Abstract
This paper examines how plant food processing techniques developed by hunter-gatherers during the Near Eastern Epipalaeolithic (ca. 23970–11990 cal b.p.) may have influenced species selection, eating habits and access to critical nutrients. A case study is presented that investigates how pulverising and thermal treatments affect the tubers of Bolboschoenus maritimus (L.) Palla (sea club-rush), a plant that is frequently recovered from ancient sites in the Levant and Anatolia. A range of microscopy techniques was employed to observe the changes in tuber microstructure caused by individual processing techniques. The results show that pulverising is a necessary step in transforming these tubers into edible products because it disrupts the cell walls, facilitating tissue softening and access to intracellular nutrients. Heating, while necessary to cook the intracellular starch, does not promote tissue softening in the tubers of this species. The results demonstrate how the biologically inherited functional properties of a species interact with specific food processing techniques to promote or hinder its edibility and nutrient bioaccessibility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the Near Eastern Epipalaeolithic (23970–11990 cal b.p.), significant changes in plant food processing systems occurred in tandem with increased exploitation of wild plant resources. Plant assemblages recovered from early Epipalaeolithic sites suggest that by this time some groups had replaced generalised foraging strategies with intensive plant collecting practices based on a wide range of resources (Colledge 2001; Kislev et al. 1992; Hillman 2000; Hillman et al. 1989; Martinoli and Jacomet 2004; Van Zeist and Bakker-Heeres 1984). Indeed, more than 250 wild plant taxa have been identified from seeds, parenchymous tissue, phytoliths, starch grains, charcoal and pollen retrieved from Epipalaeolithic sites. Many are potentially edible species that require processing, sometimes extensively, before they can be eaten and include seeds that are bitter and/or toxic such as those of legumes, and plant parts that are too hard to chew in the raw form such as many seeds and tubers. Except for the cereals, we know little about their potential food values and functional properties, that is, the ways that plant tissue responds to different processing techniques, mastication and post-harvest ripening.
Human use of fire dates from the Lower and Middle Pleistocene (Goren-Inbar et al. 2004), however, it is only during the Epipalaeolithic that the technology appears to have been tailored to plant processing. Likewise, groundstone tools such as pestles and bedrock and portable mortars, first occurred in small numbers in the Upper Palaeolithic, where they appear to have been used primarily for pounding ochre. In the Near East, the earliest evidence of these tools being used to process edible plants dates from the early Epipalaeolithic/final Upper Palaeolithic (Hillman et al. 1989; Piperno et al. 2004). The tools that are thought to indicate the intensive processing of plant foods, the deep vessel mortar and elongated pestle, were also introduced in the early Epipalaeolithic (Wright 1994, 2005). During the millennia that followed there were sequential increases in the numbers and types of groundstone tools, as well as increases in the numbers of types of hearths and pit features. Zooarchaeological evidence of bone boiling by late Pleistocene groups suggests that the boiling of edible plants was also feasible from at least the early Epipalaeolithic (Munro and Bar-Oz 2005). There is also some suggestion that Epipalaeolithic groups may have used pit-cooking (earth oven) technology (Goring-Morris 1995).
Altogether, the implications of the data are that over time Epipalaeolithic hunter-gatherers were investing more time and energy in food processing activities than their predecessors. As a result of these investments, post-harvest systems emerged that involved pulverising, grinding and heat treatments such as boiling, roasting and possibly washing, peeling, baking, pit-cooking, preservation and storage. Moreover, the data suggest that over time people were consuming more processed foods than their predecessors.
We propose that Epipalaeolithic hunter-gatherers were willing to invest time and labour in plant food processing because they recognised that it could promote abundance by producing improvements in both quality and quantity (see Stahl 1989; Wandsnider 1997; Wrangham et al. 1999). Recent studies (for example, Hotz and Gibson 2007; Stahl et al. 2002) have shown that food processing can promote greater bioaccessibility of macronutrients (proteins, lipids, carbohydrates), micronutrients (minerals, vitamins) and phytochemicals for human consumption and digestion. Bioaccessibility is defined as the fraction of a nutrient that is released from a food matrix during digestion and is therefore potentially available for absorption in the gastrointestinal tract. It is an important factor in bioavailability, which is the proportion of a nutrient that is absorbed in the digestive system to become metabolically active.
