Abstract
Bone marrow (BM)-derived T-cell progenitors differentiate into CD4 or CD8 single-positive (SP) cells in the thymus. We have previously reported that a single autosomal mutation, thid, causes a defect in the maturation of CD4 SP thymocytes and an abnormality of peripheral helper T cells in the LEC rat. In this study we attempted to identify a gene responsible for the thid mutation. We first performed genetic linkage analysis and mapped the thid locus between Myb and D1Rat392 on Chr 1. In this region we found an approximately 380-kb deletion from intron 3 of the Ptprk gene, which encodes a receptor-like protein tyrosine phosphatase type κ (RPTPκ) to intron 1 of the RGD1560849 predicted gene in the LEC rat genome. Reconstitution with syngenic BM cells transduced Ptprk but not the RGD1560849 predicted gene rescued development of CD4 SP cells in the LEC rat thymus. It is confirmed by this result that the Ptprk gene is responsible for the thid mutation in the LEC rat. Our results further suggest that RPTPκ plays a critical role in the development of CD4 SP cells in the thymus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The thymus is a major site of development of T lymphocytes. Bone marrow (BM)-derived T-cell progenitors enter the thymus and differentiate into mature T cells through sequential stages defined by expression of T-cell receptors (TCRs), expression of accessory molecules on the cell surface, and antigen-induced selection (Singer and Bosselut 2004). The most immature T cells in the thymus express neither the TCRs nor the coreceptors CD4 and CD8 (double negative, or DN). The DN thymocytes are found in the subcapsular zone and the outer cortical region of the thymus and migrate to the inner cortex (Lind et al. 2001; Porritt et al. 2003). During this step, DN thymocytes differentiate into CD4 and CD8 double-positive (DP) cells. The DP cells are selected by the extent of signaling, depending on the interactions between TCRs and self-peptide-bound major histocompatibility complex (MHC) molecules, leading to either deletion (negative selection) or development of MHC-restricted CD4 or CD8 single-positive (SP) cells (positive selection) (Ladi et al. 2006). The self MHC on the cortical thymus epithelial cells mediates positive selection (Bousso et al. 2002; Witt et al. 2005). The CD4/CD8 lineage commitment is regulated by the strength or duration of TCR-MHC binding, and the activities of the signaling pathway control the expression of CD4/CD8 molecules (He et al. 2005; Laky et al. 2006; Liu and Bosselut 2004; Sun et al. 2005). SP cells move to the medulla and are negatively selected for removal of thymocytes bearing TCRs reactive to self-antigens (Siggs et al. 2006). Negative selection is supported by the medullary thymic epithelial cells that present tissue-specific antigens on MHC molecules (Gallegos and Bevan 2004).
We have previously reported that a mutant strain of rats, LEC, exhibits a defect in T-cell maturation from DP to CD4 SP but not to CD8 SP cells in the thymus (Agui et al. 1990, 1991b). Although the thymocytes from LEC rats contain less than 1% of CD4 SP cells, some CD4+ T cells appear in the peripheral lymphoid organs. However, these peripheral CD4+ cells are not functional as helper T cells, i.e., the secretion of IL-2 after treatment with mitogenic lectin is impaired (Sakai et al. 1993). Moreover, LEC rats do not produce antibodies against T-cell-dependent antigen sheep red blood cells (Agui et al. 1990). These defects in the LEC rat are caused by a single autosomal recessive locus designated as thid (T-helper immunodeficiency) (Yamada et al. 1991). In this study we attempted to identify a gene responsible for the thid mutation by positional cloning and succeeded to show that a deletion mutation of the Ptprk gene, encoding a receptor-type protein tyrosine phosphatase type κ (RPTPκ), is responsible for the thid in the LEC rat.
