Abstract
After the beginning of metal processing at the transition from the Neolithic to the Bronze Age, further knowledge of ore mining and smelting had spread from the Near East to central Europe. In the copper ore deposits of Schwaz, in the central part of the Alps, the oldest traces of copper mining derive from the early to middle Bronze Ages. Investigation of a middle to late Bronze Age (1410–920 cal B.C.) slag-washing site in the area revealed a carbonised seed of Nigella damascena (Ranunculaceae) (love-in-a-mist) together with individual other food plants. The plant remains had become incorporated into the slag sediments by chance and had been preserved in an excellent state due to toxic copper salts contained in the soil. Nigella damascena, like N. sativa (black cumin), is traditionally used as a condiment and healing herb in southern Europe and the Near East, but has never grown in the wild in central Europe. Until now, there has been no evidence of prehistoric large-scale cultivation of N. damascena in central Europe. This leads to two possible conclusions: the find may either originate from an exchange of goods with the cultures in the Mediterranean during the Bronze Age, or indicate an introduction of the plant by an immigrant population from that area. Implicating the latter alternative together with the archaeological context of the ore processing site suggests that Nigella damascena had been introduced to the Alps by foreign miners in the course of ore exploitation during the middle to late Bronze Age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction: Investigating Bronze Age metallurgical history in the eastern Alps
The first steps in metal processing were taken in Asia Minor during the Neolithic. As of the 9th/8th millennium B.C. solid gold and copper were tempered into objects of ritual and status purposes. Subsequent improvements of metallurgical techniques are reported from the same region: mining and smelting of copper ore, casting solid copper, and creating bronze alloys (Pernicka 1990; Strahm 1994).
The spread of metallurgical knowledge throughout Europe, originating from the high cultures in the Aegean and the Near East (Pernicka 1990; Strahm 1994), is documented as of the 5th millennium B.C. Prospectors are believed to have travelled the lands in search of profitable ore deposits, and immigrants brought their metallurgical skills with them (Krause 2004). During the transition from the late Neolithic (the Chalcolithic, or Copper Age) to the Bronze Age the use of the new working material increased greatly, together with the appearance of new and more elaborate techniques of extracting and processing copper.
Copper ore deposits of prime importance are located in the central part of the Alps, as in the areas of Schwaz (Tyrol, Austria) and Mitterberg (Salzburg, Austria). The deposits around Schwaz are well-known for their predominance of tetrahedrite-tennantite type ores (Fahlerz, grey copper ore). While the use of copper in the eastern Alps is first documented from the beginning of the 4th millennium B.C. (Matuschik 1997), the oldest evidence of copper extraction from copper ore dates to the early to the middle Bronze Age, as for the Schwaz deposits (Goldenberg 1998, 2001).
Prehistoric large-scale cultural and mercantile relations between the eastern Mediterranean and central Europe are already well documented by artefact finds. Della Casa and Primas (1998), for instance, have illustrated the trade between the Aegean and the eastern Adriatic in the period between 2800 and 2700 B.C. Giardino (1998) has studied the role of the Italian area as part of metal trade routes between the Aegean and the rest of Europe: in the gulf of Naples the beginning of trade relations with the Aegean region was dated to Bronze Age B (1700–1500 B.C.).
In the Alps, however, direct evidence for cultural and trade bonds to the Aegean region during Bronze Age has only rarely been found: first of all, the finding of several gold objects in the Alpine foothills near Bernstorf (Bavaria, Germany) is to be mentioned. Archaeological and metallurgical analyses of the discovered objects, among them a large crown-like diadem and a golden belt, have shown that this jewellery had been cast in the Aegean region. Moreover, their supposed ritual purpose refers unambiguously to cultures of the eastern Mediterranean (Gebhard 1999). For the area near Schwaz, finds of imported prestige weapons serve as another fine example of Bronze Age large-scale trade: three one-piece handle daggers (“Vollgriffdolche”) have been discovered up to now in the lower Inntal. One of them is of Únětice origin, but had been forged mimicking archetypes from Italy (Schwenzer 2004).
