Abstract
The mapping resolution of the physical map for chicken Chromosome 4 (GGA4) was improved by a combination of radiation hybrid (RH) mapping and bacterial artificial chromosome (BAC) mapping. The ChickRH6 hybrid panel was used to construct an RH map of GGA4. Eleven microsatellites known to be located on GGA4 were included as anchors to the genetic linkage map for this chromosome. Based on the known conserved synteny between GGA4 and human Chromosomes 4 and X, sequences were identified for the orthologous chicken genes from these human chromosomes by BLAST analysis. These sequences were subsequently used for the development of STS markers to be typed on the RH panel. Using a logarithm of the odds (LOD) threshold of 5.0, nine linkage groups could be constructed which were aligned with the genetic linkage map of this chromosome. The resulting RH map consisted of the 11 microsatellite markers and 50 genes. To further increase the number of genes on the map and to provide additional anchor points for the physical BAC map of this chromosome, BAC clones were identified for 22 microsatellites and 99 genes. The combined RH and BAC mapping approach resulted in the mapping of 61 genes on GGA4 increasing the resolution of the chicken–human comparative map for this chromosome. This enhanced comparative mapping resolution enabled the identification of multiple rearrangements between GGA4 and human Chromosomes 4q and Xp.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Comparative genomics plays an important role in the understanding of genome dynamics during evolution and as a tool for the transfer of mapping information from species with gene-dense maps to species whose maps are less well developed (O’Brien et al. 1993, 1999). For farm animals, therefore, the human and mouse have been the logical choice as the model species used for this comparison. Medium-resolution comparative maps have been published for many of the livestock species, including pig, cattle, sheep, and horse, identifying large regions of conserved synteny between these species and man and mouse. More detailed analyses subsequently showed the presence of many internal rearrangements resulting in altered gene orders within these syntenic blocks (Sun et al. 1997, 1999; Rink et al. 2002; Larkin et al. 2003). In chicken, the first comparative maps indicated an extraordinary conservation of synteny between this species and mammals, even though these species diverged around 300–350 Myr ago (Smith et al. 1997; Groenen et al. 1998; Nanda et al. 1999; Burt et al. 1999). However, subsequent detailed mapping studies on a number of chicken chromosomes indicated that the number of intrachromosomal rearrangements was considerably higher then thus far anticipated (Suchyta et al. 2001; Crooijmans et al. 2001; Buitenhuis et al. 2002 and Jennen et al. 2002, 2003), clearly showing the need for an increased gene density on the chicken maps. Although a considerable number of genes have been mapped on the chicken linkage map, achieving the required high gene density necessary to identify the different conserved blocks within the regions of conserved synteny is not very practical because of the required polymorphism in the markers used. An alternative mapping approach that circumvents this problem is by using the radiation hybrid mapping technique (Walter et al. 1994). Originally, Goss and Harris (1975) first developed a technology for physical map generation using irradiation and fusion gene transfer (IFGT). This technique, however, was rarely used until advances in molecular genetics allowed efficient polymerase chain reaction (PCR) screening of the RH panels. Therefore, it was recently rediscovered (Cox et al. 1990; Walter et al. 1994) as an effective approach to building ordered maps of sequence-tagged sites. Since then radiation hybrid (RH) cell lines have proven to be a powerful resource for gene mapping, particularly in mammals, and they have been used to develop detailed physical gene-dense maps in human (Gyapay et al. 1996), zebrafish (Geisler et al. 1999), mouse (McCarthy et al. 1997), pig (Yerle et al. 1998), and horse (Kiguwa et al. 2000). Recently, a RH panel has also been constructed for chicken (Morisson et al. 2002), which has been used in the present study to improve the gene density on chicken Chromosome 4 (GGA4).