Materials and methods
Animals
LEC/Ncu, BN/SsN, and (BN × LEC)F1 × LEC backcrossed progenies were maintained at animal breeding rooms in the Center for Experimental Animal Science, Graduate School of Medical Sciences, Nagoya City University, and the Graduate School of Veterinary Medicine, Hokkaido University. Animal breeding rooms were kept at 23 ± 2°C and 50 ± 10% relative humidity with a 12-h light-dark cycle. Research was conducted according to the Guidelines for the Care and Use of Laboratory Animals of both the Graduate School of Medical Sciences, Nagoya City University and the Graduate School of Veterinary Medicine, Hokkaido University. The experimental protocols were approved by the Institutional Animal Care and Use Committee of both the Graduate School of Medical Sciences, Nagoya City University and the Graduate School of Veterinary Medicine, Hokkaido University.
Genome mapping
A total of 197 (BN × LEC)F1 × LEC backcrossed progenies were used for PCR-based single sequence length polymorphism (SSLP) analysis. The sequences of all microsatellite primers were based on the Rat Genome Database <http://www.rgd.mcw.edu/> (RGSC 3.4, Dec 2004). We found a 12-bp-long polymorphism in the coding region of the Myb gene between the LEC and BN rats (data not shown). The sequences of the primers for detecting this polymorphism are given in Table 1. The thid genotype was estimated from the phenotype determined by the ratio of CD4+ T cells in mesenteric lymph node cells with flow cytometry as described previously (Jung et al. 2001). Briefly, backcrossed progenies showing the normal ratio (40–50%) of CD4+ cells in mesenteric lymph node cells were classified as thid/+ genotype, whereas backcrossed progenies showing the small ratio (10–20%) of CD4+ cells were classified as thid/thid genotype. Backcrossed progenies were clearly segregated into two groups with 1:1 ratio as reported previously (Jung et al. 2001; Wei et al. 1997; Yamada et al. 1991). Linkage analysis was performed by MapManager QTXb20 software (Manly et al. 2001).
Identification of gene responsible for thid and sequencing of genomic DNA
The sequence around the Ptprk gene in the rat genome was searched in the rat genome browser of Ensembl (release 43, February 2007) (http://www.ensembl.org/index.html) and NCBI (http://www.ncbi.nlm.nih.gov/) by BLAST software (ver. 2.2.15, October 2006) with the sequence of mouse genome based on the information of the homologous synteny. The sequence obtained was used for the construction of PCR primers for genes located in the thid region. The sequences of PCR primers are given in Table 1. Sequencing of PCR products was performed with an ABI 377 genetic analyzer (Applied Biosystems, Foster City, CA).
Flow cytometry
Mesenteric lymph node cells and thymocytes (106 cells) stained with fluorescein isothiocyanate (FITC)-conjugated anti-rat CD4 (Calbiochem, San Diego, CA) and phycoerythrin (PE)-conjugated anti-rat CD8 (Calbiochem) were analyzed with an EPICS XL ADC flow cytometer (Beckman Coulter, Fullerton, CA).
Lentiviral transduction
The rat Ptprk gene and the RGD1560849 predicted gene cDNAs were amplified by RT-PCR with primers given in Table 1 using total RNA from the BN rat thymus as template. The amplified cDNAs were sequenced (GenBank accession Nos. AB297790 and XR_008922 for Ptprk and RGD1560849 predicted gene, respectively) and cloned downstream of the cytomegalovirus (CMV) promoter of pLenti6/V5 lentiviral vector (Invitrogen, Carlsbad, CA). Recombinant lentivirus was generated by using the ViraPower lentivirus expression system (Invitrogen).
Generation of BM-reconstituted rats
Recipient female LEC rats at 6–8 weeks of age were treated with 6 Gy X-irradiation, which is a lethal dose for LEC rats (Hayashi et al. 1994). BM cells (106 cells) from donor male LEC rats (8 weeks old) were infected with recombinant lentivirus and transplanted to recipient LEC rats through the tail vein on the next day. At 5–6 weeks after BM reconstitution, thymocytes were examined with flow cytometry.