Bioaccessibility and therefore bioavailability are highly influenced by the physiochemical structure and properties of the plant cell walls, which can act as physical barriers to digestion and absorption of the intracellular nutrients (Parada and Aguilera 2007; Slaughter et al. 2001). The key role of plant cell walls in hindering bioaccessibility is usefully demonstrated in a human study by Ellis et al. (2004) which shows that even after chewing, digestion and large bowel fermentation, a significant proportion of raw Amygdalus communis L. (almond) tissue is preserved intact along with the intracellular nutrients such as lipids (Fig. 1).
Heating or mechanical processing such as grinding of plant foods promotes nutrient release through cell rupture (breakage) and/or cell separation (Hather 2000; Brett and Waldron 1996). In cell rupture the plant tissue is disrupted by breakage of the cell wall thereby exposing the contents. It occurs when the cell–cell adhesion is very strong, and is typical of unripe or crisp or crunchy fruits and uncooked vegetables. In cell separation, individual cells or clusters of cells become detached from each other, so that the tissue is disrupted along the plane of the middle lamella. Separation occurs when the cell–cell adhesion is weak and there is a depolymerisation and or dissolution of cell wall polymers (usually pectic polysaccharides) bridging the middle lamella (Brett and Waldron 1996). The softening of plant tissue by thermal processing and post-harvest ripening is usually the result of cell separation.
The present study examines the effects of food processing techniques similar to those known by Epipalaeolithic groups on the microstructure of one of the edible plants that they exploited. Bolboschoenus maritimus (L.) Palla, synonym Scirpus maritimus L., (sea club-rush) was selected from among species recovered from Epipalaeolithic contexts because it is widespread at early sites (Table 1 in ESM), its archaeological occurrence spans a significant time period and both the seeds and tubers are starch-rich; therefore the plant is thought to have good food potential (Hillman et al. 1989; Wollstonecroft 2007). Ethnographic and ethnohistorical reports mention people using these tubers as food in Europe, Asia, North America and Oceania (Bryant 1783; CSIR 1972; Moerman 1998; Gott 1982, respectively).
Materials and methods
Sea club-rush (SCR) is a semi-aquatic perennial of the Cyperaceae (sedge family). It grows at low to mid elevations throughout the temperate zones of the northern and southern hemispheres, including most parts of the Near East except the Negev and Sinai (see Townsend and Guest 1985). It has possibly been similarly distributed since ca. 15300 cal b.p., when CO2 conditions had become more favourable for C3 plants (Hillman et al. 2001).
SCR is a helophyte, a semi-aquatic plant in which the perennating organs (tubers, rhizomes and rootlets) lie in soil or mud below the water level, while the aerial shoots (stems, leaves, florets) protrude above the water. It is a clonal species, reproducing and expanding through its underground network of rhizomes and tubers (Clevering et al. 1995). The perennial rhizome tubers range from 2 to 19 g (fresh weight). The tuber nutrient content is as follows: moisture 77%, protein 1.4%, lipid 0.2%, total carbohydrate 20% (including dietary fibre and starch) and ash (minerals) 0.8%. These values were obtained using standard methods for determining chemical composition of plant materials (Kirk and Sawyer 1991).
All SCR tubers discussed in this paper were collected from Pevensey Marsh in East Sussex. They were collected in the month of September to ensure comparability. At that time of the year (late summer/early autumn) SCR tubers are replenished with nutrients that have been translocated from the dying above-ground parts (Clevering et al. 1995; Wollstonecroft 2007). Following harvesting, samples intended for processing were kept moist, stored overnight at room temperature, and processed the next day.