Genes mapped on the chicken linkage map for GGA4 (Groenen et al. 2000; Schmid et al. 2000) indicated that most of this chromosome showed synteny with human Chr 4 and the q arm of the human X chromosome. In addition, genes located on a number of different human chromosomes (HSA2, 3, and 5) mapped to the end of the linkage group of GGA4, most likely representing the tip of the q arm of this chromosome. These results were further confirmed by zoo-FISH experiments between HSA4 and GGA4 (Chowdhary and Raudsepp 2000). These results indicated that the region from GGA4q1.1 to GGA4q2.6 is syntenic with HSA4.
Recently, large collections of chicken gene sequences have become available in the form of expressed sequenced tags (EST) (Tirunaguru et al. 2000; Abdrakhmanov et al. 2000; Boardman et al. 2002). Clustering of these ESTs followed by sequence comparisons to human genes indicates that the chance of finding a chicken ortholog for a particular human gene is around 2 out of 3. This resource of chicken EST sequences was used in the current study to improve the gene density on GGA4 both by using the ChickRH6 panel and the chicken BAC library constructed in Wageningen (Crooijmans et al. 2000).
Materials and methods
Selection of markers and genes
For type II markers, 23 chicken microsatellite markers covering the p and q arms of GGA4 were selected from the published chicken genetic map (Groenen et al. 2000). Primer information for microsatellite markers located on GGA4, such as a primer sequence and PCR conditions, can be found at ARKdb farm animal database (http://www.thearkdb.org/) and ChickAce (http://acedb.asg.wur.nl/).
For type I markers, 127 primer pairs derived from EST sequences representing chicken orthologs to genes located on HSA4 (102 genes) and HSAX (25 genes) were selected. Potential chicken orthologous sequences were first identified by a BLAST database search (BLAST v2.0 software; http://www.ncbi.nlm.nih.gov/blast) with the human mRNA sequences representing all the genes known to be located on HSA4 and HSAX. The BLAST analysis was performed against a local chicken EST database containing all publicly available chicken EST sequences. Homologous chicken ESTs were subsequently used in a BLAST search against all human mRNAs (E-values at least e −50) to distinguish between orthologous and paralogous sequences. Only those chicken EST sequences that most likely represented the chicken ortholog of a gene located in human on HSA4 and HSAX were used for further analysis. Primer pairs were designed preferably within a single exon. For those cases where the resulting PCR product would be too small (<100 bp), primers were designed in adjacent exons spanning the intervening intron. In these cases, preferably the smaller introns were chosen. Primers were designed with the PRIMER3 program (http://frodo.wi.mit.edu/) (Table 1). Amplification conditions for each marker were optimized by varying the annealing temperature to produce a single amplicon of the predicted length with chicken genomic DNA and no amplification with genomic hamster DNA. Only primer pairs that gave a clear amplification product with the chicken and not with the hamster DNA were used for RH typing.
RH panel screening
The ChickRH6 panel (Morisson et al. 2002) consists of a total of 90 hybrids. Chicken and hamster genomic DNA and TE buffer were used as positive and negative controls, respectively. Ten to 25 ng of each panel DNA was amplified in a 384-well plate in a 6-μl mixture containing 1.5 mM MgCl2, 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 1 mM tetramethylammoniumchloride (TMAC), 0.1% Triton X-100, 0.01% gelatin, 0.2 mM of each dNTP, 0.125 U Silverstar polymerase (Eurogentec, Liege, Belgium) and 1.2 pmol of each primer. Amplification products were separated by electrophoresis on 1.5% ethidium bromide-stained agarose gels in 0.5 TBE buffer (44.5 mM Tris, 44.5 mM boric acid, 1 mM EDTA, pH 8.3), and reactions were scored for the presence or absence of the specific amplification product. Each marker was typed independently in duplicate.
BAC library screening
The BAC library was screened for type I and type II markers by two-dimensional PCR (Crooijmans et al. 2000). In the first round, positive plates were identified followed by the PCR analysis of the row and column pools of the positive plates. At least one BAC clone from each of the markers was identified.
PCR conditions
For both RH panel and BAC screening, the PCR reactions were started with 5 min at 95°C followed by 35 cycles for 30 sec at 95°C, 45 sec at 45°C, 50°C or 55°C annealing temperature, and 60 sec at 72°C, followed by a final elongation step at 72°C for 10 min, and finally stop step at 4°C. PCR reactions were performed in PCR system Biometra® using 384-well plates.