Results
Genetic linkage analysis of the thid locus
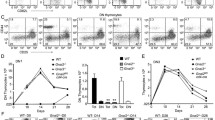
It has been reported that the thid locus is mapped between markers D1Mgh17 and D1Mgh3 on Chr 1 (Wei et al. 1997). To identify the precise position of the thid locus, we performed genetic linkage analysis using markers closer to the thid locus than the previous markers. We produced 197 (BN × LEC)F1 × LEC backcrossed progenies. The ratio of peripheral CD4+ T cells in the mesenteric lymph nodes was examined by flow cytometry in all progenies to classify thid/thid or thid/+ phenotype (Fig. 1A). Genotyping with markers on Chr 1 was also performed in all progenies (Fig. 1B) and a linkage panel was generated (Fig. 1C). We found that the thid locus locates between markers Myb and D1Rat392 at the interval of 2 cM. The rat genomic sequence data (RGSC 3.4, December 2004) between Myb and D1Rat392 and the homologous region of the mouse genome in terms of the synteny indicated that there are six genes and two predicted genes in the thid locus. These genes were positioned as follows: Myb (16.5 Mb)-Hbs1l (16.7 Mb)-LOC683474 (Aldh8a1) (16.75 Mb)-RGD1560849-predicted (17.2 Mb)-Ptprk (17.8 Mb)-RGD1560695-predicted (18.1 Mb)-Lama2 (18.4 Mb)-Arhgap18 (19.0 Mb)-L3mbtl3 (19.5 Mb)-D1Rat392 (19.6 Mb).
Genetic mapping of the thid locus. A FACS analysis of mesenteric lymph node cells. Backcrossed progenies possessing normal ratio of CD4+ cells (40–50%) were classified as thid/+ genotype, while those with a smaller ratio of CD4+ cells (10–20%) were classified as thid/thid genotype. Figure is representative of each genotype. B Genotyping panel generated from 197 (BN × LEC)F1 × LEC backcrossed progenies. Open and filled squares indicate BN/LEC heterozygous and LEC/LEC homozygous genotypes, respectively. The values under the squares indicate the numbers of progenies. C Linkage map generated from the data of genotyping panel. The values indicate the genetic distance (cM) ± standard error between the two loci
Identification of the thid mutation
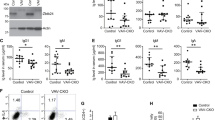
We examined the expressions of all genes located in the thid region in the BM and thymus and compared them between BN control and LEC rats (Fig. 2). Among genes located in the critical region, the Ptprk gene and the RGD1560849 predicted gene were found to be expressed differently in BN and LEC rats. The Ptprk gene was expressed in both BM and thymus of BN rats but not in those of LEC rats, while the RGD1560849 predicted gene was expressed in the thymus of BN rats but not in that of LEC rats. Therefore, we next analyzed the genomic structure of Ptprk and RGD1560849 predicted gene in the LEC rat. Because complete sequence data of the region around the rat Ptprk gene was not registered in the rat genome database, we searched sequence data in the trace archive of the rat genome by comparing it with the sequence of the mouse genome using BLAST. Using these sequence data, we designed several PCR primers and performed genomic PCR. Among them, the data for A, B, C, and D shown in Fig. 3A were informative. Both A and B sites are located approximately 3 kb downstream from the donor site of exon 1 of the RGD1560849 predicted gene, which is located downstream of the Ptprk gene. Sites C and D are located approximately 17 kb downstream from the donor site of exon 3 of the Ptprk gene (Fig. 3A). Sites A and D could be amplified when genomic DNA of LEC rats was used as a template as well as BN control rats. We used LEA and WKAH rats as additional controls because the LEA rat has been established from the Long-Evans closed colony simultaneously with the LEC rat and is the most genetically closed strain to the LEC rat. The WKAH rat was used as a nonrelevant control because we and others have been used it as a control strain for the LEC rat in previous reports (Agui et al. 1990; Hayashi et al. 1994; Sakai et al. 1993; Wei et al. 1997; Yamada et al. 1991). In contrast, sites B and C could not be amplified in the LEC rat only (Fig. 3B). These results suggest that the region expanding from the intron 3 of the Ptprk gene to the intron 1 of the RGD1560849 predicted gene is largely deleted in the LEC rat genome. Because the genomic region between B and C could be amplified using the forward primer of B and the reverse primer of C in the LEC rat, we sequenced the PCR product to confirm the breakpoint of the thid mutation. The PCR product of the LEC rat contained the sequence of parts of both the Ptprk intron 3 and the RGD1560849 predicted intron 1, as expected (Fig. 3C). Because the Ptprk gene and the RGD1560849 predicted gene are not completely assembled in the rat genome database at present, the precise size of the deleted region in the thid locus is unknown. However, the size is estimated to be approximately 380 kb, considering the homologous region of the mouse genome.
