Abstract
In order to evaluate the genetic structure of purebred dogs, six Y chromosome microsatellite markers were used to analyze DNA samples from 824 unrelated dogs from 50 recognized breeds. A relatively small number of haplotypes (67) were identified in this large sample set due to extensive sharing of haplotypes between breeds and low haplotype diversity within breeds. Fifteen breeds were characterized by a single Y chromosome haplotype. Breed-specific haplotypes were identified for 26 of the 50 breeds, and haplotype sharing between some breeds indicated a common history. A molecular variance analysis (AMOVA) demonstrated significant genetic variation across breeds (63.7%) and with geographic origin of the breeds (11.5%). A network analysis of the haplotypes revealed further relationships between the breeds as well as deep rooting of many of the breed-specific haplotypes, particularly among breeds of African origin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Analysis of the Y chromosome has proven to be a valuable tool for understanding the origin of modern humans as well as the relationships between existing human populations (Forster et al. 2000; Underhill et al. 2000). Both single nucleotide poly-morphisms (SNP) and simple tandem repeat (STR) markers have been used for analyses of the human Y chromosome and have revealed that the human Y chromosome extant today migrated out of Africa ∼50,000 YBP (Cavalli–Sforza and Feldman 2003). This powerful approach to understanding evolutionary and historical relationships (reflected by paternal lineages) has thus far been under-utilized in animals. The Y chromosome might be particularly informative in domesticated animals as a limited number of males can have an enormous impact on highly selected domestic animal populations.
The dog is considered one of the most morphologically diverse of all domesticated species, with over 1000 breeds recognized worldwide (Morris 2001). Morphologically distinct types of dogs have been associated with certain geographic locations for thousands of years (Brewer et al. 2001). These distinct phenotypes have been purposely bred over the last couple of hundred years to create modern pure or pedigreed breeds. Given the fact that males can sire many more offspring than females, it is likely that extensive male founder effects exist in modern purebreds and that paternal lineage may be informative in genetically defining breeds.
Archeological evidence dates a close relationship between humans and the domestic dog for at least 14,000 years in locations as widespread as Europe and the Middle East (Davis and Valla 1978; Nobis 1979; Valla 1990; Tchernov and Valla 1997). Mitochondrial sequence analysis, which traces maternal lineages, has been used to study genetic relationships between dogs and wild canids. Mitochondrial sequence analysis indicates that the dog was domesticated from the wolf and was probably the earliest domesticated species (Vila et al. l997; Savolainen et al. 2002). However, it has not been helpful in genetically defining breeds possibly because of the slow mutation rate of DNA combined with the short amount of time that dog breeds have existed.
A collection of microsatellites has recently been used for a phylogenetic analysis of purebred dog breeds and to investigate relatedness between breeds. Using autosomal microsatellites, only 30% of the total genetic variation could be attributed to breed differences. However, breeds could be clustered into groups of related breeds which corresponded to geographic origin, morphology, or role in human activities (Parker et al. 2004). The study of the paternal lineage of dogs should compliment mitochondrial analysis which reflects maternal lineage as well as the autosomal marker studies. Based on the lack of recombination and the smaller number of males used in domestic animal breeding programs, we predicted that Y haplotype analysis would provide a powerful tool to study breed origins and relationships.
Materials and Methods
A total of 824 dogs were genotyped for the six microsatellite markers. A minimum of five individuals per breed was used for the analysis. Each breed’s country of origin was taken from Dogs (Morris 2001). The majority of the samples (531) were obtained from blood drawn from patients of the University of California at Davis, Veterinary Medical Teaching Hospital. Breed information was obtained from the animal’s medical record. Twenty-five randomly bred dogs from the Veterinary Medical Teaching Hospital were also used for comparison. The remaining samples (293) were submitted voluntarily by owners to the Veterinary Genetics Laboratory (University of California, Davis). Samples submitted by different owners that were unrelated in the first generation were selected for this analysis. These samples were submitted as buccal swabs and DNA was isolated as described (Oberbauer et al. 2003)
Canine microsatellite markers were developed from the Y chromosome by using three single copy probes to screen a canine BAC library (RPCI81) for Y chromosome inserts. Hybridization probes were obtained from two nucleotide segments (650 and 990) of the canine Y chromosome by polymerase chain reaction (PCR) as described (Olivier and Lust 1998). A segment of the SRY gene that had been localized to the canine Y chromosome was isolated using primers designed with Primer 3 (Whitehead Institute for Biomedical Research) (GenBank AF107021).