Statistical analysis and map construction
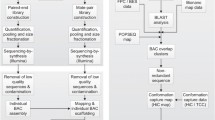
The CarthaGene program (Schiex and Gaspin 1997) was used to analyze and construct the RH map for GGA4. CarthaGene is a maximum-likelihood multipoint RH and genetic data-mapping tool (available at http://www.inra.fr/bia/T/CarthaGene/). Markers disrupting good map ordering were identified in the best sets of map orders produced and by examining the consistency of patterns for two-point LOD scores (higher than 5) of markers in their assigned order against each other across the chromosome to get the final frame work groups (Fig. 1). Therefore, final map distances were calculated by using CarthaGene order as input for the RH map for maximum likelihood.
Alignment of RH maps of chicken Chr 4 with the framework genetic linkage map of GGA4 map. Microsatellites that are also located on the genetic linkage map are indicated in bold. Genes indicated by an asterisk have been mapped by using BAC information. RH linkage groups shown are supported by a two-point LOD score >5. The map within each group was identified as the best order using the “Nice-map” analysis within CarthaGene.
Results
RH mapping
We initially started with the 127 STS markers representing likely orthologs of genes from human Chrs 4 and X and 23 chicken microsatellite markers that had previously been mapped to GGA4. These markers were tested for successful amplification on chicken genomic DNA and on the DNA from the RH panel. Successful amplification was defined as a single amplification product as visualized on agarose gel electrophoresis and the absence of that fragment using the hamster genomic DNA as a control. This was eventually achieved for 77 genes (60.63%) and 15 of the microsatellite markers (65.22%). These markers were typed on the RH panel and used for further linkage analysis.
The first step in building the chicken Chr 4 RH map was to group markers within separate linkage groups using a LOD threshold of 5.0. This allowed us to create an initial set of 9 RH linkage groups, containing 61 of the genes and 11 of the microsatellite markers. For 22 markers (randomly distributed along HSA4 and HSAX), no linkage was observed with any of the other markers, even after lowering the LOD threshold to 2.0. For the linkage groups 1–9 (Fig. 1), the locus order was investigated by using the build option of the CarthaGene program. The retention frequency (Rf) was calculated for all markers used and was found to vary enormously. Although the average Rf for the markers was 24%, the lowest Rf observed was 7% for the KAL1 gene whereas the highest Rf (82%) was observed for marker ADL0317 (Table 1).
BAC library
To further increase the number of genes on GGA4 and to increase the number of anchor points between the RH maps of Chr 4 and the BAC contig map currently under construction, the Wageningen BAC library (Table 1) was screened with 127 of the STS markers described above. For 99.99 markers at least one BAC clone was identified (77.95%). Because several of the STSs appeared to be positive for the same BAC(s), an additional 5 genes could be assigned to the RH linkage maps (linkage groups 3, 4, 5, 7, and 8). Furthermore, an additional RH linkage 180 group (linkage group 6) could be anchored to the genetic linkage map of GGA4 (Fig. 1). The BAC clone bW040D21 identified for microsatellite marker LEI0388 was also positive for the marker developed within the gene GABRA4, allowing the positioning of RH linkage group 6 containing the genes STIM2, GABRA4, TEC, SGCB, PDGFRA, TPARL, and PPAT to the region around position 180 cM on the genetic linkage map for GGA4.
The five genes (KLHL2, PGRMC2, REST, UGT8, and D4S234E) that were added to RH linkage groups 3, 4, 5, 7 and 8, respectively, based on the identified BACs are labeled in Figure 1 by an asterisk. In order to avoid inflation of the map size of these linkage groups, we chose to project these additional markers at their most likely location without altering the multipoint distance between framework markers (Fig. 1).