Expression of genes located in the thid locus. RT-PCR was performed with cDNAs prepared from the thymus and BM tissues of BN and LEC rats. Primers for each gene are given in Table 1
Identification of the thid mutation. A Genomic structure around the thid locus on rat Chr 1. A deleted region between region A in intron 1 of the RGD1560849 predicted gene and region D in intron 3 of the Ptprk gene is indicated as a filled column. B PCR amplification of regions A through D indicated in panel A. Genomic DNAs from WKAH, LEA, BN, and LEC rats were used to amplify regions A through D. B–C indicates the PCR products of the region between B and C. C DNA sequencing of PCR products. The sequence of the BN rat was derived from the PCR product of region C in panel B. The sequence of the LEC rat was derived from the PCR product of region B–C in panel B
Ptprk expression rescues T-helper immunodeficiency phenotype in LEC rats
Our results indicate that two genes, Ptprk and RGD1560849 predicted, are defective in the LEC rat. Ptprk encodes a receptor protein tyrosine phosphatase κ (Jiang et al. 1993), and RGD1560849 predicted gene encodes a hypothetical protein similar to E430004N04Rik of the mouse and whose function is unknown. We performed BM reconstitution with syngenic BM cells transduced with Ptprk or RGD1560849 predicted gene using the lentiviral gene expression system in X-irradiated recipient LEC rats, since it has been reported that BM cells but not thymic epithelial cells are defective due to the thid mutation (Agui et al. 1990a). When Ptprk or RGD1560849 predicted gene was transduced into LEC-derived BM cells, BM cells sufficiently expressed each gene (Fig. 4A). Next, these BM cells were reconstituted into X-irradiated recipient LEC rats. After 5–6 weeks, recipient rats were sacrificed and expression of these genes in the thymus and spleen was examined. As shown in Fig. 4B, each gene was sufficiently expressed, indicating that the reconstitution with exogenous gene-transduced BM cells succeeded. Thymocyte subsets were then examined by flow cytmetry (Fig. 4C). When RGD1560849 predicted gene-transduced donor BM cells were reconstituted, CD4 SP cells in the thymus were as few as that in LEC rats. In contrast, when Ptprk-transduced donor BM cells were reconstituted, CD4 SP cells apparently appeared as consisting of 3.7% of the total thymocytes, which corresponds to half of normal rat CD4 SP cells. These results indicate that Ptprk is a gene responsible for the defect in the development of CD4 SP cells in the LEC rat thymus, and further suggest that Ptprk expression in BM cells is a prerequisite for the development of CD4 SP cell lineage in the thymus.
Rescue experiment by transplantation of BM cells transduced with the Ptprk gene or the RGD1560849 predicted gene. A Expression of Ptprk gene and RGD1560849 predicted gene in LEC rat BM cells, which had been transduced by these genes using the lentiviral vector. Lane 1: RGD1560849 predicted gene-transduced BM cells; lane 2: Ptprk gene-transduced BM cells. B Expression of Ptprk gene and RGD1560849 predicted gene in the thymus and spleen of LEC rats transplanted with Ptprk- or RGD1560849 predicted gene-transduced BM cells. Ptprk+ and RGD1560849+ indicate the data from recipient LEC rats transplanted with BM cells, which had been transduced by the respective genes. Note that the Ptprk gene was expressed in both thymus and spleen, whereas the RGD1560849 predicted gene was expressed in the thymus but not in the spleen. C Flow cytometry of thymocytes from LEC rats transplanted with Ptprk- or RGD1560849 predicted gene-transduced BM cells. BN and LEC indicate the data from untreated rats
Discussion
In the present study we have identified a deletion mutation of the Ptprk gene and the RGD1560849 predicted gene in the thid locus of the LEC rat. Reconstitution with Ptprk-transduced but not RGD1560849 predicted gene-transduced BM cells rescued development of CD4 SP cells in the thymus. Therefore, we conclude that a deletion mutation of the Ptprk gene is responsible for T-helper immunodeficiency in the LEC rat.
