Abstract
Genetic factors independent of those affecting plasma lipid levels are a major contributor to risk for atherosclerosis in humans, yet the basis for these is poorly understood. This study examined plasma lipids and diet-induced atherosclerosis in 16-month-old female mice of strains C56BL/6J and DBA/2J. Mice of the parental strains, from recombinant inbred strains derived from these (BXD RI), and F2 progeny were fed an atherogenic diet for 16 weeks, beginning at 1 year of age. This induced atherosclerotic lesion formation in both parental strains, accompanied by increased plasma LDL levels. However, individual BXD RI strains and the BXD F2 mice demonstrated a range of atherosclerotic lesion formation that was not or at best weakly correlated with plasma lipid levels. Quantitative trait locus (QTL) analysis of the BXD F2 mice identified a locus with significant linkage (lod 4.5) for aortic lesion size on Chromosome (Chr) 10 that was independent of plasma lipids. Other loci with suggestive or significant linkage for various plasma lipid measures were identified on Chr 2, 3, 4, 5, 6, 7, 11, and 17. In this intercross, the genes primarily influencing atherosclerosis are distinct from those controlling plasma lipid levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis is a chronic inflammatory disease of the elastic and muscular arteries characterized by intimal thickening with lipid deposition, leading ultimately to obstruction of the affected vessel by thrombosis or progressive lesion enlargement (Lusis 2000). Oxidized lipids in the artery wall are believed to be an important inflammatory stimulus contributing to lesion development and progression (Berliner et al. 1995). Epidemiological and clinical studies have identified numerous risk factors, both environmental and genetic, contributing to atherosclerosis (Ellsworth et al. 1999). Among the significant components associated with cardiovascular disease are cholesterol levels and obesity. Although a few distinct genetic disorders predispose to atherosclerosis, such as LDL receptor deficiency, the genetic basis for most cases is multifactorial, and the contributing genes are not known with certainty. This is particularly true for genes that may act at the level of the vessel wall, because of the relative inaccessibility of tissue for study.
Mouse models have been useful for the dissection of such complex traits (Moore and Nagle 2000). The availability of a large number of inbred strains with variation in the relevant traits has allowed for the construction of mouse crosses, which can be used to map quantitative trait loci (QTLs). Atherosclerosis itself, and cholesterol homeostasis and obesity as risk factors, are complex traits known to be controlled by multiple genes in mice and in humans, as we and others have established (Dansky et al. 2002; Mehrabian et al. 2001; Mu et al. 1999; Nishina et al. 1993; Paigen 1995; Pitman et al. 1998; Purcell et al. 2001; Welch et al. 2001). This study presents results of QTL analyses for aortic fatty lesion size and plasma lipid levels in a C57BL/6J × DBA/2J (BXD) intercross, for which we have previously reported analyses of femoral bone density and morphometry and obesity (Drake et al. 2001a, 2001b). This cross was constructed owing in part to preliminary findings in BXD recombinant inbred (RI) strains fed an atherogenic diet. Among BXD RI strains, both aortic lesions and plasma lipids showed significant variation, yet they were discordant among strains, suggesting that the genetic basis for lesion development was independent of that for hyperlipidemia. We report the identification of a locus on Chr 10 contributing to aortic fatty lesion development, independent of multiple QTLs linked to plasma lipid and/or obesity phenotypes. The results suggest that, in this cross, the genes primarily influencing atherosclerosis are unique from those controlling cholesterol and adipose tissue.