Discussion
RH mapping
Because GGA4 showed conservation of synteny mainly to HSAX and HSA4 (Schmid et al. 2000), chicken ESTs orthologous to genes located in man on HSAX and HSA4 were identified. The combined RH mapping and BAC identification approach eventually resulted in the successful mapping of 61 genes to GGA4, 54 of which were homologous to genes located on HSA4 and 7 of which were homologous to genes located on HSAX. Although the resulting RH map consists of 9 independent linkage groups, all are linked by a marker to the genetic linkage map of GGA4 and therefore could be ordered with respect to each other. However, since most of these linkage maps are connected to the genetic linkage map by only a single marker, the relative orientation of them is not known.
There were some initial difficulties at arriving at the gene order for the 9 radiation hybrid maps for GGA4. For example, although PDGFRA has a low two-point LOD score with SGCB, GABRA4, and PPAT (only 2.0, 0.2, and 3.3, respectively), it has a high two-point LOD score with TEC and TPARL (7.0 and 8.0, respectively), clearly indicating that it is located on the same linkage group. The average observed Rf for the markers mapped to GGA4 is 24%, which is similar to the results described by Morisson et al. (2002) who used two microsatellite markers from GGA4 (MCW0085 and MCW0099). The Rfs for these two markers were 20% and 7%, respectively. In another study using this RH panel (Jennen et al. 2004), which focused on GGA15, the observed average Rf was also found to be in the same range (18%). However, the observed Rf of the markers used in this study varied considerably along the chromosome (Fig. 2). The Rf was particularly high in the region around position 15 and 80 cM of the consensus linkage map (around map positions 27 and 134 cR of linkage groups 1 and 2, respectively). The differences in Rf are observed for markers that are not just randomly distributed along GGA4 but that are located in the same region on the chromosome. This indicates that the observed retention frequencies reflect the actual retention of these chromosomal fragments in the cell lines from the radiation hybrid cell panel and are not caused by nonspecific amplification of the marker.
BAC library
The BAC clones that were identified by screening the Wageningen BAC library resulted in the mapping of several additional genes on GGA4, i.e., KLHL2, PGRMC2, REST, UGT8, and D4S234E. Furthermore, the identification of BAC bW040D21 for markers LEI0338 and GABRA4 resulted in the positioning of RH linkage group 6 around position 180 on the genetic linkage map of GGA4, thereby placing another 7 genes on this chromosome (STIM2, GABRA4, TEC, SGCB, PDGFRA, TPARL, and PPAT) (marker details can be found at http://acedb.asg.wur.nl/). BACs that have been identified with markers mapped on GGA4 are also key elements that help to integrate the cytogenetic and linkage maps with the physical BAC contig map. BAC end sequencing of the Wageningen BAC clones is currently in progress and this will aid in the further integration of the RH, linkage, and BAC mapping data with chicken genome sequence contigs.
Comparative map
A comparison of the genes mapped on GGA4 to the chromosomal location of the orthologous genes in human is shown in Figure 3. The available comparative mapping data clearly show that the major part of GGA4 shows conserved synteny to the q arm of HSAX and the q arm of HSA4. In addition, regions syntenic to the p arm of HSA4 and to small regions on HSA21q, HSA3p, HSA2p, and HSA5q have also been described (Groenen et al. 2000; Schmid et al. 2000; Matsishima et al. 2000). However, in those cases where such conserved synteny is based on only a single gene, one has to pay particular caution as many of these links may be the result of incorrect previous identifications of orthologs versus paralogs. The conserved synteny between GGA4 and HSA5, for example, is based on the FISH mapping of the CTNN1 gene to the distal tip of the q arm of GGA4 (Suzuki et al. 1999). Interestingly, the related CTNN2 gene has also been mapped to HSA2p12–11.1, already indicating that the gene mapped in chicken probably is the ortholog of CTNN2 (Groenen and Crooijmans 2003). This led us to reexamine the FISH mapping results by Suzuki et al. (1999) and a BLASTN search was done with the sequence of the cDNA clone used in the FISH experiment (accession number D11090). The results clearly showed that this cDNA was the orthologous gene of the human CTNNA2 gene that is located on GGA4 (with a sequence identity of 82%). The other example on GGA4 is the TGFBR2 gene, which is located on HSA3 and on MMU9. No other genes currently mapped on GGA4 are located on these chromosomes in man and mouse. A close reexamination of the data for the consensus linkage map showed that the localization of this gene on GGA4 on the consensus map (Groenen et al. 2000) has been erroneous. Finally, the link between the distal part of GGA4p to HSA21q needs to be regarded as doubtful. This presumed syntenic link was established by mapping the microsatellite MCW0047 to position 23 cM on the linkage map of GGA4 (Crooijmans et al. 1995; Groenen et al. 2000). This microsatellite was derived from a genomic clone (accession number M20817) containing part of the HMGN1 gene. However, a close examination of a BLASTN database search using this sequence showed that this sequence also has sequence identity of 94–97% to several other human clones derived from a number of different human chromosomes (11, 12, 13, 14, 15, 17, 21, and 22) including HSAX.