The Ptprk gene encodes a receptor-like protein tyrosine phosphatase type κ (RPTPκ) protein. RPTPκ contains a meprin/A5 antigen/RPTPμ (MAM) domain, an immunoglobulin-like (Ig) domain, and four fibronectin type III (FN) repeats in the extracellular region, and two protein tyrosine phosphatase domains in the cytoplasmic region (Jiang et al. 1993). The Ptprk gene in the LEC rat loses exons 4–31. It is unknown whether the truncated mRNA or protein of Ptprk is produced. However, it seems unlikely since the Ptprk mRNA is anticipated not to possess the polyadenylation signal. Furthermore, even if we assume that the truncated mRNA or protein would be present, it loses important domains such as Ig, FN, and tyrosine phosphatase. Thus, the LEC rat is considered to possess a null mutation of the Ptprk gene.
The extracellular region of RPTPκ plays a critical role in homophilic binding, which leads to cell-cell adhesion mediated by RPTPκ (Sap et al. 1994; Zondag et al. 1995). RPTPκ is upregulated by transforming growth factor (TGF)-β, associated with epidermal growth factor (EGF) receptor, and reduces phosphorylation of EGF receptor in a mammary epithelial cell line, MCF10A. Consequently, cell proliferation is reduced when RPTPκ is activated (Wang et al. 2005). In contrast, downregulation of RPTPκ modulates Src and FAK phosphorylation and prevents TGF-β-mediated cell adhesion and migration (Wang et al. 2005). In addition, it is suggested that RPTPκ plays a role in tumor suppression. Previous reports indicate that human Chr 6q, which contains the PTPRK gene, has been deleted frequently in various tumors (Cooney et al. 1996, Theile et al. 1996). Indeed, loss of heterozygosity of 6q22-23, in which PTPRK is located, was found in primary central nervous system lymphomas (Nakamura et al. 2003). Moreover, PTPRK mRNA was downregulated in some melanoma cells (McArdle et al. 2001). It is reported that PTPRK mRNA is expressed in various tissues such as spleen, prostate, ovary, brain, kidney, liver, and epithelial cell line (Jiang et al. 1993; Shen et al. 1999; Yang et al. 1997). In contrast, a low expression of PTPRK mRNA was observed in human thymus and was not seen in peripheral blood leukocytes (Yang et al. 1997). To date, the expression and the biological function of RPTPκ in the immune cells is unknown. The present study is the first report describing the function of RPTPκ in the immune system. RPTPκ may play a role in the regulation of adhesion, proliferation, and migration of T-precursor cells in the thymus.
Ptprk-deficient mice were generated by gene trap (Shen et al. 1999; Skarnes et al. 1995). However, they were viable, fertile, and absent of overt phenotypes. Abnormality of the immune system was not examined. There are four homologous proteins, RPTPκ, RPTPλ, RPTPμ and RPTPρ in the protein tyrosine phosphatase family that contain MAM, Ig, and FN extracellular domains in humans and rodents (Alonso et al. 2004). Therefore, it is suggested that other members compensate the biological functions when one is deficient. Indeed, homozygous mice in which Ptprm is gene-trapped are viable and appear to be normal like Ptprk-disrupted mice (Koop et al. 2003). However, our results indicate for the first time that a deficiency of a member of the protein tyrosine phosphatase family, Ptprk, is not compensated by the other three homologs, showing a deficient phenotype with respect to the development of CD4 SP cells in the thymus.
After we submitted this article, a similar report was published elsewhere (Kose et al. 2007). Although they reached the conclusion that the Ptprk gene is responsible for the thid mutation, their report is incomplete. Thus, they did not show the deleted region in the LEC rat genome nor verify their result by the rescue experiment.