The traces of prehistoric mining are still visible everywhere on the south bank of the lower Inntal. Palaeoethnobotanical investigation of a slag-washing site near Schwaz has now supplied plant material that proved an unexpected insight into Bronze Age mining history.
Materials and methods
The site
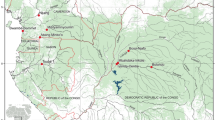
In the years 1997 and 2000 excavations were carried out in the low mountain ranges of the Inntal near Schwaz in Tyrol, Austria (Fig. 1). Archaeological investigation of a small southern tributary valley, the Maukengraben, revealed a huge slag-dump beneath the humus of a Picea abies (spruce) stand at about 900 m a.s.l. The dimensions of the slag-dump were estimated to an area of approximately 1000 m2, its thickness reaching from about 50 cm up to 1 m.
Location of the site in Tyrol (Skamen 2002 onwards, modified; small map from Spiess 2002, modified)
Several samples of wood and charcoal were taken from the sediments and sent to the VERA (Vienna Environmental Research Accelerator) laboratory at the Institute for Isotope Research and Nuclear Physics of the University of Vienna for radiocarbon dating. The results (Table 1) gave evidence that the slag heap derived from a middle to late Bronze Age (1410–920 B.C.) slag-washing site where miners had post-processed crude ore.
The first excavation campaign in 1997 revealed a wooden trough embedded in the slag layers (Fig. 2). Its walls consisted of wooden planks in an extraordinarily good state of preservation. The two lateral planks were later identified as Larix decidua (larch), but the front plank was not analysed. The bottom of the supposed flotation device consisted of a massive clay layer obviously thick enough to keep the water inside. From behind the southern plank, shreds of rough cloth were retrieved—perhaps the remnants of a sieve-like device for fractioning the slag grains.
According to the excavation director, G. Goldenberg, the area with the wooden trough had most probably been covered by the sediments of a second slag washing device, situated further uphill, which explains why the trough itself was embedded within the slag. This second site has not been located yet.
Sampling and sample processing
The material was obtained by purposively sampling the slag layers inside the trough, and in its perimeter, and the basal clay layers were also sampled. Twelve soil samples and seven single macro remains were retrieved from the slag dump, making a total of 21.5 l (21.1 kg) of material. The samples contained both carbonised and uncarbonised plant remains. During sample-taking we were already able to judge the exceptionally good state of preservation of the uncarbonised macro remains, when several intact leaves and large leaf fragments of Fagus sylvatica (beech) in perfect condition became visible in the slag material. The slag’s high content of toxic heavy metal compounds (salts of copper, antimony, and arsenic, for instance) had added anti-microbial properties to the soil layers. Due to direct proximity of the site to a small brook, the Maukenbach, the slag sediments were waterlogged. Both conditions had caused a greatly slowed down decomposition of uncarbonised plant macro remains.
All samples were immediately enclosed in airtight polyethylene bags and stored at +5°C until analysis. The macro remains were extracted using the flotation method. Staggered sieves at mesh sizes of 0.25, 0.5, 1 and 2 mm were used for separation by particle size. Sub-sampling was done according to standard methods (Jacomet and Kreuz 1999) depending on the amount of plant material. Uncarbonised macro remains were preserved using Strasburger’s preserving agent (Gerlach 1969) consisting of equal amounts of distilled water, 96% ethanol, and glycerine. A small amount of phenol was added (0.4 - 0.6% by weight), serving as a fungicide.