The tubers were prepared as three types of food products: mush/gruel, flat bread and as a boiled vegetable. According to the ethnographies the tubers were typically pulverised into flour which was baked into bread or boiled into a mush/gruel. Although no ethnographic or other information was found to suggest that SCR tubers can be eaten whole after simply boiling or steaming without first pulverising them, boiling experiments were included in the study because SCR is a stem tuber and edible stem tubers such as Solanum tuberosum L. (potato) and Eleocharis dulcis (Burm. f.) Hensch. (Chinese water chestnut) are commonly prepared for consumption by boiling them.
Prior to processing, the tubers were washed and the outer layers (endodermis, cortex and epidermis) removed.
Pulverising
Samples prepared into bread and gruel were first pulverised into flour using wooden mortars and pestles. Samples for microscopy were collected after 5, 15 and 20 min of pulverising.
Thermal processing: boiling and baking
Table 2 (in ESM) summarises the details of the thermal processing.
Boiled SCR
SCR tubers measuring approximately 3 cm in diameter were placed in boiling water within a 2 l glass saucepan on a home gas cooker, using a large gas burner with a 3.0 kW nominal heat capacity and a 750 W net heat capacity. For comparative purposes small new potatoes (variety Charlotte), of the same size as the SCR specimens, were boiled. Samples for microscopy were collected from both SCR and potato after 10 and 30 min boiling.
SCR gruel
Peeled SCR tubers were pounded for up to 15 min into the consistency of a meal (but not sieved) and water added. The mixture was composed of two parts SCR meal to five parts water. The mixture was cooked until it thickened and a gruel-like consistency was obtained after 10 min. Samples for microscopy were collected after 10 min cooking.
SCR bread
Previous experiments by Wollstonecroft and Erkal (1999) demonstrated that SCR tuber flour can be prepared into leavened bread when mixed with bread wheat and baked in an oven. The aim of the present experiment was to produce bread made from 100% SCR flour, with no other ingredients added except water. The absence of gluten or other raising agents determined that the proposed product would be a flatbread. To obtain a fine particle size, the tubers were pulverised for up to 20 min and sieved through a 500 µm mesh. A batter was prepared from 80 ml water and 230 g SCR flour. Three batches of dough were poured onto aluminium foil, and baked at 250°C for 30 min in an electric home oven with a 48 l size capacity and 3.19 kW heat capacity. Three samples were collected for microscopy: the flour, the flour mixed with water and soaked 20 min, and the baked bread.
Sensory analysis
Informal sensory testing was carried out primarily for the purpose of obtaining information about the texture and flavour (palatability) of the SCR food products. The authors and one other individual participated in these tests.
Microstructural analysis
Compound light microscopy (LM), transmission electron microscopy (TEM), and scanning electron microscopy (SEM) were used. SEM studies were conducted with a Hitachi S-570 and a Philips SEM501B microscope using standard SEM techniques described by Hather (2000). Raw and processed samples for TEM and LM were fixed immediately (within 5 min of harvesting or processing) in a solution of 2.5% (w/v) glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2). In the laboratory the fixed samples were washed twice, for 30 min each time, in 0.1 M sodium cacodylate or phosphate buffer and then postfixed in 1% (w/v) osmium tetroxide for 2 h. Subsequently samples were dehydrated in graded acetone or ethanol serial dilutions (50, 70 and 90% by volume, acetone or ethanol and distilled water) for 30 min for each solution and then in a 100% acetone or ethanol for 30 min (three times).
To prepare sections for viewing with TEM and LM, samples were infiltrated with Spurr resin, embedded in moulds and polymerised at 60°C. For TEM, ultrathin sections (~70 nm) were cut and examined with a JEOL 100CX MK II transmission electron microscope and images were recorded onto black and white negative film. For LM, 1 µm sections were cut, mounted on glass slides and stained with Toluidine blue and Schiff’s method, following methods described by Kiernan (1990). We examined these samples using a Zeiss Axioskop two mot plus microscope, with a magnification range of 100×–400×.
