygous according to the sequenced samples. Thirty mutant and 30 wild-type mice of the third generation and 20 ICR mice were randomly selected for genotyping.
Computer-assisted prediction of the biochemical properties of the mutated proteins
The biochemical analyses were performed using the Proteomics tools of the ExPASy Molecular Biology server (http://www.expasy.ch; provided in the public domain by the Swiss Institute of Bioinformatics, Geneva, Switzerland). Protein models were calculated using the SWISS-MODEL (Arnold et al. 2006; Schwede et al. 2003; Guex and Peitsch 1997). In particular, we used the InterPro program for characterization of additional biochemical features.
Results
Phenotype and lens morphology
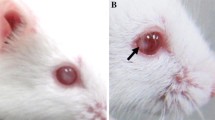
In cataractous mice, the opacity of the lens appeared at eye opening after birth. Both in homozygous and heterozygous mutant mice, lens opacities were concentrated in the nuclear region, whereas in heterozygous mutant mice, the opacity was also found throughout the eye (Fig. 1).
The histological analysis of 8-day-old cataractous lenses demonstrated that polygon epithelial cells were seen in the cortex underneath (Fig. 2a–d) and equatorial region (Fig. 2e–h) and that vacuole and sponge-like degeneration was observed in the cortical area underneath the posterior lens capsule (Fig. 2i–l). In the nucleus of the lens, fibers lost their normal arrangement and large vacuole-like degeneration was observed in homozygous mutants. However, in heterozygous mice, less vacuole-like degeneration was observed (Fig. 2m–p). There was a blank zone in the equatorial region in the entire eye in the 8-day-old mice (Fig. 2q–t). However, there was a certain degree of atrophy in cornea tension and retina in the lens in 3-month-old mice. In the homozygous mutant mice, the lens was severely damaged (Fig. 2u–x).
Histological sections of eyes of 8-day-old and 3-month-old mice. In histological sections of eyes from 8-day-old BALB/cJ (a, e, i, m, q) and ICR mice (b, f, j, n, r), no pathological changes are observed. In contrast, polygon epithelial cells in the anterior lens capsule are seen in homozygous mutant mice lenses. Sponge-like degeneration is seen in the cortical area underneath the anterior lens capsule in mutant mice. Epithelial cells overproliferate and become polygon in shape in the equatorial region in mutant mice, and sponge-like and vacuole-like degeneration in the cortical area under the posterior lens capsule is seen in heterozygous and homozygous mutant mice (arrow), respectively. Pink-stained embryonic nucleus of the lens and the fibers become swollen and lose the normal arrangement in the nucleus (arrow). c, d Anterior lens capsule from heterozygous and homozygous mice. g, h Equatorial region from heterozygous and homozygous mice. k, l Posterior lens capsule from heterozygous and homozygous mice. o, p Nucleus of the lens from heterozygous and homozygous mice. Heterozygous lens (s) and homozygous lens (t) are smaller in the histological sections of whole eyes at 8 days old. u, v Natural eyes from BALB/cJ and ICR mice. The heterozygous (w) and homozygous lens (x) in the histological sections of whole eyes at 3 month old and homozygous lens have been damaged. Original magnification: ×400 (a–p), ×40 (q–x). C cornea, L lens, LB lens bow, LE lens epithelium, R retina
Linkage analysis
Among the 318 progeny of the third generation, 143 cataractous mice and 175 wild-type mice were morphologically identified. In the 143 cataractous mice, there were 71 females and 72 males, which indicates an autosomal dominant inheritance.
The mutation was mapped to chromosome 1 between the markers D1Mit410 and D1Mit102. The members of the Cryg gene cluster (position = 32 cM from the centromere) and Cryba2 (position = 40.8 cM from the centromere) were considered to be candidate genes for cataractous mutation based on their known functions (Kratochvilova and Favor 1992). The detailed haplotype analysis of five markers was used which culminated with the cataract gene in an 11-cM interval on chromosome 1 between D1Mit236 and D1Mit19 (Fig. 3). Therefore, the Cryg gene cluster was selected as an attractive candidate gene underlying the pathology.
Haplotype analysis localized the Crygc mutation to chromosome 1. The heterozygous carriers were backcrossed to wild-type C3H/HeJ mice. Among the offspring, only the cataractous mice were analyzed for their parental genotypes with respect to a variety of microsatellite markers; results are given for those at chromosome 1. The total number of progeny scored for each locus is given on the right of the boxes, including the calculated distances between the loci (in cM). The number of progeny that inherited each haplotype is given below the boxes. The number of STR and theoretical distances is given on the left
Sequence analysis
All six members of the Cryg gene cluster were amplified successfully by PCR from lens cDNA. cDNA sequence analysis revealed a 1-bp deletion in exon 3 of the Crygc gene in mutants, leading to seven novel amino acids from 70 to 76 amino acids and a stop codon at the 76th amino acid of exon 3 of the Crygc gene, which produces a truncated protein (Fig. 4). Sequence homology analysis revealed that the deletion is derived from the ICR strain. Confirmed that the deletion is the cause of cataracts, all mutants were identified as heterozygous, while all wild-type mice, including ICR mice, were homologous after genotyping (see Fig. 5).