PCR reactions were performed in a PTC-100 (MJ Research, San Francisco, CA). Male canine DNA (1 μl) isolated using QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA) was used in 20 μl of reaction volume. The PCR reaction mix included GeneAmp 10 × buffer with 1.5 mM Mg2+, 2.5 mM dNTP, 1 pM of each primer, and 0.5 U Amplitaq Gold (Applied Biosystems, Foster City, CA). PCR reaction conditions started with a 12-min denaturation step at 94°C followed by 35 cycles of amplification and a final incubation step of 20 min at 72°C. The cycling conditions were 60 sec at 94°C, followed by 45 sec at 58°C and 60 sec at 72°C for 650; 30 sec at 94°C followed by 45 sec at 60°C and 60 sec at 72°C for 990; 45 sec at 94°C followed by 45 sec at 60°C and 60 sec at 72°C for SRY. PCR products were purified and labeled with a random hexamer labeling kit (Rediprime II, Amersham Biosciences, Piscataway, NJ). To determine if probes were single copy and male-specific, Southern analysis was performed using male and female canine genomic DNA. One bacterial artificial chromosome (BAC) for each probe was identified and used for subsequent subcloning. The three BACs were used as fluorescence in situ hybridization (FISH) probes in a three-color analysis. All three BACs colocalized to the canine Y chromosome (data not shown). Each BAC clone was partially digested with Sau3A and subcloned into the pBluescript II SK vector (Stratagene, La Jolla, CA). Subclones were screened for microsatellites by hybridization with both (CA)18 and (GAAA)5. Positive clones were purified and sequenced. Primers were designed to amplify microsatellites for eight candidate loci. Only three (650-79.2, 650-79.3, and 990-35) were polymorphic in 16 dogs of different breeds.
Two Y-specific microsatellites, MS34 and MS41, were previously published; (Olivier et al. 1999) however, primers amplified two PCR products in males, rendering them unusable for haplotype analysis. New locus-specific reverse primers (MS34CA and MS34TT) were developed for MS34 by cloning and sequencing each of the PCR products. Locus-specific primers for MS41, MS41A, and MS41B were developed by others (Sundqvist et al. 2001), but only MS41B was included in this study as MS41A was monomorphic. Microsatellite primers developed for this study as well as the allelic range and the repeat type are shown in Table 1. Sequence data from this article have been deposited with GenBank Data Libraries under accession Nos. AY466397, AY466398, and AY466399.
The six microsatellite markers were amplified in four PCR reactions. MS41B was amplified by itself as described (Sundqvist et al. 2001). Two sets of markers [(650-79.2 and 990-35) and (650-79.3 and MS34CA)] were amplified together using 0.5 μM of each PCR primer. MS34TT was amplified by itself using 1 μM of each primer. PCR reaction conditions for the microsatellites included a 12-min denaturation step at 94°C followed by 35 cycles of 10 sec at 94°C, 15 sec at 60°C, and 20 sec at 72°C and a final elongation step for 20 min at 72°C. Genotyping was performed on an ABI 3100 using Rox 400HD (Applied Biosystems, Foster City, CA) as a size standard. Alleles were scored using the STRand program (Toonen and Hughes 2001).
The Arlequin software package (Schneideir et al. 2000) was used to calculate the haplotype diversity, to identify haplotypes shared between breeds, and for an analysis of molecular variance (AMOVA). AMOVA was performed using RST, an analog of FST, which uses both haplotype frequency as well as the differences between haplotypes as the basis of the variation between breeds. The significance of the covariance components was tested using nonparametric permutation procedures using 10,000 permutations as described in the supporting documents for Arlequin. Dog breeds were grouped according to continent of origin as shown in Table 2. Based on the AMOVA results which showed highly significant geographic differences in Y haplotypes, the frequency of haplotypes based on geographic region was calculated.