Materials and methods
Genetic cross for QTL analysis
C57BL/6J (B6), DBA/2J (DBA), and BXDRI mice were purchased from The Jackson Laboratory (Bar Harbor, Me.) and were housed under conditions meeting the guidelines of the Association for Accreditation of Laboratory Animal Care. F1 animals derived from crossing B6 females and DBA males were used to generate F2 progeny. The F2 mice were weaned at 21 days, and only females were selected and placed on a rodent chow diet containing 17.5% protein and 11% fat (Purina 5001). At 12 months of age the BXD F2 mice were transferred to a high-fat, high-cholesterol diet (HF) containing 75% chow supplemented with 7.5% cocoa butter, 2.5% dextrose, 1.625% sucrose, 1.625% dextrin, 1.25% cholesterol, and 0.5% sodium cholate (Diet # 90221; Harlan Teklad, Madison, Wis.) for 16 weeks. All mice were fed ad libitum and maintained on a 12-h light/dark cycle. The F2 mice studied are the same as those reported previously (Drake et al. 2001a, 2001b). The BXDRI strain mice (all females) received the same diet under similar conditions, except that it was initiated at 3 months of age and continued for 15 weeks. At completion of the study periods, mice were fasted overnight, with body weight and length measurements obtained and blood collected immediately prior to sacrifice. The heart and ascending aortae were removed en bloc and were embedded in OCT compound and frozen at −70°C for subsequent sectioning. Kidneys and spleens were collected for DNA isolation, and fat pads (omental, retroperitoneal, parametrial, and subcutaneous) were removed and weighed.
Experimental measurements
For plasma lipid levels, blood was collected by retro-orbital bleeding under isoflurane anesthesia from mice fasted for 14 h. Samples were collected prior to placement of the mice on the HF diet (chow diet samples) and at the time of sacrifice (high-fat, or HF, diet samples). Plasma was separated from cells by centrifugation of blood at 12,000 g for 3 min. Enzymatic assays for total cholesterol, HDL cholesterol, triglycerides, and unesterified free fatty acids were performed as previously described (Mehrabian et al. 1993). Combined VLDL and LDL cholesterol levels were calculated as the difference in total cholesterol levels and HDL cholesterol levels, and are referred to in figures as LDL.
Aortic fatty lesion quantitation
Quantitation of aortic fatty lesion formation was performed as previously described (Qiao et al. 1994). Serial cryosections were made of the hearts and attached aortae up to the level of the aortic arch. Every fifth section was collected on poly-D-lysine-coated slides up to the level of the aortic valve, and every other section was collected through the region of the aortic root. Samples were stained with oil red O and hematoxylin for routine analysis. Slides were examined by light microscopy, and aortic intimal fatty lesions were quantitated by morphometry as described.
Linkage and data analyses
A linkage map for all chromosomes was completed with microsatellite markers spanning an average density of approximately 13 cM. PCR primers were purchased from Research Genetics (Huntsville, Ala.). Concentrated DNA was serially diluted to 20 ng/µL in water, and genotyping was performed by PCR amplification as previously described (Machleder et al. 1997). PCR products were run on 5% polyacrylamide gels and detected by autoradiography. A linkage map was constructed by using Map Manager QTXb13, following procedures described in the accompanying manual (Manly and Olson 1999). QTL analyses were performed by using QTL Cartographer version 1.16 (Basten and Zeng 1994, 2002). Composite interval mapping (CIM) was performed with model 6 in the Zmapqtl program, multitrait analysis by the JZmapqtl program, and multiple interval mapping (MIM) performed using the MImapqtl program, following procedures described in the accompanying manual. MIM analysis with MImapqtl incorporates identification of the presence and nature of epistatic interactions between loci influencing a specific trait (Zeng et al. 1999). Phenotypic traits were transformed as needed, and in a few instances 1 or 2 outliers (>3 SD) were excluded, to induce approximate normality of the residuals, which involved either taking the natural log or in some cases the square root of the trait values. Normality of the distribution before and after transformation was assessed with the S statistic as calculated by the Qstats program of QTL Cartographer. ANOVA and correlation analyses were done with Statview v.5.0 (SAS Institute Inc, Berkeley, Calif.). QTL analysis of BXD RI strain data was performed by MapManager QTXb13 with the marker set assembled by Williams and colleagues (Williams et al. 2001).
The BXD F2 study was performed in a two-step process. To the extent possible, all traits were measured, and full genotyping of all chromosomes as described above was performed on the first 142 mice. For technical reasons (that did not result in selection bias), lesion scores were obtained on 123 of these mice. After QTL analysis identified loci controlling aortic lesion size on Chrs 7 and 10 as described below, aortic lesions were measured, and additional markers on Chrs 7 and 10 were determined on an additional 144 mice to confirm the significance of these loci.