Sequence analysis of the Crygc mutant. The Crygc DNA sequence from wild-type mice is compared to that from the Crygc mutants. The γC-crystallin amino acid composition is given above the cDNA sequence in parentheses. Below the cDNA sequence, a further putative γC-crystallin amino acid composition is predicted. The deletion of 1 bp at the 76th amino acid of exon 3 of the Crygc gene is shown in vertical lines and shaded in gray. The stop codons are underlined. Triangle is the position of the 1-bp deletion the wild-type mouse
Biochemical analysis
A 1-bp deletion in exon 3 of the Crygc gene produced the mutated protein, which consisted of 160 amino acids (wild-type γC-crystallin theoretically has 175 amino acids) and the loss of part of the four Greek key motifs. The calculated molecular weight of the mutant protein was 19 kDa (wild-type γC-crystallin has a theoretical molecular weight of 21 kDa).
From domain analysis using InterPro software, four fingerprints of BGCRYSTALLIN, a mark of 4-element fingerprints for the β- and γ-crystallin family, are present in wild-type γC-crystallin, while only three fingerprints exist in mutant γC-crystallin. Likewise, in the three-dimensional structure analysis, the C-terminal of the mutant protein loses a β-sheet, and the C-terminal conserved region affects the γC-crystallin in the correct folding (Fig. 6). The modeled residue range is from 2 to 175 amino acids in wild-type γC-crystallin, but from 2 to 153 in mutant γC-crystallin, indicating the seven novel amino acids are not included in the protein structural domain. Moreover, in the detection of atomic empirical mean force potential of three structural models using the SWISS-MODEL workspace, the final total energy of the wild type γC-crystallin structure was −13518.472 KJ/mol but it was –11494.698 KJ/mol in the mutant γC-crystallin structure. As a result, the highly symmetric structure of γC-crystallin is disrupted when accepting the loop from 2 to 2 of γC-crystallin as result of a 1-bp deletion mutation.
Structural modeling of the wild-type and mutant γC-crystallins. The structural modeling is based on the X-ray-determined coordinates of mouse γC-crystallin chain A using SWISS-MODEL. a A structural model of the wild-type γC-crystallin with 99.425% sequence identity. b A structural alteration of the mutant γC-crystallin with 99.342% sequence identity. Highly symmetric structure of γC-crystallin is disrupted when 15 amino acids are removed from the COOH-terminus of γC-crystallin as result of a 1-bp deletion mutation
Experimentally, we investigated whether the novel protein is expressed in the eye lens and whether it is stable. Therefore, the onset of Crygc expression was investigated by RT-PCR at various stages of lens development (Fig. 7). Although the RT-PCR results show that mRNA transcription of Crygc is normal, the mutation is located in the conserved fourth Greek key motif which is shortened in the mutant mice. When mRNA is translated into comprised protein, the function of the gene is changed.
Discussion
Mutation in the Crygc gene leads to a nuclear cataract. In the present study, histological observations demonstrated that the mutated γC-crystallin gene can influence the normal development of the lens. It promotes overproliferation of the lens epithelia and forms polygon epithelial cells. There is also obviously disturbance of the arrangement and shape of fiber cells, which leads to the formation of cataract. In heterozygous and homozygous mutant mice, the deeply stained pink nucleus in the central region of the lens indicates that organization of the lens and its molecular components is disrupted. This suggests that γC-crystallin may be involved in epithelial cell growth, which in turn may contribute to lens fiber organization (Ji et al. 2007).
cDNA sequencing reveals a 1-bp deletion in Crygc leading to a stop codon at the 76th amino acid of exon 3 of the Crygc gene. As reported, base substitution, base deletion, and nucleotide insertion may result in lens-specific gene transcription termination (Bu et al. 2002; Matteson et al. 2008; Sandilands et al. 2004; Talamas et al.2006). The new deletion is obviously different from other alleles that affect the Crygc gene, such as Crygc Chl3 (Graw et al. 2002) or Crygc MNU8 (Graw et al. 2004). The Crygc Chl3 mutation is affected by a 6-bp deletion in exon 3 of the Crygc gene, and the Crygc MNU8 mutation is affected by the substitution of the regular G with an A at position 471 of the Crygc gene. So far, a high number of Cryg/CRYG mutations have been observed in mouse and man, making this gene cluster a hot spot for autosomal dominant cataracts (Graw et al. 2001, 2004; Klopp et al. 1998; Li et al. 2008), but no recessive mutation has been reported (Graw and Löster 2003). Furthermore, a single-base difference among wild-type, heterozygous, and homozygous mutants can be easily distinguished by PCR (Fig. 5). A 100% penetrance of the deletion confirmed the cause of cataracts, by all phenotypes of mice in accordance with the genotypes.