Comparative map between chicken Chr 4 and human Chr 2, 4, and X. Estimated positions for human genes are given in cM, according to the Map Viewer from Entrez Genomes (http://www.ncbi.nlm.nih.gov/). The numbers located on the right of the vertical bar of GGA4 represent the relative positions in cM of the chicken loci. Microsatellite markers are in bold; genes labeled with an asterisk are mapped based on the BAC screening. Genes mapped by RH are in italics.
Although the exact map location of the genes that were mapped in this study to GGA4 is not known yet, the approximate positions of these genes already clearly indicate that multiple rearrangements have occurred within the syntenic regions on chicken Chr 4 and human Chr 4, X, and 2 (Fig. 3). The comparative data that are currently available for GGA4 indicate that this chromosome contains at least 20 CSOs (Conserved Segments Ordered) when compared to human. These findings are in agreement with previous studies on chicken Chr 10 and 15 where a large number of rearrangements were observed within regions of conserved synteny between man and chicken (Crooijmans et al. 2001; Jennen et al. 2003). In these studies the average size of these CSOs was between 4 and 6 cM, which is even smaller than that observed for GGA4. This, however, is due to the density of the genes currently mapped on these chromosomes, and it is expected that the number of conserved segments will increase as the number of genes mapped in chicken increases. Based on the currently available data, we expect that the number of conserved regions between chicken and human might be as high as 300 (20 CSOs with a size of GGA4 approximately 7% of the chicken genome) to 1000 (19 CSOs with a size of GGA15 approximately 1.8% of the chicken genome; Jennen et al. 2004) with an average size of just a few cM. A more accurate estimation of this number has to await the completion of the physical and sequence maps of the chicken genome expected to become available in 2004.
References
I Abdrakhomanov D Lodygin P Geroth H Arakawa et al. (2000) ArticleTitleA large database of chicken bursal ESTs as a resource for the analysis of vertebrate gene function Genome Res 10 2062–2069 Occurrence Handle10.1101/gr.10.12.2062 Occurrence Handle11116100
J Aerts RPMA Crooijmans S Cornelissen K Hemmatian et al. (2003) ArticleTitleIntegration of chicken genomic resources to enable whole-genome sequencing Cytogenet Genome Res 102 297–303 Occurrence Handle10.1159/000075766 Occurrence Handle1:CAS:528:DC%2BD2cXhtlejtbs%3D Occurrence Handle14970720
PE Boardman J Sanz–Ezquerro JM Overton DW Burt et al. (2002) ArticleTitleA comprehensive collection of chicken cDNAs Curr Biol 12 1965–1969 Occurrence Handle10.1016/S0960-9822(02)01296-4 Occurrence Handle12445392
AJ Buitenhuis RPMA Crooijmans L Bruijnesteijn van Coppenraet A Veenendaal et al. (2002) ArticleTitleImprovement of the comparative map of chicken chromosome 13 Anim Genet 33 249–254 Occurrence Handle10.1046/j.1365-2052.2002.00861.x Occurrence Handle1:CAS:528:DC%2BD38XmvVWrtb8%3D Occurrence Handle12139503