Results
The informal sensory analyses determined that the most successful SCR food products were the gruel and the bread. These foods were found to be easily chewed and swallowed and to have pleasant flavours, although the gruel was thought to be slightly undercooked because it had a “starchy” flavour. Boiling whole SCR tubers for up to 30 min did not result in edible products because the tissue remained too hard to bite into. Potatoes, which were boiled for the same time as SCR, were adequately softened.
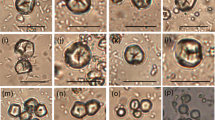
Viewed with microscopy (Fig. 2), no cell separation or cell rupture is evident in the tissue of the whole boiled SCR tubers. In the raw SCR tissue (Fig. 2a, d), native starch filled the intracellular spaces. In the boiled tissue (Fig. 2b, c, e, f) the intracellular starch was disrupted, and appeared to be fully gelatinised and pasted, but remained encapsulated within the cells (not released).
Raw and boiled SCR parenchyma tissue: a, d raw tissue; b, e tissue boiled for 10 min; c, f tissue boiled for 30 min. Microscopy: upper row a–c, LM of tissue stained with Toluidine blue to highlight the cw and intracellular starch; lower row d–f, TEM. (Note: the starch granules in d are wrinkled due to shrinkage caused by the fixative.) csg Compound native starch granule, cw cell wall, i intercellular space, s starch (gelatinized), sg single native starch granule
These patterns contrast with the thermal behaviour of potato (Fig. 3) which exhibited both cell rupture and cell separation after only a few minutes of boiling. Our experiments and reported studies on the potato (Loh et al. 1982) show that cell separation occurs after only 1 min of steaming/boiling at 100°C, followed soon after by cell rupture. The starch was released from the cells and appeared fully gelatinised and pasted in samples collected after 10 and 30 min boiling (Fig. 3b, c, e, f).
Raw and boiled Solanum tuberosum (potato) parenchyma tissue: a, d raw tissue; b, e tissue boiled for 10 min; c, f tissue boiled for 30 min. Microscopy: upper row a–c, LM of tissue stained with Schiff’s method; lower row d–f, TEM. (Note: the starch granules in a, d are wrinkled due to shrinkage caused by the fixative.) cw Cell wall, i intercellular space, s starch
SEM images of pulverised SCR (Fig. 4) show the tissue broken into particles comprised of clusters of tightly bonded cells that are ruptured on the fracture surface only (the first layer of cells). Starch granules that have been released from the ruptured cells are visible on the tissue surface. Other changes that resulted from pulverising include the deformation of cell shape, some (minor) cell separation along the middle lamella, and fissures along the middle lamella of cells that had not separated (Fig. 4b–d). Longer pulverising times (20 vs. 5 min) did not promote increased cell separation but did promote increased cell rupture, resulting in a smaller particle size and more surface area and intracellular nutrients being exposed.
The application of heat to the pulverised tissue produced distinctly different results than the application of heat to whole tubers. The micrographs of SCR gruel show that after 10 min boiling, no additional cell wall separation or cell wall rupturing occurred (Fig. 5). But there are other changes to the tissue including an increased deformation of overall cell shape, and the starch is also significantly changed from the native state, exhibiting a loss of structural integrity (shown with TEM and LM in Fig. 5d, f). However, in the SEM micrograph (Fig. 5b), the starch granules appear only partially gelatinised, which accords with our earlier inference from sensory tests on the gruel, that the starch was not fully cooked (for an explanation of starch gelatinisation and pasting, see Buléon et al. 1998; Colonna et al. 1992).