Sequence alignment indicates that the C-terminal of γC-crystallin is a highly conserved sequence in mouse and other species, suggesting that the COOH-terminal may be essential for the normal function of γC-crystallin. Without the last 15 amino acid residues from the COOH-terminus of γC-crystallin (normally 175 amino acids), the incomplete Greek key motifs of the γC-crystallin form truncated protein. Illustrated in the three-dimensional structure analysis of protein (Fig. 6), the C-terminal of the mutant protein loses a β-sheet, which causes the conserved region of the second structural domain to be damaged. A novel nonsense mutation in CRYGC was detected one and a half Greek key motifs at the C-terminal but was absent in the three-dimensional structural model of the mutant γC-crystallin (Yao et al. 2008). Based on the amino acid sequence information, a shortened conservative segment affects the function of the lens proteins and leads to the formation of cataracts.
For the Crygc gene, Crygc Chl3 (Graw et al. 2002) and the Crygc MNU8 (Graw et al. 2004) were obtained from mutagenesis, but no disease alleles have yet been found in cataracts caused by spontaneous mutations. The Crygc Chl3 mutation expressed a nuclear and radial cataract and the Crygc MNU8 mutation expressed a dense nuclear and subcortical opacity, which is different from the present study. All mutants characterized in human CRYG genes were found in CRYGC, CRYGD, and CRYGS, but there is no report about CRYGA or CRYGB to date (Graw 2009). Mutations in human CRYGC genes are thought to be associated with the formation of the Coppock-like cataract (Héon et al. 1999), the nuclear cataract (Yao et al. 2008), and the variable zonular pulverulent cataract (Ren et al. 2000). A nuclear congenital cataract is caused by mutations in CRYGC but is associated with the phenotype of a lamellar cataract (Gonzalez-Huerta et al. 2007). Three other autosomal dominant congenital cataracts with a lamellar cataract phenotype and a central nuclear cataract are also caused by mutations in CRYGC or CRYGD genes (Santana et al. 2009; Santhiya et al. 2002).The γ-crystallins are monomeric with a molecular mass of 21 kDa and comprise about 40% of the total proteins in the mouse lens and 25% in the human lens. Hence, γ-crystallins play an important role for lens transparency in both mouse and human (Graw 1997; Wistow and Piatigorsky 1988).
References
Arnold K, Bordoli L, Kopp J, Schwede T (2006) The SWISS-MODEL Workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201
Brown SD, Balling R (2001) Systematic approaches to mouse mutagenesis. Curr Opin Genet Dev 11:268–273
Bu L, Yan S, Jin M, Jin Y, Yu C (2002) The gamma S-crystallin gene is mutated in autosomal recessive cataract in mouse. Genomics 80:38–44
Gonzalez-Huerta LM, Messina-Baas OM, Cuevas-Covarrubias SA (2007) A family with autosomal dominant primary congenital cataract associated with a CRYGC mutation: evidence of clinical heterogeneity. Mol Vis 13:1333–1338
Graw J (1997) The crystallins: genes, proteins, and diseases. Biol Chem 378:1331–1348
Graw J (2004) Congenital hereditary cataracts. Int J Dev Biol 48:1031–1044
Graw J (2009) Mouse models of cataract. J Genet 88:469–486
Graw J, Löster J (2003) Developmental genetics in ophthalmology. Ophthalmic Genet 24:1–33
Graw J, Klopp N, Löster J, Soewarto D, Fuchs H et al (2001) ENU-induced mutation in mice leads to the expression of a novel protein in the eye and to dominant cataracts. Genetics 157:1313–1320
Graw J, Neubäuser-Klaus A, Löster J, Favor J (2002) A 6-bp deletion in the Cryg gene leading to a nuclear and radial cataract in the mouse. Invest Ophthalmol Vis Sci 43:236–240
Graw J, Neubäuser-Klaus A, Klopp N, Selby PB, Löster J et al (2004) Genetic and allelic heterogeneity of Cryg mutations in eight distinct forms of dominant cataract in the mouse. Invest Ophthalmol Vis Sci 45:1202–1213
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modelling. Electrophoresis 18:2714–2723
Héon E, Priston M, Schorderet DF, Billingsley GD, Girard PO et al (1999) The γ-crystallins and human cataracts: a puzzle made clearer. Am J Hum Genet 65:1261–1267