In summary, we show that the T-helper immunodeficiency mutation in the LEC rat is attributed to a deletion mutation of the Ptprk gene, which encodes a receptor-like protein tyrosine phosphatase type κ. It was verified by the data that BM reconstitution with Ptprk-transduced BM cells could rescue the T-helper immunodeficiency phenotype in LEC rats. Thus, the present study proposes a crucial role for the RPTPκ in the positive selection and/or maintenance of CD4 SP cells in the thymus.
References
Agui T, Oka M, Yamada T, Sakai T, Izumi K, et al. (1990) Maturational arrest from CD4+8+ to CD4+8- thymocytes in a mutant strain (LEC) of rat. J Exp Med 172:1615–1624
Agui T, Sakai T, Himeno K, Matsumoto K (1991a) Bone marrow-derived progenitor T cells convey the origin of maturational arrest from CD4+CD8+ to CD4+CD8- thymocytes in LEC mutant rats. Eur J Immunol 21:2277–2280
Agui T, Sakai T, Matsumoto K (1991b) Ontogeny of T cell maturation in LEC mutant rats which bear a congenital arrest of maturation from CD4+CD8+ to CD4+CD8- thymocytes. Eur J Immunol 21:2537–2541
Alonso A, Sasin J, Bottini N, Friedberg I, Osterman A, et al. (2004) Protein tyrosine phosphatases in the human genome. Cell 117:699–711
Bousso P, Bhakta NR, Lewis RS, Robey E (2002) Dynamics of thymocyte-stromal cell interactions visualized by two-photon microscopy. Science 296:1876–1880
Cooney KA, Wetzel JC, Consolino CM, Wonjo KJ (1996) Identification and characterization of proximal 6q deletion in prostate cancer. Cancer Res 56:4150–4153
Gallegos AM, Bevan MJ (2004) Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med 200:1039–1049
Hayashi M, Okui T, Endoh D, Sato F, Kasai N, et al. (1994) Radiation hypersensitivity of LEC strain rats controlled by a single autosomal recessive gene. Mutat Res 314:135–142
He X, Dave VP, Zhang Y, Hua X, Nicolas E, et al. (2005) The zinc finger transcription factor Th-POK regulates CD4 versus CD8 T-cell lineage commitment. Nature 433:826–833
Jiang YP, Wang H, D’Eustachio P, Musacchio JM, Schlessinger J, et al. (1993) Cloning and characterization of R-PTP-kappa, a new member of the receptor protein tyrosine phosphatase family with a proteolytically cleaved cellular adhesion molecule-like extracellular region. Mol Cell Biol 13:2942–2951
Jung C-G, Miyamoto T, Tsumagari T, Agui T (2001) Genetic association between low expression phenotype of CD62L (L-selectin) in peripheral CD4+ T cells and the thid (T-helper immunodeficiency) phenotype in the LEC rat. Exp Anim 50:337–340
Koop EA, Lopes SM, Feiken E, Bluyssen HA, van der Valk M, et al. (2003) Receptor protein tyrosine phosphatase mu expression as a marker for endothelial cell heterogeneity; analysis of RPTPmu gene expression using LacZ knock-in mice. Int J Dev Biol 47:345–354
Kose H, Sakai T, Tsukumo S, Wei K, Yamada T, et al. (2007) Maturational arrest of thymocyte development is caused by a deletion in the receptor-like protein tyrosine phosphatase κ gene in LEC rats. Genomics 89:673–677
Ladi E, Yin X, Chtanova T, Robey EA (2006) Thymic microenvironments for T cell differentiation and selection. Nat Immunol 7:338–343
Laky K, Fleischacker C, Fowlkes B (2006) TCR and Notch signaling in CD4 and CD8 T-cell development. Immunol Rev 209:274–283
Lind EF, Prockop SE, Porritt HE, Petrie HT (2001) Mapping precursor movement through the postnatal thymus reveals specific microenvironments supporting defined stages of early lymphoid development. J Exp Med 194:127–134
Liu X, Bosselut R (2004) Duration of TCR signaling controls CD4-CD8 lineage differentiation in vivo. Nat Immunol 5:280–288