DW Burt C Bruley IC Dunn CT Jones et al. (1999) ArticleTitleThe dynamics of chromosome evolution in birds and mammals Nature 402 411–413 Occurrence Handle10.1038/46555 Occurrence Handle1:CAS:528:DyaK1MXnvVyku7g%3D Occurrence Handle10586880
BP Chowdhary T Raudsepp (2000) ArticleTitleHSA4 and GGA4: Remarkable conservation despite 300-Myr divergence Genomics 64 102–105 Occurrence Handle10.1006/geno.1999.6085 Occurrence Handle1:CAS:528:DC%2BD3cXhsFKisbk%3D Occurrence Handle10708523
DR Cox M Burmeister ER Price S Kim RM Myers (1990) ArticleTitleRadiation hybrid mapping: A somatic cell genetic method for constructing high-resolution maps of mammalian chromosomes Science 250 245–250 Occurrence Handle1:CAS:528:DyaK3cXmt1Gkur4%3D Occurrence Handle2218528
RPMA Crooijmans JJ Poel Particlevan der MAM Groenen (1995) ArticleTitleFunctional genes mapped on the chicken genome Anim Genet 26 73–78 Occurrence Handle1:STN:280:ByqB2M7itFI%3D Occurrence Handle7733510
RPMA Crooijmans J Verbalove RJM Dijkhof JJ Poel ParticleVan Der MAM Groenen (2000) ArticleTitleTwo-dimensional screening of the wageningen chicken BAC library Mamm Genome 11 360–363 Occurrence Handle10.1007/s003350010068 Occurrence Handle1:CAS:528:DC%2BD3cXjt1Kjtbg%3D Occurrence Handle10790534
RPMA Crooijmans RJM Dijkhof T Veenendaal JJ Poel Particlevan der et al. (2001) ArticleTitleThe gene orders on human chromosome 15 and chicken chromosome 10 reveal multiple inter- and intrachromosomal rearrangements Mol Biol Evol 18 2102–2109 Occurrence Handle1:CAS:528:DC%2BD3MXotVSlsbc%3D Occurrence Handle11606706
R Geisler G-J Rauch H Baier F Bebber ParticleVan et al. (1999) ArticleTitleA radiation hybrid map of the zebrafish genome Nat Genet 23 86–89 Occurrence Handle10.1038/12692 Occurrence Handle1:CAS:528:DyaK1MXlvFWhtr0%3D Occurrence Handle10471505
S Goss H Harris (1975) ArticleTitleNew method for mapping genes in human chromosomes Nature 255 680–684 Occurrence Handle1:CAS:528:DyaE2MXlt1ynsbw%3D Occurrence Handle1169690
MAM Groenen RPMA Crooijmans (2003) Structural genomics: integrating linkage, physical and sequence maps WM Muir RK Koehn (Eds) Poultry genetics, breeding and technology CAB International Cambridge, MA 497–536
MAM Groenen RPMA Crooijmans A Veenendaal HH Cheng et al. (1998) ArticleTitleA comprehensive microsatellite linkage map of the chicken genome Genomics 49 265–274 Occurrence Handle10.1006/geno.1998.5225 Occurrence Handle1:CAS:528:DyaK1cXjtlajsb4%3D Occurrence Handle9598314
MAM Groenen HH Cheng N Bumstead B Benkel et al. (2000) ArticleTitleConsensus linkage map of the chicken genome Genome Res 10 137–147 Occurrence Handle1:CAS:528:DC%2BD3cXpsl2juw%3D%3D Occurrence Handle10645958
G Gyapay K Schmitt C Fizames H Jones et al. (1996) ArticleTitleA radiation hybrid map of the human genome Hum Mol Genet 5 339–346 Occurrence Handle10.1093/hmg/5.3.339 Occurrence Handle1:CAS:528:DyaK28XhsFyntLs%3D Occurrence Handle8852657