Ji Y, Lu Y, Kong X, Yan S (2007) The anatomical and pathological changes in the crystalline lens of a congenital γS-crystallin gene mutated mouse model. Chin J Optom Ophthalmol 9:145–148
Kang M, Cho JW, Kim JK, Kim E, Kim JY et al (2008) Fine localization of a new cataract locus, Kec, on mouse chromosome 14 and exclusion of candidate genes as the gene that causes cataract in the Kec mouse. BMP Rep 41:651–656
Klopp N, Favor J, Löster J, Lutz RB, Neuhäuser-Klaus A et al (1998) Three murine cataract mutants (Cat2) are defective in different γ-crystallin genes. Genomics 52:152–158
Kratochvilova J, Favor J (1992) Allelism tests of 15 dominant cataract mutations in mice. Genet Res 59:199–203
Li L, Chang B, Cheng C, Chang D, Hawes NL et al (2008) Dense nuclear cataract caused by the gamma B-crystallin S11R point mutation. Invest Ophthalmol Vis Sci 49:304–309
Liu Y, Zhang X, Luo L, Wu M, Zeng R et al (2006) A novel alphaB-crystallin mutation associated with autosomal dominant congenital lamellar cataract. Invest Ophthalmol Vis Sci 47:1069–1075
Matteson PG, Desai J, Korstanje R, Lazar G, Borsuk TE et al (2008) The orphan G protein-coupled recptor, Gpr161, encodes the vacuolated lens locus and controls neurulation and lens development. Physiol Genomics 105:2088–2093
Omi N, Kiyokawa E, Matsuda M, Kinoshita K, Yamada S et al (2008) Mutation of Dock5, a member of the guanine exchange factor Dock180 superfamily, in the rupture of lens cataract mouse. Exp Eye Res 86:828–834
Ren Z, Li A, Shastry BS, Padma T, Ayyagari R et al (2000) A 5-base insertion in the γC-crystallin gene is associated with autosomal dominant variable zonular pulverulent cataract. Hum Genet 106:531–537
Sandilands A, Hutcheson AM, Long HA, Prescott AR, Vrensen G et al (2002) Altered aggregation 105: properties of mutant γ-crystallins cause inherited cataract. EMBO J 21:6005–6014
Sandilands A, Wang X, Hutcheson AM, James J, Prescott AR et al (2004) Bfsp2 mutation found in mouse 129 strains causes the loss of CP49 and induces vimentin-dependent changes in the lens fibre cell cytoskeleton. Exp Eye Res 78:109–123
Santana A, Waiswol M, Arcieri ES, Cabral de Vasconcellos JP, Barbosa de Melo M (2009) Mutation analysis of CRYAA, CRYGC, and CRYGD associated with autosomal dominant congenital cataract in Brazilian families. Mol Vis 15:793–800
Santhiya ST, Manohar MS, Rawlley D, Vijayalakshmi P, Namperumalsamy P et al (2002) Novel mutations in the γ-crystallin genes cause autosomal dominant congenital cataracts. J Med Genet 39:352–358
Schwede T, Kopp J, Guex N, Peitsch MC (2003) SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res 31:3381–3385
Shi X, Cui B, Wang Z, Weng L, Xu Z et al (2009) Removal of Hsf4 leads to cataract development in mice through down-regulation of gammaS-crystallin and Bfsp expression. BMC Mol Biol 10:10
Slingsby C, Clout NJ (1999) Structure of the crystallins. Eye 13:395–402
Talamas E, Jackson L, Koeberl M, Jackson T, McElwee JL et al (2006) Early transposable element insertion in intron 9 of the Hsf4 gene results in autosomal recessive cataracts in lop11 and ldisl mice. Genomics 88:44–51
Wistow GJ, Piatigorsky J (1988) Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem 57:479–504
Yao K, Jin C, Zhu N, Wang W, Wu R et al (2008) A nonsense mutation in CRYGC associated with autosomal dominant congenital nuclear cataract in a Chinese family. Mol Vis 14:1272–1276
Zhao G, Yang Y, Zhang R, Gu J, Xu P et al (2009) Cultivation of a mouse model of inherited cataract (BALB/c-Cat)―A preliminary report. Chin J Comput Med 19:63–65
Zhao L, Bao S, Zhao G, Liang Y, Li K et al (2010) Mutation, distribution, positional cloning for cataract in house mouse. Chin J Comput Med 20:62–66
Acknowledgments
The authors are grateful to the Eye Ear Nose and Throat Hospital affiliated with Fudan University for histological analysis. This work was supported by grants from Key Programs of the Science and Technology Commission Foundation of Shanghai (No. 09140901100) and National Natural Science Foundation of China (30700529).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, L., Li, K., Bao, S. et al. A 1-bp deletion in the γC-crystallin leads to dominant cataracts in mice. Mamm Genome 21, 361–369 (2010). https://doi.org/10.1007/s00335-010-9275-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00335-010-9275-5