Results
Female parental mice of both B6 and DBA/2 strains were fed an atherogenic diet for 16 weeks, and lipoprotein levels were measured for animals on both a chow and a high-fat diet (Table 1). When cholesterol levels were compared among the same strains on different diets (HF diet vs. chow diet), both of the parental mice had a dramatic increase in total cholesterol levels when maintained on the HF diet. However, on the same diet, plasma lipid levels were not significantly different between the two parental strains. Similarly, aortic fatty lesions developed in both strains although the extent of lesion formation tended to be less in the DBA mice, as has been described by others (Nishina et al. 1993)
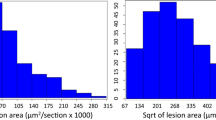
In contrast, evaluation of these traits among 18 BXD RI strains (two to four mice each) showed significant differences, indicating the presence of multigenic determinants (Fig. 1). There was no significant correlation between aortic lesion scores and any of the plasma lipids (Spearman correlation coefficients all <0.1 with p > 0.1). QTL analysis did not reveal any suggestive or significant linkages for any of the traits.
Distribution of phenotypes examined in 18 individual BXD RI strains (aortic lesions; total, HDL, and LDL cholesterol on a high-fat diet; and HDL and LDL cholesterol on a chow diet), presented in order of increasing aortic lesion score. From left to right in each graph, the individual strain No.s are: 29, 12, 18, 16, 8, 22, 14, 9, 25, 28, 19, 1, 27, 23, 30, 6, 24, 11. Bar height represents the mean value derived from two to four animals.
A BXD F2 population was constructed to further examine the relationship of plasma lipids with atherosclerotic lesions, and to identify genetic loci controlling these. Administration of the high-fat diet for 16 weeks caused significant changes in plasma lipids (all p < 0.0001 by paired t-test), including elevation in total and LDL/VLDL cholesterol and depression of HDL cholesterol, triglycerides, and free fatty acids (Table 1). The only statistically significant correlation between aortic lesions and plasma lipids was with plasma LDL/VLDL on the high-fat diet, and that was relatively weak (Table 2).
Genome-wide linkage analyses were performed on these traits by both composite interval mapping (CIM) and multiple interval mapping (MIM) analyses, as implemented in the QTL Cartographer suite of programs (Table 3).
Data for aortic fatty lesion size were available from 127 mice for genome-wide QTL analysis. Approximately 36% of total variance was genetic. CIM analysis identified three loci with significant or suggestive linkage, on Chrs 7, 8, and 10 (Table 3), which together accounted for 30% of trait variance. MIM analysis identified an additional lesser locus on mid Chr 2 at approximately 68 cM. The locus with the greatest effect (contributing approximately 11% of total variance, and having a peak CIM lod score of 4.51) was located on mid Chr 10 (Fig. 2A). The locus exhibited an additive inheritance pattern, with the DBA allele associated with greater lesion size (Fig. 2B). Mean lesion size in mice homozygous for the B6 alleles at this locus was only approximately one-third that of mice homozygous for the DBA alleles. A second locus accounting for approximately 10% of total variance was identified on proximal Chr 7 (peak CIM lod score 3.3). No epistatic effects were detected, although the relatively small sample size does not allow for much power to detect such interactions. To confirm the significance of the loci on Chrs 7 and 10, the intercross was extended to include an additional 146 mice, which were analyzed for aortic lesion scores and linkage with markers on Chrs 7 and 10. The strength of the linkage remained consistent for the Chr 10 locus (simple interval mapping lod 4.3), but decreased for the Chr 7 locus (lod 2.1). The Chr 10 locus for atherosclerotic lesions did not coincide with any of the loci described below for plasma lipids (Table 3), nor were plasma lipid traits for either chow or high-fat diets influenced by this locus (Fig. 2B).
Locus on Chr 10 contributing to the development of aortic fatty lesions in BXD F2 mice on a high-fat diet. (A) Lod curve for log aortic lesion scores, and HDL and LDL cholesterol levels on the high-fat diet. Arrowheads indicate location of typed markers. (B) Size of aortic fatty lesion by genotypes at the peak marker D10Mit42.