Stages of gruel making: raw tissue pounded 15 min shown in a SEM, c LM, and e TEM, (pulverised) tissue boiled for 10 min shown in b SEM, d LM, and f TEM. In the LM images c, d the tissue is stained with Toluidine blue to highlight the cw and intracellular starch. (Note: the starch granules in e are wrinkled due to shrinkage caused by the fixative.) cw Cell wall, i intercellular space, ml middle lamella, p cell wall pitting, s starch, v vascular tissue
The micrographs of SCR bread making are shown in Fig. 6. Samples collected after the second stage, in which water was added to the already pulverised tissue and the mixture left to soak for 20 min (Fig. 6b, e), produced no significant increase in cell separation compared with tissue that was pulverised only, discussed above. Changes to the tissue include swelling/distortion along the middle lamella (Fig. 6e), the emptying of starch from ruptured cells, and a melting or pasting of starch into a single mass within intact cells.
Stages of bread making: raw tissue pounded for 20 min shown in a LM, and d TEM, mixed with water and left to stand for 20 min shown in b LM, and e TEM, baked for 30 min shown in c LM, and f TEM. In the LM images c, d the tissue is stained with Toluidine blue to highlight the cw and intracellular starch. (Note: the starch granules in d, e are wrinkled due to shrinkage caused by the fixative.) csg compound native starch granule, cw cell wall, gs gelatinized starch, i intercellular space, ml middle lamella, s single native starch granule
Samples collected after the third stage of bread making, 30 min baking (Fig. 6c, f), exhibited no significant changes from the previous stage in terms of cell wall rupture or separation. The most obvious change is that the starch granules have lost their structural integrity and become amorphous. As seen in the SEM micrograph (Fig. 7) the starch appears to be fully gelatinised and pasted.
SEM micrograph of the SCR bread showing the ruptured cells and gelatinised intracellular starch. Compared with raw tissue (Fig. 5a) the cells appear flattened and the cw is thinner. cw Cell wall, s starch
Discussion
Pulverising is clearly an important step in SCR tissue softening and nutrient release. It caused cell wall rupture, which in turn led to a reduction of the relative particle size of the tissue and an increase in surface area. Some cell separation along the middle lamella also occurred. Fissures along the middle lamella of cells that did not otherwise separate may have contributed to tissue softening and/or made the tissue more susceptible to thermal softening during subsequent baking and boiling. Significantly, by disrupting cell wall structures, pulverising exposed the intracellular starch granules.
Heating alone did not promote a softening of the SCR tuber tissue. Heating was observed to disrupt the starch structure through swelling and gelatinisation, a process that modifies it into a form that is more susceptible to breakdown by α-amylase during digestion (Slaughter et al. 2001). In SCR tubers that were boiled whole, the gelatinised and pasted starch remained encapsulated within intact cells. It appears that a longer cooking time would not result in SCR tissue softening because tissue boiled for 30 min exhibited no more cell separation than tissue collected after 10 min of boiling, although some swelling of the cell wall appears to have occurred in the former. In contrast, potatoes sustained both cell separation and cell rupture during boiling and the intracellular starch was released.
Interestingly, the thermal behaviour of SCR is similar to that reported for its relative, the Chinese water chestnut (Eleocharis dulcis), also a member of the Cyperaceae family. Chinese water chestnut is among several tubers that are known to be inherently resistant to thermal softening because the chemical properties of the middle lamella of the cell promote cell adhesion during heating (Parker et al. 2003; Parr et al. 1996).
The raw tissues of potato and Chinese water chestnut are similar in chemical composition and have comparable tensile strength and toughness (Loh et al. 1982). However, potato and Chinese water chestnut respond very differently to heating because the cell wall of the latter does not separate (Loh and Breene 1982). Investigations of the thermal behaviours of these two taxa by Mudahar and Jen (1991) demonstrate that after 10 min boiling in water, the degree of structural integrity retained by the potato cell wall diminishes from 100% to about 15%, but in the same period the degree of structural integrity retained by the Chinese water chestnut diminishes by only 10%. They further observed that after 30 min boiling the degree of structural integrity retained by the potato is negligible, while that of Chinese water chestnut diminishes to no more than 65%. Physical differences between the two tubers, for example the larger and less uniform cells and thinner cell walls of the potato, do not explain their disparate thermal behaviours. In fact, compression studies by Mudahar and Jen (1991) revealed that, in the raw state, the potato is slightly harder (tougher) than Chinese water chestnut, and has a higher degree of tensile strength (structural integrity).