A network analysis of canine Y haplotypes was performed using the default settings in the program Network 3.0 of r = 2 and ε = 0 (Bandelt et al. 1999). Forty-two common haplotypes that occurred more than once, and with a frequency greater than 0.2 within a breed, were analyzed. A reduced median analysis was performed first, and then the output data were analyzed using neighbor-joining analysis. The markers were weighted according to their variance as follows: 650-79.2 had a variance of 0.596 and was assigned a weight of 5; 650-79.3 had a variance of 1.572 and was given a weight of 2; 990-35 had a variance of 0.349 and was weighted 9; MS34CA had a variance of 0.742 and was weighted 6; MS34TT had a variance of 1.318 and was weighted 3; and MS41B had a variance of 3.416 and was weighted 1.
Results
A panel of polymorphic Y chromosome microsatellite markers was used to investigate genetic relationships within and between dog breeds originating in various parts of the world. Six dinucleotide microsatellite markers were used to genotype 824 male dogs from 50 different breeds. Sixteen dogs, on average, were tested for each breed (minimum of five dogs). A list of the breeds, the number of individuals tested, and the continent of origin of each breed is in Table 2. The full list of haplotypes, with repeat sizes as well as the allele frequency at each locus, is available in Supplemental Table 1 and Fig. 1. The variance in allele sizes ranged from a low of 0.349 for marker 990-35 to a high of 3.41 for marker MS41B.
DOI for Supplemental material: 10.1007/s00335-004-0054z
Y chromosome haplotype diversity and haplotype sharing between breeds was evaluated. A total of 67 distinct haplotypes were generated for each animal, based on alleles of six microsatellite loci. Twenty-six breeds had haplotypes unique to the breed (45 total unique haplotypes or 67% of the total haplotypes). Eighteen of these unique haplotypes were detected only once (27% of the total number of haplotypes). Twenty-one breeds had haplotypes unique to the breed that occurred in more than one individual (27 multiple unique haplotypes or 40% of the total number of haplotypes). A significant amount of Y chromosome haplotype sharing occurred between certain breeds, indicating a common or shared origin for some breeds (Fig. 1). Seven breeds did not share haplotypes with any other breeds, including the American Cocker Spaniel, Afghan Hound, Basenji, Brittany, Norwegian Elkhound, Rhodesian Ridgeback, and Tibetan Terrier.
Haplotype sharing between breeds. Haplotypes that were shared between breeds are listed along the top. Haplotypes that were unique were not included. The breeds listed in the first column are the breeds that did not have entirely unique haplotypes (43/50 breeds). Shared haplotypes are shaded in gray. The number within the box is the haplotype frequency within that breed. Unique haplotypes in breeds that had other shared haplotypes are not shaded.
Y chromosome diversity measured for each breed was based upon the probability that two randomly chosen haplotypes from one breed would differ from each other. This measure is equivalent to heterozygosity for diploid markers. Diversity values were obtained for each of the 50 breeds of dog and are listed in Table 2. The average diversity for the 50 breeds was 0.38, while the average diversity obtained in a group of 25 mongrel dogs (mixed-breed origin) was 0.95 ± 0.03. Six haplotypes were identified in the mixed-breed dogs that were not identified in the purebred dog population. Fifteen breeds had only a single Y chromosome haplotype, thus giving them zero diversity. One of these breeds was the Golden Retriever (n = 57), one of the most popular breeds in the world. The highest Y chromosome diversity value was 0.91 ± 0.10 in the Canaan Dogs, making it one of the most genetically diverse breeds based on Y haplotypes.
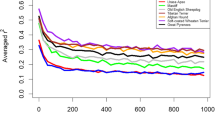
The geographic specificity of the Y chromosome haplotypes in purebred dogs from various continents was investigated. Figure 2 shows the haplotype frequencies by continent of origin of the breeds for haplotypes that occurred with a frequency greater than 0.01. There were continent-specific haplotypes as well as haplotypes shared between breeds from different continents. To quantify the genetic variation within and between breeds as well as to investigate the amount of genetic affinity based on geographic location of the origin of the breed, an AMOVA (Schneider et al. 2000) was performed. The amount of genetic variation within breeds, among breeds, and among breeds from different geographic regions is shown in Table 3.
Haplotype frequency based on continent of origin of the breed. The haplotype number is on the x axis and the frequency within each geographic region is on the y axis. The haplotype numbers represent the same haplotypes as in Fig. 1. African haplotypes represent 49 individuals from 5 breeds, Asian haplotypes represent 104 individuals from 8 breeds, American haplotypes represent 134 individuals from 6 breeds, and European haplotypes represent 531 individuals from 30 breeds.