Plasma lipids were measured from animals at 1 year of age while on a chow diet, and prior to sacrifice at 16 months of age, after 16 weeks on an atherogenic diet, and QTL analyses were performed for each of these. The fraction of total variance attributable to genetic effects was greater for lipid levels on a chow diet than on the high-fat diet (Table 3). The loci identified for lipid levels on the chow diet were in almost all cases clearly distinct from loci from the high-fat diet samples, and, with the exception of loci on proximal Chr 3 (Fig. 3), loci for HDL were distinct from those for LDL. Total, HDL and LDL cholesterol levels on the chow diet all had coincident QTL on proximal Chromosome 3. LDL on the high fat diet also showed a weak QTL at the same region. For each of these traits, the B6 allele was associated with higher levels and exhibited both additive and dominant effects. Application of a test for close linkage vs pleiotropy (Drake et al. 2001b) indicated that the loci could not be identified as distinct at a significance level of 0.05, consistent with the presence of one locus with pleiotropic effects. Also consistent with this hypothesis was the finding of a significant increase in the lod score to nearly 10 when multitrait analysis as implemented in the JZmapqtl program of QTL cartographer was performed for chow HDL, chow LDL, and HF LDL (Fig. 3A).
Other loci with significant linkage (lod > 4.3) for lipid traits were distal Chr 6 (chow total and HDL cholesterol; Fig. 3B) and mid Chr 17 (chow FFA). Among the loci listed in Table 3, few interactions were detected by the MIM analysis. Those that were identified involved loci on Chrs 2 and 3 for chow LDL (dominant by dominant effect), and Chrs 2 and 7 for HDL on the high-fat diet (additive by additive effect). These contributed 4.5 and 2.2% to genetic variance respectively. Total cholesterol on a chow diet had the highest level of genetic variance as determined by the MIM analysis. In addition to the three loci listed in Table 3 that were identified by CIM analysis as having lod scores > 2.8, MIM analysis identified loci on Chrs 4, 10, 14, and 16, which in aggregate contributed approximately 22% to total variance. Also, interactive effects between four pairs of loci made an additional contribution to overall variance: loci on Chrs 10 and 14 (5.1%); 16 and 17 (5.6%); 17 and 10 (2.0%); and 4 and 10 (6.8%).
Discussion
Mice are naturally resistant to the development of atherosclerosis, but the lesions can be induced in the B6 and DBA strains fed an atherogenic diet, as we have done here. Administration of the diet is accompanied by an elevation of plasma LDL cholesterol and a decrease of HDL to levels more akin to those found in human populations. Although administration of the diet is an essential prerequisite for lesion development, lesion size varies considerably among the F2 population and is only weakly correlated with the degree of LDL elevation. Data from BXD RI strains were consistent. Therefore, other factors besides plasma lipids must influence lesion development. In this study we have identified a significant locus on Chr 10 that influences aortic lesion size independent of plasma lipids. This (and other loci for other traits) was identified in the QTL analyses of the F2 population, but not in the RI strain set, which has more limited power. Two other loci with suggestive linkage were also identified. It is of interest that for the primary locus on Chr 10, the DBA allele was associated with greater lesion size, even though among the parentals, the aggregate of published data suggests that the B6 strain has larger lesions. One of the lesser loci (Chr 8) did show an association of larger lesions with the B6 allele, and presumably there are multiple other loci that this study lacked the power to detect that contribute to the larger lesion size in B6 mice. We did not detect significant interactions among the loci identified as a potential explanation for this, although, as noted, the sample size limits the power to detect them.