More recent investigations into the thermal behaviour of Chinese water chestnut (Parker et al. 2003; Parr et al. 1996) reveal that it is caused by the presence of specific phenolic substances known as ferulic acids, which promote cell adhesion by cross-linking the polysaccharides of adjacent cell walls. Ferulic acids are more common in monocotyledons but also occur in several economically important dicotyledons, for example Beta vulgaris L. (beetroot) and Manihot esculenta Crantz (cassava) (Buschmann et al. 2002). In plants that thrive in wet conditions, which describes both Chinese water chestnut and SCR, ferulic acids may serve to protect the tubers from pathogens (Parr et al. 1996). It is possible that SCR tubers contain phenolic substances similar to those of Chinese water chestnut. Clearly this is an area that warrants further investigation.
Conclusions
The case study on sea club-rush demonstrates that these tubers do not soften during thermal processing, a functional behaviour that we argue is best explained by a thermal stability of the parenchyma tissue, which causes the cell wall to be prone to adhesion rather than cell separation during heating. However, the results also show that sea club-rush tubers can be prepared into edible products by applying sequences of processing techniques, including pulverising, the addition of water and baking or boiling. Pulverising is a necessary step because it disrupts the cell walls, facilitating tissue softening and access to intracellular nutrients. These results support our argument that food-processing systems developed by Near Eastern Epipalaeolithic groups provided them with an ability to obtain greater amounts of energy and other nutrients from plants within their foraging territories. These results have implications about the resource decisions of Near Eastern Epipalaeolithic and also Neolithic groups, suggesting that the functional properties of the available plants were key variables in species selection, eating habits and access to critical nutrients.
This study draws attention to potential discrepancies between the inherent nutrient values of edible plants and the bioaccessibility of those nutrients, and therefore, calls into question archaeological optimisation models that are based solely on energy and nutrient values reported in food composition tables.
References
Brett CT, Waldron KW (1996) Physiology and biochemistry of plant cell walls. Chapman & Hall, London
Bryant C (1783) Flora diaetetica. B. White, London
Buléon A, Colonna P, Planchot V, Ball S (1998) Starch granules: structure and biosynthesis. Int J Biol Macromol 23:85–112
Buschmann H, Potter U, Beeching J (2002) Ultrastructure of cassava root studied by TEM and SEM. Microsc Anal 16:9–11
Clevering OA, Van Vierssen W, Blom CWPM (1995) Growth, photosynthesis and carbohydrate utilization in submerged Scirpus maritimus L. during spring growth. New Phytol 130:105–116
Colledge S (2001) Plant exploitation on Epipalaeolithic and Early Neolithic sites in the Levant (B.A.R. International Series 986). Archaeopress, Oxford
Colonna P, Leloup V, Buléon A (1992) Limiting factors of starch hydrolysis. Eur J Clin Nutr 46:17–32
CSIR (Council of Scientific, Industrial Research) (1972) The wealth of India, a dictionary of Indian raw materials and industrial products, vol 9. Council of Scientific and Industrial Research, New Delhi
Ellis PR, Kendall CWC, Ren Y, Parker C, Pacy JF, Waldron KW, Jenkins DJA (2004) Role of cell walls in the bioaccessibility of lipids in almond seeds. Am J Clin Nutr 80:604–613
Goren-Inbar N, Alperson N, Kislev M, Simchoni O, Melamed Y, Ben-Nun A, Werker E (2004) Evidence of hominid control of fire at Gesher Benot Ya’aqov, Israel. Science 304:725–727