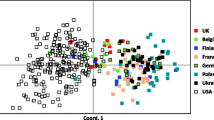
To further explore the relationships of the haplotypes to each other, a network analysis of haplotypes was created. The network represents the most likely relationships of the haplotypes to each other based on the molecular differences between the alleles of the 6 loci that make up the haplotypes. Because the Y chromosome haplotypes were often breed-specific, a breed could be represented by a single haplotype. The network shown in Fig. 3 is star-like with a number of deeply rooted branches with unique geographic origins. Three unique haplotypes were identified in the Basenji dogs sampled. These three haplotypes were the most deeply rooted haplotypes (one of the three haplotypes is not shown in Fig. 3 since it occurred in only one individual). The other two, H29 and H37, are 14 and 15 mutation steps, respectively, from the center. The next most divergent haplotypes, H35 and H14, are only 12 mutation steps from the center of the network.
Network analysis of canine Y chromosome haplotypes. Arrows point in the direction of an increase of a repeat number; hence, each arrow represents a presumed historical mutation event. The geographic origin of the haplotypes is coded by color as indicated in the figure (common haplotypes occurred in breeds from two different regions). The haplotype numbers represent the same haplotypes as they did in Fig. 1 and 2. Haplotypes that were unique to their breeds and were therefore not included in Fig. 1 are listed here along with the breed and the frequency of that haplotype; 8 American Cocker Spaniel (0.89), 21 Norwegian Elkhound (0.92), 26 Rhodesian Ridgeback (1.0), 28 Afghan Hound (1.0), 29 Basenji (0.6), 32 Brittany (1.0), 36 Tibetan terrier (0.6), 37 Basenji (0.3), and 38 Tibetan Terrier (0.4).
Discussion
The diversity identified within purebred breeds was very low compared with a set of randomly bred dogs, the latter having values more comparable to humans. No human population has demonstrated a loss of Y chromosome diversity of the magnitude observed within purebred dogs. The lowest human Y chromosome diversity was 0.8636 (Swiss), while the highest was 0.9968 (Han-Chinese) (Kayser et al. 2001). Two important caveats to this analysis were the small sample sizes used and the geographic sampling bias introduced by obtaining the majority of our samples from a California-based teaching hospital. The high level of breed specificity of the Y chromosome haplotypes of purebred dogs and the associated loss of diversity were anticipated, but not to this degree. Domestication and breed development involve inbreeding but the extreme to which males were overused in the creation of most modern dog breeds is striking.
The limited number of haplotypes (67) identified in purebred dogs is in sharp contrast to humans, where 598 different haplotypes were identified among 986 people from 20 distinct ethnic groups (Kayser et al. 2001). Furthermore, many more multiple unique haplotypes occurred in purebred dogs than among humans. Only 11.4% of the haplotypes were multiple unique in human populations compared with 40% in purebred dogs, yielding many breed-specific haplotypes (Kayser et al. 2001).
A significant amount of Y chromosome haplotype sharing occurred between certain breeds, indicating a common or shared origin. Some of these relationships were predicted by breed histories. For example, the Australian Shepherd was derived partially from the Border Collie, and they share three Y chromosome haplotypes. The Dalmatian was used to create the Australian Cattle dog, and therefore it is not surprising that they share a haplotype. Some haplotype sharing seems unlikely at first glance. The Doberman Pinscher and the Pomeranian share Y chromosome haplotypes even though the two breeds differ greatly in size. However, those two breeds originated in Germany and the Doberman Pinscher was created by cross-breeding dogs of unrecorded breed type. Our findings support the fact that the Pomeranian may have been used in the creation of the Doberman Pinscher. Alternatively, the Pomeranian and the Doberman Pinscher could share a common male ancestor. Another explanation for the haplotype sharing that occurred between different breeds is that the haplotypes are identical-by-state rather than being identical-by-descent. In order to evaluate this more fully, mutation rates should be determined for these microsatellites. The haplotypes could also be expanded to include more markers.