A number of QTL have been reported that influence atherogenesis, frequently unrelated to plasma lipid levels (Dansky et al. 2002; Mehrabian et al. 2001; Mu et al. 1999; Nishina et al. 1993; Paigen 1995; Pitman et al. 1998; Purcell et al. 2001; Welch et al. 2001). The locus described in our current study is sufficiently near that described by Dansky on proximal Chr 10 (Dansky et al. 2002) that it may represent the same underlying gene or genes. In addition to the lipid traits reported here, this locus is independent of loci for adipose tissue mass on Chrs 2 and 6, which we have previously reported on for this intercross (Drake et al. 2001b). This is relevant, as adipose tissue mass is strongly inversely correlated with lesion size in this cross, and it has been shown that mice with obesity-inducing mutations have decreased lesion size relative to the isogenic background strains (Nishina et al. 1994). Clinical studies in humans have suggested that non-lipid related factors play an important role in atherosclerosis risk (Robinson and Loscalzo 1998). Although some are characterized at the phenotypic level, such as elevated homocysteine, and association studies are suggestive for others, such as PAI1, none are as yet unequivocally defined at the genetic level (Lusis et al. 1998). Studies of mice with induced mutations in genes such as PON1 and myeloperoxidase clearly demonstrate that alteration in non-lipid related genes can significantly influence atherogenesis (Brennan et al. 2001; Shih et al. 2000). Identification of the gene responsible for the Chr 10 locus will, therefore, be of value in helping elucidate non-lipid related potential genetic risk factors for atherosclerosis in human.
This study also contributes the identification of several novel loci controlling plasma HDL levels and, for some loci, other plasma lipid traits as well. The locus on proximal Chr 3 showing linkage with multiple lipid measures coincides with a locus we identified previously in an intercross of strains B6 and C3H for plasma HDL on an atherogenic diet and 7-alpha hydroxylase mRNA levels in liver. Also, the Chr 7 locus, which was found for HDL cholesterol on the high-fat diet, is an interesting QTL since it corresponds to an HDL cholesterol QTL in a (C57BL/6XSpretus) × C57BL/6 F2 population previously identified (Warden et al. 1993). Loci identified on Chrs 4 and 6 that had significant linkage for plasma HDL on high fat and chow diets respectively have not been previously identified, nor have the other loci not mentioned above, to our knowledge.
Confirmation of the loci described here through the use of congenic strains is the next step, in conjunction with finer mapping, also utilizing congenic strains. The recent development of a genome-wide set of congenic strains (designated “genome tagged mice” or GTM in short) carrying DBA segments on a B6 background will greatly speed the continued evaluation of these and other loci identified in BXD intercross, backcross, or RI strain studies (Iakoubova et al. 2001).
References
CJ WB Basten Z-B Zeng (1994) Zmap-a QTL cartographer. GJ Smith J Benkel W Chesnais JP Fairfull BW Kennedy (Eds) et al. 5th World Congress on Genetics Applied to Livestock Production: Computing Strategies and Software Organizing Committee, 5th World Congress on Genetics Applied to Livestock Production Guelph, Ontario, Canada 65–66
CJ WB Basten Z-B Zeng (2002) QTL Cartographer. Department of Statistics, North Carolina State University Raleigh, N.C.