Results
Remains of wild and cultivated plants were extracted from the samples (Table 2). The species spectra in the soil samples did not give any evidence of an intentional plant deposit (as for example in refuse or storage pits) but indicated a rather “natural” sedimentation of plant remains from the surrounding vegetation. The wood and charcoal findings, as distinct remains of human activity, did not indicate a preference of the miners for certain woody taxa. For this reason the identified wild plants were used to characterise the site’s surrounding vegetation during the middle and late Bronze Age (Heiss 2001). Parts of the area had been covered by a zonal climax forest dominated by Picea abies (spruce), Abies alba (fir), and Fagus sylvatica (beech), with Pinus sp. (pine) and Larix decidua (larch) occurring sporadically. The presence of treeless patches was indicated in the samples by the presence of plants such as Urtica dioica (stinging nettle) and Sambucus nigra (elder), and the woody pioneer taxa Betula sp. (birch) and Salix sp. (willow).
Cultivated plants were represented by a carbonised caryopsis of Panicum miliaceum (common millet). Though a cereal of only minor importance, the cultivation of common millet in the eastern Alpine region during the Bronze Age is documented by archaeological evidence (Oeggl 1992; Swidrak and Oeggl 1998). Lacking gluten protein and thus unsuitable for baking bread, P. miliaceum was usually consumed as millet gruel.
Among the plant remains a carbonised seed of Nigella damascena (love-in-a-mist) was also found (Fig. 3). It was identified by its characteristic features: a triangular-ovate seed with tubercled surface, three longitudinal and several transverse ridges (crests) forming a conspicuous reticulum. Besides standard literature, reference material from several Nigella species was used for validating the identification (Fig. 4). However, according to A. Weber (pers. comm.) differentiation between the seeds of N. damascena and N. elata, which are both in the subsection Erobathos (see section on taxonomy and Table 3), is only possible by their surface structure: N. damascena is characterised by the presence of a small, nipple-like elevation on each of the faces’ convex cells (Fig. 3c).
General information on the genus Nigella, and on N. damascena in particular
Etymology
The genus Nigella L. (nigella, fennel flower) was named after its seeds which, as a characteristic, are black in most Nigella species. The Latin term nigellus is diminutive of niger, meaning ‘black’ (Hegi 1975). The ancient Greek name μɛλάνθιον (melanthion, ‘black [seeded] flower’) for Nigella, allegedly for N. sativa, derives from the same attribute (Aufmesser 2002).
The species epithet of Nigella damascena is named after the Syrian capital Damascus, referring to the plant’s eastern Mediterranean origin. Common names of N. damascena allude either to this geographical context, to the plant’s use as a condiment, or to its morphology (see below).
Morphology
Nigella (s.l.) species are characterised by the following features: all are annual plants with pinnatisect (rarely simple) leaves, their segments more or less linear. The hermaphrodite flowers are pentamerous and tetracyclic with conspicuous white to bluish or yellowish sepals persisting for a time on the ripening fruit. The nectariferous petals (honey leaves) are of reduced size, and of characteristic shape: each consists of a lower petaloid, bifid lip secreting nectar and an upper scale-like lip acting as a protective “lid” (Fig. 5b). The five carpels are partly united forming aggregate follicles (Hegi 1975; Zohary 1983).
Line drawing of Nigella damascena L. (Pabst 1887, 1889; modified). a sepal, b petal (honey leaf), c, d capsule, e seeds
Slightly different morphological features can be recognised in Nigella subsection Erobathos, containing the two species N. damascena and N. elata. The number of carpels is 10, and they are entirely fused into a capsule (Fig. 5c, d; Hegi 1975; Tutin et al. 1993). Another typical feature is the involucre of finely dissected leaves enclosing the inflorescence. Hence originated some of the common names of N. damascena, such as ‘love-in-a-mist’, or ‘devil-in-the-bush’.
Taxonomy
The presence of the characteristic honey leaves mentioned above differentiates, among other things, the subtribe Nigellinae from the other Ranunculaceae. The Nigellinae were traditionally placed within the subfamily Helleboroideae, tribe Caltheae (Hegi 1975). However, modern multidisciplinary approaches to Ranunculaceae taxonomy suggest that Nigella and its subtribe are to be placed within the subfamily Ranunculoideae, as done by the Angiosperm Phylogeny Group (APG, Stevens 2001 onwards) and by Frohne and Jensen (1998).