DGJ Jennen RPMA Crooijmans B Kamps A Acar et al. (2002) ArticleTitleA comparative map of chicken chromosome 24 and human chromosome 11 Anim Genet 33 205–210 Occurrence Handle10.1046/j.1365-2052.2002.00860.x Occurrence Handle1:CAS:528:DC%2BD38Xlt1Kjs7c%3D Occurrence Handle12030924
DGJ Jennen RPMA Crooijmans B Kamps R Açar et al. (2003) ArticleTitleComparative map between chicken chromosome 15 and human chromosomal region 12q24 and 22q11-q12 Mamm Genome 14 629–639 Occurrence Handle10.1007/s00335-003-3007-z Occurrence Handle1:CAS:528:DC%2BD3sXnslGmsr0%3D Occurrence Handle14629113
DGJ Jennen RPMA Crooijmans M Morisson AE Grootemaat et al. (2004) ArticleTitleA radiation hybrid map of chicken chromosome 15 Anim Genet 35 63–65 Occurrence Handle1:CAS:528:DC%2BD2cXitVKltrw%3D Occurrence Handle14731233
SL Kiguwa P Hextall AL Smith R Critcher et al. (2000) ArticleTitleCharacterization of whole genome radiation hybrid panel: Chromosomes 1 and 10 preliminary maps Mamm Genome 11 803–805 Occurrence Handle10.1007/s003350010146 Occurrence Handle1:CAS:528:DC%2BD3cXnt1Olu7s%3D Occurrence Handle10967144
DM Larkin A Everts–van der wind WM Rebeiz PA Schweitzer et al. (2003) ArticleTitleA cattle–human comparative map built with cattle BAC-ends and human genome sequence Genome Res 13 1966–1972 Occurrence Handle12902387
Y Matsishima K Matsumura S Ishii Y Suzuki et al. (2000) ArticleTitleChromosomal assignment of the chicken NP220 gene encoding a large DNA-binding nuclear protein and its targeted disruption in chicken DT40 cells Cytotechnology 33 109–115 Occurrence Handle10.1023/A:1008107227862
LC McCarthy J Terrett ME Davis AL Smith et al. (1997) ArticleTitleA first-generation whole-genome radiation hybrid map spanning the mouse genome Genome Res 7 1153–1161 Occurrence Handle1:CAS:528:DyaK1cXkvFWkug%3D%3D Occurrence Handle9414320
M Morisson A Lemière S Bosc M Galan et al. (2002) ArticleTitleChickRH6: a chicken whole-genome radiation hybrid panel Genet Sel Evol 34 521–533 Occurrence Handle10.1051/gse:2002021 Occurrence Handle1:CAS:528:DC%2BD38Xns1enu7Y%3D Occurrence Handle12270108
I Nanda M Koehler M Schartl DW Burt et al. (1999) ArticleTitle300 million years of conserved synteny between chicken Z and human chromosome 9 Nat Genet 21 258–259 Occurrence Handle10.1038/6769 Occurrence Handle1:CAS:528:DyaK1MXitVCit7k%3D Occurrence Handle10080173
SJ O’Brien JE Womack LA Lyons KJ Moore et al. (1993) ArticleTitleAnchored reference loci for comparative genome mapping in mammals Nat Genet 3 103–112 Occurrence Handle1:CAS:528:DyaK3sXhtFyjsL4%3D Occurrence Handle8499943
SJ O’Brien MM Raymond WJ Murphy WG Nash et al. (1999) ArticleTitleThe promise of comparative genomics in mammals Science 286 458–481 Occurrence Handle10.1126/science.286.5439.458 Occurrence Handle1:CAS:528:DyaK1MXmvVOht74%3D Occurrence Handle10521336
A Rink EM Santschi KM Eyer B Roelofs et al. (2002) ArticleTitleA first generation EST RH comparative map of porcine and human genome Mamm Genome 13 578–587 Occurrence Handle10.1007/s00335-002-2192-5 Occurrence Handle1:CAS:528:DC%2BD38Xos1amu7c%3D Occurrence Handle12420136