JA Berliner M Navab AM Fogelman JS Frank LL Demer et al. (1995) ArticleTitleAtherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation 91 2488–2496 Occurrence Handle1:CAS:528:DyaK2MXlvFCrsrY%3D Occurrence Handle7729036
ML Brennan MM Anderson DM Shih XD Qu X Wang et al. (2001) ArticleTitleIncreased atherosclerosis in myeloperoxidase-deficient mice. J Clin Invest 107 419–430 Occurrence Handle1:CAS:528:DC%2BD3MXhtlWktLs%3D Occurrence Handle11181641
HM Dansky P Shu M Donavan J Montagno et al. (2002) ArticleTitleA phenotype-sensitizing Apoe-deficient genetic background reveals novel atherosclerosis predisposition loci in the mouse. Genetics 160 1599–1608 Occurrence Handle1:CAS:528:DC%2BD38XktFahtr4%3D Occurrence Handle11973313
TA Drake K Hannani JM Kabo V Villa K Krass et al. (2001a) ArticleTitleGenetic loci influencing natural variations in femoral bone morphometry in mice. J Orthop Res 19 511–517 Occurrence Handle1:CAS:528:DC%2BD3MXmvVaiurw%3D
TA Drake E Schadt K Hannani JM Kabo K Krass et al. (2001b) ArticleTitleGenetic loci determining bone density in mice with diet-induced atherosclerosis. Physiol Genomics 5 205–215 Occurrence Handle1:CAS:528:DC%2BD3MXjs12quro%3D
DL Ellsworth P Sholinsky C Jaquish RR Fabsitz TA Manolio (1999) ArticleTitleCoronary heart disease. At the interface of molecular genetics and preventive medicine. Am J Prev Med 16 122–133 Occurrence Handle10.1016/S0749-3797(98)00138-X Occurrence Handle1:STN:280:DyaK1M3nsFemtQ%3D%3D Occurrence Handle10343889
OA Iakoubova CL Olsson KM Dains DA Ross A Andalibi et al. (2001) ArticleTitleGenome-tagged mice (GTM): two sets of genome-wide congenic strains. Genomics 74 89–104 Occurrence Handle10.1006/geno.2000.6497 Occurrence Handle1:CAS:528:DC%2BD3MXjslKhtbo%3D Occurrence Handle11374905
AJ Lusis (2000) ArticleTitleAtherosclerosis. Nature 407 233–241 Occurrence Handle10.1038/35025203 Occurrence Handle1:CAS:528:DC%2BD3cXmvVSlsrk%3D Occurrence Handle11001066
A Lusis A Weinreb TA Drake (1998) Genetics of atherosclerosis. E Topol (Eds) Textbook of Cardiovascular Medicine. Lippincott-Raven Publishers Philadelphia 2389–2414
D Machleder B Ivandic C Welch L Castellani K Reue et al. (1997) ArticleTitleComplex genetic control of HDL levels in mice in response to an atherogenic diet. Coordinate regulation of HDL levels and bile acid metabolism. J Clin Invest 99 1406–1419 Occurrence Handle1:CAS:528:DyaK2sXitVWktLo%3D Occurrence Handle9077551
KF Manly JM Olson (1999) ArticleTitleOverview of QTL mapping software and introduction to map manager QT. Mamm Genome 10 327–334 Occurrence Handle10087288
M Mehrabian JH Qiao R Hyman D Ruddle C Laughton et al. (1993) ArticleTitleInfluence of the apoA-II gene locus on HDL levels and fatty streak development in mice. Arterioscler Thromb 13 1–10 Occurrence Handle1:CAS:528:DyaK3sXkt1GksL0%3D Occurrence Handle8422330
M Mehrabian J Wong X Wang Z Jiang W Shi et al. (2001) ArticleTitleGenetic locus in mice that blocks development of atherosclerosis despite extreme hyperlipidemia. Circ Res 89 125–130 Occurrence Handle1:CAS:528:DC%2BD3MXlsFWhu7s%3D Occurrence Handle11463718
KJ Moore DL Nagle (2000) ArticleTitleComplex trait analysis in the mouse: the strengths, the limitations and the promise yet to come. Annu Rev Genet 34 653–686 Occurrence Handle1:CAS:528:DC%2BD3MXlvFOjuw%3D%3D Occurrence Handle11092842
JL Mu JK Naggert KL Svenson GB Collin JH Kim et al. (1999) ArticleTitleQuantitative trait loci analysis for the differences in susceptibility to atherosclerosis and diabetes between inbred mouse strains C57BL/6J and C57BLKS/J. J Lipid Res 40 1328–1335 Occurrence Handle1:CAS:528:DyaK1MXksVKiu70%3D Occurrence Handle10393218