Many European breeds share haplotypes, which correlates with the historical account of the increase of purebred dog breeds in Europe in the mid-1800s (Morris 2001). Seven breeds did not share haplotypes with any of the other 50 breeds. This could be due to a breed sampling bias. For example, the American Cocker Spaniel is closely related to the English Cocker Spaniel, which was not among the breeds sampled. Breed-specific haplotypes and a lack of haplotype sharing among breeds indicate a long independent history. Three African breeds do not share haplotypes as well as one Asian breed. The Norwegian Elkhound also does not share haplotypes with other breeds. Interestingly, the Norwegian Elkhound was identified by mitochondrial sequence analysis to form a unique clade. This breed is reported to have ancient origins (Vila et al. 1997; Morris 2001).
Based on the high number of breed-specific haplotypes identified in this analysis, a greater variation was anticipated between breeds than within breeds. Indeed, the variation between breeds (63.7%) using Y chromosome haplotyping was double the amount detected using autosomal microsatellites (Parker et al. 2004). However, the samples used in this work and that of Parker et al. (2004) differ in the number of breeds evaluated as well as the number of individual animals evaluated in each breed. Even more compelling was the significant portion of the total genetic variation that could be attributed to the breed’s continent of origin (11.45%). When we grouped the samples based on AKC grouping or size, there was no significant variation due to group or size (data not shown). Although our samples were collected predominantly in California, the significant variation due to origin of the breeds gives us confidence that these samples are representative of the breed.
The network allowed relationships between breeds to be evaluated based on the similarities of the haplotypes. A Bulldog and a Mastiff haplotype (23 and 35 in Fig. 3) are very similar, as are the Rhodesian Ridgeback and an Africanis haplotype (41 and 26 in Fig. 3), two South African breeds. A Keeshond haplotype (27 in Fig. 3) is also related to the Akita breed (24 and 25 in Fig. 3). These breeds are morphologically similar in their spitz-like body types. Interestingly, the five most deeply rooted haplotypes listed in Fig. 3 correspond to different morphological body types: Akita/Spitz type (25 in Fig. 3), Shih Tzu and Pug/small dog (14 in Fig. 3), Basenji/small sight hound (29 and 37 in Fig. 3), Bulldog and Mastiff/Mastiff type (23 and 35 in Fig. 3), Africanis and Rhodesian Ridgeback/large sight hound (26 and 41 in Fig. 3).
African haplotypes appear to be the most ancient based on their distance from the center (deep rooting). In this regard, the canine network was remarkably similar to the network of human Y chromosome haplotypes (Forster et al. 2000). Although there were other deeply rooted haplotypes among breeds from the Far East and Europe, haplotypes from these other regions were not as deeply rooted as the African breeds. Mitochondrial evidence supported an Asian domestication of the dog from wolves (Savolainen et al. 2002). Y chromosome network analysis reported herein demonstrates an ancient origin of both Asian and African breeds, with the most divergent Y haplotypes observed in the Basenji, a small African sight hound. In conclusion, analysis of the Y chromosome in dogs provided remarkable genetic distinction among breeds as well as illuminated relationships between breeds. The significant amount of variation due to continent of origin of the breeds was consistent with the close association between dogs and people for thousands of years.
References
HJ Bandelt P Forster A Rohl (1999) ArticleTitleMedian-joining networks for inferring intraspecific phylogenies Mol Biol Evol 16 37–48 Occurrence Handle1:CAS:528:DyaK1MXjvVGltA%3D%3D Occurrence Handle10331250
D Brewer T Clark A Phillips (2001) Dogs in Antiquity Anubis to Cerberus The Origins of the Domestic Dog Aris and Phillips Warminster
LL Cavalli–Sforza MW Feldman (2003) ArticleTitleThe application of molecular genetic approaches to the study of human evolution Nat Genet 33 IssueIDSuppl 266–275 Occurrence Handle10.1038/ng1113 Occurrence Handle1:CAS:528:DC%2BD3sXhsV2ktL0%3D Occurrence Handle12610536