Manly KF, Cudmore RH Jr, Meer JM (2001) Map Manager QTX, cross-platform software for genetic mapping. Mamm Genome 12:930–932
McArdle L, Rafferty M, Mælandsmo GM, Bergin O, Farr CJ, et al. (2001) Protein tyrosine phosphatase genes downregulated in melanoma. J Invest Dermatol 117:1255–1260
Nakamura M, Kishi M, Sakaki T, Hashimoto H, Nakase H, et al. (2003) Novel tumor suppressor loci on 6q22-23 in primary central nervous system lymphomas. Cancer Res 63:737–741
Porritt HE, Gordon K, Petrie HT (2003) Kinetics of steady-state differentiation and mapping of intrathymic-signaling environments by stem cell transplantation in nonirradiated mice. J Exp Med 198:957–962
Sakai T, Agui T, Muramatsu Y, Yamada T, Matsumoto K (1993) Dissociation of the interleukin-2 production from the cell activation in response to the mitogenic lectin in peripheral CD4+ cells of LEC mutant rats. Immunology 79:491–497
Sap J, Jiang YP, Friedlander D, Grumet M, Schlessinger J (1994) Receptor tyrosine phosphatase R-PTP-kappa mediates homophilic binding. Mol Cell Biol 14:1–9
Shen P, Canoll P, Sap J, Musacchio JM (1999) Expression of a truncated receptor protein tyrosine phosphatase kappa in the brain of an adult transgenic mouse. Brain Res 826:157–171
Siggs OM, Makaroff LE, Liston A (2006) The why and how of thymocyte negative selection. Curr Opin Immunol 18:175–183
Singer A, Bosselut R (2004) CD4/CD8 coreceptors in thymocyte development, selection, and lineage commitment: analysis of the CD4/CD8 lineage decision. Adv Immunol 83:91–131
Skarnes WC, Moss JM, Hurtley SM, Beddington RS (1995) Capturing genes encoding membrane and secreted proteins important for mouse development. Proc Natl Acad Sci USA 92:6592–6596
Sun G, Liu X, Mercado P, Jenkinson SR, Kypriotou M, et al. (2005) The zinc finger protein cKrox directs CD4 lineage differentiation during intrathymic T cell positive selection. Nat Immunol 6:373–381
Theile M, Seitz S, Arnold W, Jandrig B, Frege R, et al. (1996) A defined chromosome 6q fragment (at D6S310) harbors a putative tumor suppressor gene for breast cancer. Oncogene 13:677–685
Wang SE, Wu FY, Shin I, Qu S, Arteaga CL (2005) Transforming growth factor β (TGF-β)-Smad target gene protein tyrosine phosphatase receptor type kappa is required for TGF-β function. Mol Cell Biol 25:4703–4715
Wei K, Muramatsu Y, Sakai T, Yamada T, Matsumoto K (1997) Chromosomal mapping of the T-helper immunodeficiency (thid) locus in LEC rats. Immunogenetics 47:99–102
Witt CM, Raychaudhuri S, Schaefer B, Chakraborty AK, Robey EA (2005) Directed migration of positively selected thymocytes visualized in real time. PLoS Biol 3:e160
Yamada T, Natori T, Izumi K, Sakai T, Agui T, et al. (1991) Inheritance of T-helper immunodeficiency (thid) in LEC mutant rats. Immunogenetics 33:216–219
Yang Y, Gil MC, Choi EY, Park SH, Pyun KH, et al. (1997) Molecular cloning and chromosomal localization of a human gene homologous to the murine R-PTP-kappa, a receptor-type protein tyrosine phosphatase. Gene 186:77–82
Zondag GC, Koningstein GM, Jiang YP, Sap J, Moolenaar WH, et al. (1995) Homophilic interactions mediated by receptor tyrosine phosphatases mu and kappa. A critical role for the novel extracellular MAM domain. J Biol Chem 270:14247–14250
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Asano, A., Tsubomatsu, K., Jung, CG. et al. A deletion mutation of the protein tyrosine phosphatase kappa (Ptprk) gene is responsible for T-helper immunodeficiency (thid) in the LEC rat. Mamm Genome 18, 779–786 (2007). https://doi.org/10.1007/s00335-007-9062-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00335-007-9062-0