Goring-Morris N (1995) Complex hunter-gatherers at the end of the Palaeolithic (20,000–10,000 b.p.). In: Levy TE (ed) The archaeology of society in the holy land. Leicester University Press, Leicester, pp 141–168
Gott B (1982) The ecology of root use by the Aborigines of Southern Australia. Archaeol Oceania 17:59–67
Hather JG (1995) Parenchymous tissues from the early Neolithic site E-75-6 at Nabta Playa, Western Desert, South Egypt. Acta Palaeobot 35:157–162
Hather JG (2000) Archaeological parenchyma. Archetype Publications, London
Hillman GC (2000) The plant food economy of Abu Hureyra 1 and 2. In: Moore AMT, Hillman GC, Legge AJ (eds) Village on the Euphrates: from foraging to farming at Abu Hureyra. Oxford University Press, Oxford, pp 327–398
Hillman GC, Madeyska E, Hather JG (1989) Wild plant foods and diet of Late Palaeolithic Wadi Kubbaniya: the evidence from charred remains. In: Wendorf F, Schild R, Close A (eds) The prehistory of Wadi Kubbaniya (vol 2) stratigraphy palaeoeconomy and environment. Southern Methodist University Press, Dallas, pp 162–242
Hillman G, Hedges R, Moore A, Colledge S, Pettitt P (2001) New evidence of lateglacial cereal cultivation at Abu Hureyra on the Euphrates. Holocene 11:383–393
Hotz C, Gibson RS (2007) Traditional food-processing and preparation practices to enhance the bioavailability of micronutrients in plant-based diets. J Nutr 137:1097–1100
Kiernan JA (1990) Histological and histochemical methods: theory and practice. Pergamon Press, Oxford
Kirk RS, Sawyer R (1991) Pearson’s composition and analysis of foods. Longman, Essex
Kislev ME, Nadel D, Carmi I (1992) Epipalaeolithic (19000 b.p.) cereal and fruit diet at Ohalo II, Sea of Galilee, Israel. Rev Palaeobot Palynol 73:161–166
Loh J, Breene WM (1982) Between-species differences in fracturability loss: comparison of the thermal behaviour of pectin and cell wall substances in potato and Chinese water chestnut. J Texture Stud 13:381–396
Loh J, Breene WM, Davis EA (1982) Between-species differences in fracturability loss: microscopic and chemical comparison of potato and Chinese water chestnut. J Texture Stud 13:325–347
Martinoli D, Jacomet S (2004) Plant food economy put in context: from Epipalaeolithic Southwest Anatolia. In: 69th Annual meeting of the Society of American Archaeology, Montreal, 31 March–4 April
Moerman DE (1998) Native American ethnobotany. Timber Press, Portland
Mudahar GS, Jen JJ (1991) Texture of raw and canned jicama (Pachyrrhizus tuberosus) and Chinese water chestnut (Eleocharis dulcis). J Food Sci 56:977–980
Munro ND, Bar-Oz G (2005) Gazelle bone fat processing the Levantine Epipalaeolithic. J Archaeol Sci 32:223–239
Parada J, Aguilera JM (2007) Food microstructure affects the bioavailability of several nutrients. J Food Sci 72:21–32
Parker CC, Parker ML, Smith AC, Waldron KW (2003) Thermal stability of texture in Chinese water chestnut may be dependent on 8 8′-diferulic acid. J Agric Food Chem 51:2034–2039
Parr A, Waldron KW, Ng A, Parker ML (1996) The wall-bound phenolics of Chinese water chestnut (Eleocharis dulcis). J Sci Food Agric 71:501–507
Piperno DR, Weiss E, Holst I, Nadel D (2004) Processing of wild cereal grains in the Upper Palaeolithic revealed by starch grain analysis. Nature 430:670–673
Slaughter SL, Ellis PR, Butterworth PJ (2001) An investigation of the action of porcine pancreatic α-amylase on native and gelatinised starches. Biochim Biophys Acta 1525:29–36
Stahl AB (1989) Plant-food processing: implications for dietary quality. In: Harris DR, Hillman GC (eds) Foraging and farming: the evolution of plant exploitation. Unwin Hyman, London, pp 171–196
Stahl W, Van den Berg H, Arthur J, Bast A, Dainty J, Faulks RM, Gartner C, Haenen G, Hollman P, Holst B, Kelly FJ, Polidori MC, Rice-Evans C, Southon S, Van Vliet T, Vina-Ribes J, Williamson G, Astley SB (2002) Bioavailabilty and metabolism. Mol Aspects Med 23:39–100