PM Nishina J Wang W Toyofuku FA Kuypers BY Ishida et al. (1993) ArticleTitleAtherosclerosis and plasma and liver lipids in nine inbred strains of mice. Lipids 28 599–605 Occurrence Handle1:CAS:528:DyaK3sXmt1ehsLc%3D Occurrence Handle8355588
PM Nishina JK Naggert J Verstuyft B Paigen (1994) ArticleTitleAtherosclerosis in genetically obese mice: the mutants obese, diabetes, fat, tubby, and lethal yellow. Metabolism 43 554–558 Occurrence Handle1:CAS:528:DyaK2cXitFChsbs%3D Occurrence Handle8177043
B Paigen (1995) ArticleTitleGenetics of responsiveness to high-fat and high-cholesterol diets in the mouse. Am J Clin Nutr 62 458S–462S Occurrence Handle1:CAS:528:DyaK2MXnsV2qs7o%3D Occurrence Handle7625360
WA Pitman MH Hunt C McFarland B Paigen (1998) ArticleTitleGenetic analysis of the difference in diet-induced atherosclerosis between the inbred mouse strains SM/J and NZB/BINJ. Arterioscler Thromb Vasc Biol 18 615–620 Occurrence Handle1:STN:280:DyaK1c3hsFWmsA%3D%3D Occurrence Handle9555867
MK Purcell JL Mu DC Higgins R Elango H Whitmore et al. (2001) ArticleTitleFine mapping of Ath6, a quantitative trait locus for atherosclerosis in mice. Mamm Genome 12 495–500 Occurrence Handle10.1007/s00335001-0006-9 Occurrence Handle1:CAS:528:DC%2BD3MXks1ajsrg%3D Occurrence Handle11420610
JH Qiao PZ Xie MC Fishbein J Kreuzer TA Drake et al. (1994) ArticleTitlePathology of atheromatous lesions in inbred and genetically engineered mice. Genetic determination of arterial calcification. Arterioscler Thromb 14 1480–1497 Occurrence Handle1:CAS:528:DyaK2cXmt1OktbY%3D Occurrence Handle8068611
K Robinson J Loscalzo (1998) Other risk factors for coronary artery disease: homocysteine, lipoprotein(a), fibrinogen, and plasminogen activator inhibitor. E. Topol (Eds) Textbook of Cardiovascular Medicine. Philadelphia Lippincott-Raven Publishers 231–248
DM Shih YR Xia XP Wang E Miller LW Castellani et al. (2000) ArticleTitleCombined serum paraoxonase knockout/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. Biol Chem 275 17527–17535 Occurrence Handle10.1074/jbc.M910376199 Occurrence Handle1:CAS:528:DC%2BD3cXktFaksbk%3D
CH Warden JS Fisler MJ Pace KL Svenson AJ Lusis (1993) ArticleTitleCoincidence of genetic loci for plasma cholesterol levels and obesity in a multifactorial mouse model. J Clin Invest 92 773–779 Occurrence Handle1:CAS:528:DyaK2cXkslWjug%3D%3D Occurrence Handle8349816
CL Welch S Bretschger N Latib M Bezouevski Y Guo et al. (2001) ArticleTitleLocalization of atherosclerosis susceptibility loci to Chrs 4 and 6 using the Ldlr knockout mouse model. Proc Natl Acad Sci USA 98 7946–7951 Occurrence Handle10.1073/pnas.141239098 Occurrence Handle1:CAS:528:DC%2BD3MXlt1KntL0%3D Occurrence Handle11438740
RW Williams J Gu S Qi L Lu (2001) ArticleTitleThe genetic structure of recombinant inbred mice: high-resolution consensus maps for complex trait analysis. Genome Biol 2 Occurrence Handle11521680
ZB Zeng CH Kao CJ Basten (1999) ArticleTitleEstimating the genetic architecture of quantitative traits. Genet Res 74 279–289 Occurrence Handle1:STN:280:DC%2BD3c7lsVeqtw%3D%3D Occurrence Handle10689805
Acknowledgements
The assistance of Larry Castellani, Sharda Charungundla, Marjon Jahromi, Kathy Salcedo, and Yishou Shi is gratefully acknowledged. This work was supported by NIH grant HL30568 (A.J. Lusis and T.A. Drake) and by the Laubisch Fund, UCLA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Colinayo, V.V., Qiao, JH., Wang, X. et al. Genetic loci for diet-induced atherosclerotic lesions and plasma lipids in mice . Mamm Genome 14, 464–471 (2003). https://doi.org/10.1007/s00335-002-2187-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00335-002-2187-2