T Schiex C Gaspin (1997) ArticleTitleCARTHAGENE: constructing and joining maximum likelihood genetic maps Proc Int Conf Intell Syst Mol Biol 5 258–267 Occurrence Handle1:STN:280:ByiH28zis1c%3D Occurrence Handle9322047
M Schmid I Nanda M Guttenbach C Steinlein et al. (2000) ArticleTitleFirst report on chicken genes and chromosomes 2000 Cytogenet Cell Genet 90 169–218 Occurrence Handle10.1159/000056772 Occurrence Handle1:STN:280:DC%2BD3M7gtFCksw%3D%3D Occurrence Handle11124517
EJ Smith LA Lyons HH Cheng SP Suchyta (1997) ArticleTitleComparative mapping of the chicken genome using the East Lansing reference population Poultry Sci 76 743–747 Occurrence Handle1:CAS:528:DyaK2sXjvFyjsLY%3D
SP Suchyta HH Cheng J Burnside JB Dodgson (2001) ArticleTitleComparative mapping of chicken anchor loci orthologous to genes on human chromosomes 1, 4 and 9 Anim Genet 32 12–18 Occurrence Handle10.1046/j.1365-2052.2001.00717.x Occurrence Handle1:CAS:528:DC%2BD3MXkvVOhsrY%3D Occurrence Handle11419339
HS Sun L Cai SK Davis JF Taylor et al. (1997) ArticleTitleComparative linkage mapping of human chromosome 13 and bovine chromosome 12 Genomics 39 47–54 Occurrence Handle10.1006/geno.1996.4481 Occurrence Handle1:CAS:528:DyaK2sXosFemtg%3D%3D Occurrence Handle9027485
HS Sun M Yerle P Pinton P Chardon et al. (1999) ArticleTitlePhysical assignments of human chromosome 13 genes on pig chromosome 11 demonstrate extensive synteny and gene order conservation between pig and human Anim Genet 30 304–308 Occurrence Handle10.1046/j.1365-2052.1999.00474.x Occurrence Handle1:CAS:528:DyaK1MXlvVagurg%3D Occurrence Handle10467706
T Suzuki T Kurosaki K Shimada N Kansaku et al. (1999) ArticleTitleCytogenetic mapping of 31 functional genes on chicken chromosomes by direct R-banding FISH Cytogenet Cell Genet 87 32–40 Occurrence Handle10.1159/000015388 Occurrence Handle1:CAS:528:DC%2BD3cXltlWmtQ%3D%3D Occurrence Handle10640808
VG Tirunaguru L Sofer J Cui J Burnside (2000) ArticleTitleAn expressed sequence tag database of T-cell-enriched activated chicken splenocytes: sequence analysis of 5251 clones Genomics 66 144–151 Occurrence Handle10.1006/geno.2000.6189 Occurrence Handle10860659
MA Walter DJ Spillett P Tomas J Weissenbach PN Goodfellow (1994) ArticleTitleA method for constructing radiation hybrid maps of whole genomes Nat Genet 7 22–28 Occurrence Handle1:CAS:528:DyaK2cXkt1yjsLs%3D Occurrence Handle8075634
M Yerle P Pinton A Robic A Alfonso et al. (1998) ArticleTitleConstruction of a whole-genome radiation hybrid panel for high-resolution gene mapping in pigs Cytogenet Cell Genet 82 182–188 Occurrence Handle10.1159/000015095 Occurrence Handle1:CAS:528:DyaK1MXkslyqtQ%3D%3D Occurrence Handle9858812
Acknowledgments
The authors would like to thank the Egyptian Ministry of Higher Education and Scientific Research for financial support (Project No. 29110/416000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rabie, T.S., Crooijmans, R.P., Morisson, M. et al. A radiation hybrid map of chicken Chromosome 4. Mamm Genome 15, 560–569 (2004). https://doi.org/10.1007/s00335-004-2362-8
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00335-004-2362-8