SJM Davis FR Valla (1978) ArticleTitleEvidence for domestication of the dog 12,000 years ago in the natufian of Israel Nature 276 608–610
P Forster A Rohl P Lunnemann C Brinkmann T Zerjal et al. (2000) ArticleTitleshort tandem repeat-based phylogeny for the human Y chromosome Am J Hum Genet 67 182–196 Occurrence Handle1:CAS:528:DC%2BD3cXntVyks7w%3D Occurrence Handle10827105
M Kayser M Krawczak L Excoffier P Dieltjes D Corach et al. (2001) ArticleTitleAn extensive analysis of Y-chromosomal microsatellite haplotypes in globally dispersed human populations Am J Hum Genet 68 990–1018 Occurrence Handle1:CAS:528:DC%2BD3MXkvFars7c%3D Occurrence Handle11254455
D Morris (2001) Dogs Trafalgar Square North Pomfret
G Nobis (1979) ArticleTitleDer alteste haushand lebte vor 14.000 jahren Umschau 79 610
AM Oberbauer DI Grossman ML Eggleston DN Irion AL Schaffer et al. (2003) ArticleTitleAlternatives to blood as a source of DNA for large-scale scanning studies of canine genome linkages Vet Res Commun 27 27–38 Occurrence Handle1:STN:280:DC%2BD3s7ht1Squw%3D%3D Occurrence Handle12625401
M Oliver G Lust (1998) ArticleTitleTwo DNA sequence specific for the canine Y chromosome Anim Genet 29 146–149 Occurrence Handle9699278
M Olivier M Breen MM Binns G Lust (1999) ArticleTitleLocalization and characterization of nucleotide sequences from the canine Y chromosome Chromosome Res 7 223–233 Occurrence Handle1:CAS:528:DyaK1MXktFagsb4%3D Occurrence Handle10421382
HG Parker LV Kim NB Sutter S Carlson TD Lorentzen et al. (2004) ArticleTitleGenetic structure of the purebred domestic dog Science 304 1160–1164 Occurrence Handle10.1126/science.1097406 Occurrence Handle1:CAS:528:DC%2BD2cXktVyit7Y%3D Occurrence Handle15155949
P Savolainen YP Zhang J Luo J Lundeberg T Leitner (2002) ArticleTitleGenetic evidence for an East Asian origin of domestic dogs Science 298 1610–1613 Occurrence Handle1:CAS:528:DC%2BD38Xosl2ktbg%3D Occurrence Handle12446907
Schneider S, Roessli, D, Excoffier L (2000) Arlequin: A software for population genetics data analysis, Ver 2.000. Genetics and Biometry Lab, Department of Anthropology, University of Geneva, Geneva, Switzerland
AK Sundqvist H Ellegren M Olivier C Vila (2001) ArticleTitleY chromosome haplotyping in Scandinavian wolves (Canis lupus) based on microsatellite markers Mol Ecol 10 1959–1966 Occurrence Handle1:CAS:528:DC%2BD3MXmslyhtrY%3D Occurrence Handle11555240
E Tchernov FR Valla (1997) ArticleTitleTwo new dogs and other Natufian dogs form the southern levant J Archeol Sci 24 65–95
RJ Toonen S Hughes (2001) ArticleTitleIncreased throughput for fragment analysis on ABI PRISM 377 automated sequencer using a membrane comb and STR and software Biotechniques 31 1320–1324 Occurrence Handle1:CAS:528:DC%2BD3MXptV2lsLY%3D Occurrence Handle11768661
PA Underhill P Shen AA Lin L Jin G Passarino et al. (2000) ArticleTitleY chromosome sequence variation and the history of human populations Nat Genet 26 358–361 Occurrence Handle10.1038/81685 Occurrence Handle1:CAS:528:DC%2BD3cXotVWhtr4%3D Occurrence Handle11062480
FR Valla (1990) ArticleTitleLe Natufien: une autre facon de comprendre le monde J Israel Prehistoric Soc 23 171–175
C Vila P Savolainen JE Maldonado JE Amorim IR Rice et al. (1997) ArticleTitleMultiple and ancient origins of the domestic dog Science 276 1687–1689 Occurrence Handle10.1126/science.276.5319.1687 Occurrence Handle9180076
Acknowledgments
This project was supported by funding from the American Kennel Club, the Canine Health Foundation, and the Center for Companion Animal Health, School of Veterinary Medicine, University of California at Davis. The authors thank Rob Tryon, Amy Young, Cathy Rinaldo, and Wendy Chuang for technical support and Alison Ruhe and Katy Robertson for DNA samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Michael J. Bannasch and Jeanne R. Ryun contributed equally to this work.
Rights and permissions
About this article
Cite this article
Bannasch, D.L., Bannasch, M.J., Ryun, J.R. et al. Y chromosome haplotype analysis in purebred dogs. Mamm Genome 16, 273–280 (2005). https://doi.org/10.1007/s00335-004-2435-8
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00335-004-2435-8