Townsend CC, Guest E (1985) Flora of Iraq (vol 8) monocotyledons. Ministry of Agriculture and Agrarian Reform, Baghdad
Van Zeist W, Bakker-Heeres JAH (1984) Archaeobotanical studies in the Levant 3: Late Palaeolithic Mureybit. Palaeohistoria 26:171–199
Wandsnider L (1997) The roasted and the boiled: food composition and heat treatment with special emphasis on pit-hearth cooking. J Anthropol Archaeol 16:1–48
Wollstonecroft M (2007) Post-harvest intensification in Late Pleistocene Southwest Asia: plant food processing as a critical variable in Epipalaeolithic subsistence and subsistence change. Unpublished doctoral thesis, University College London
Wollstonecroft M, Erkal A (1999) Summary of plant-processing experiments 1999. In: Hodder I, Catalhöyük Research Trust (eds) Catalhöyük 1999 archive report. http://www.catalhoyuk.com/archive_reports/1999/index.html
Wrangham R, Jones JH, Laden G, Pilbeam D, Congklin-Brittain NL (1999) The raw and the stolen: cooking and the ecology of human origins. Curr Anthropol 40:567–594
Wright K (1994) Groundstone tools and hunter-gatherer subsistence in Southwest Asia: implications for transition to farming. Am Antiq 59:238–263
Wright K (2005) The emergence of cooking in western Asia. Archaeol Int 2004-2005:33–37
Acknowledgments
The authors thank Tony Brain and John Pacy of King’s College London for assistance with the microscopy, Tina van Gaalen for assistance with the micrographs and figure formatting and Delwen Samuel for useful discussion of the results of the experiments. Particular thanks are given to our two anonymous reviewers for their insightful and detailed comments. The American Journal of Clinical Nutrition kindly permitted the use of the Ellis et al. (2004) graphic (Fig. 1, above). This study is part of MW’s doctoral research which was funded by a University College London Graduate School Research Scholarship, the Overseas Research Students Award (ORS) Program, the University of London Central Research Fund, and The Institute of Archaeology (UCL).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Willcox.
Electronic supplementary material
Below is the link to the electronic supplementary material.
334_2008_162_MOESM1_ESM.doc
Table 1 Late Pleistocene and early Holocene sites in southwest Asia where the tubers and seeds of Bolboschoenus maritimus/Scirpus maritimus and other Scirpus species have been found. Scirpus other than SCR are included here to account for possible mis-identifications due to taxonomic problems. (T) = Tubers, (S) = Seeds. References: Colledge (2001), Hather (1995), Hillman, Madeyska and Hather (1989), Martinoli and Jacomet (2004), and the database compiled as part of AHRB/C funded project, based at the Institute of Archaeology, UCL (2001-4): ‘The origin and spread of Neolithic plant economies in the Near East and Europe’ (PIs: S. Shennan and J. Conolly; RA: S. Colledge) (DOC 51 kb)
334_2008_162_MOESM2_ESM.doc
Table 2 Summary of thermal processing techniques: methods, apparatus, cooking time and temperatures. Temperatures were measured with an RS 53 K-type handheld digital thermometer attached to an external thermocouple, with a range of −50°C to 1,300°C (DOC 30 kb)
Rights and permissions
About this article
Cite this article
Wollstonecroft, M.M., Ellis, P.R., Hillman, G.C. et al. Advances in plant food processing in the Near Eastern Epipalaeolithic and implications for improved edibility and nutrient bioaccessibility: an experimental assessment of Bolboschoenus maritimus (L.) Palla (sea club-rush). Veget Hist Archaeobot 17 (Suppl 1), 19–27 (2008). https://doi.org/10.1007/s00334-008-0162-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00334-008-0162-x