Apart from Nigella, the subtribe Nigellinae contains two other genera: Garidella (with G. unguicularis Lam. and G. nigellastrum L.) and monotypic Komaroffia (K. diversifolia Ktze).
Illustrating Nigella‘s sub-generic taxonomy is, however, somewhat more intricate. Differing taxonomic approaches have resulted in an assemblage of many synonyms, some of them ambiguous. Linnaeus, when he first described this rather small genus, recorded six species (Linnaeus 1753, cited by Zohary 1983). Referring to Zohary (1983), the number of species names published up to now in the genus Nigella is at least 80 (!).
-
According to the Flora Europaea (Tutin et al. 1993), 14 native European Nigella species including six subspecies are differentiated today.
-
Strid (1970, cited by Zohary 1983) investigated many populations of the extremely polymorphic N. arvensis L. in the eastern Mediterranean, and split the taxon into several separate species.
-
Zohary (1983) described 14 species. But in contrast to the Flora Europaea he “lumped” all three Nigellinae genera into the genus Nigella at the rank of sections and also included some non-European taxa in his work. Furthermore, many species and all of the subspecies were reduced to the rank of varieties. The current work is based on Zohary’s taxonomic point of view.
Table 3 gives an overview of the Nigella taxa treated by Zohary (1983), providing their geographical distribution and their most important synonyms (Tutin et al. 1993; Hooker and Jackson 1893 et seq.).
Ecology and distribution
All Nigella species are short-lived annuals, and are generally to be found in summer annual vegetation of semi-arid areas. They frequently occur on disturbed soils. The whole subtribe Nigellinae (Nigella s.l.), in its native distribution, is limited to the meridional and southern temperate zones of Eurasia and northern Africa, particularly in the eastern Mediterranean and Near East. Hence this region is believed to be the taxon’s centre of diversity (Strid 1970, cited by Zohary 1983; Kästner et al. 2001). Most of the species can be found between the western Mediterranean-continental areas (Iberian Peninsula) and the Irano-Turanian zone (Turkey, Syria, and Israel). Only the following Nigella taxa occur outside of Europe:
-
N. glandulifera Freyn: China
-
N. media Pakh.: Central Asia
-
N. integrifolia Regel, syn. Komaroffia diversifolia Ktze.: Irano-Turanian zone to central Asia
-
N. stellaris Boiss.: Syria
-
N. arvensis s.l.: Morocco to Near East; archaeophyte (introduced by 15th century) in Europe (Kästner et al. 2001).
Although being the most dispersed group within the genus, N. arvensis (s.l.) does not occur on the Iberian Peninsula. However, it is the only Nigella taxon growing wild in central Europe.
N. damascena, initially a plant with an eastern Mediterranean distribution, is today a common ruderal plant in many parts of the Mediterranean. Outside this area, this species may only form ephemeral populations after escaping from cultivation. Just like N. sativa, N. damascena has been introduced to central Europe. Though neither species is to be found there in the wild (Tutin et al. 1993), both provide satisfying crop yields when cultivated in the area, as shown by modern experiments conducted in Thüringen, Germany (Biertümpfel et al. 2001).
Prehistoric, historical, and present cultivation and use
The time when Nigella damascena was introduced to central Europe is unknown. Until now no archaeological data at all has been available on that species (Kroll 2004, pers. comm.). The seed from the site near Schwaz is actually the oldest archaeological evidence of N. damascena in central Europe so far.
The evaluation of historical documents on plant cultivation and utilisation requires a high degree of caution. Due to inaccurate or (more commonly) completely lacking morphological descriptions it is often hard to identify the genera mentioned by antique and medieval authors.
Of the ancient Greek authors, Theophrastos does not give any account about Nigella in his “Enquiry into Plants” from the 4th century B.C. (Hort 1916). In Hippocrates the plant melanthion (see section on etymology) is recommended for a small number of medical uses as, for instance, to promote pregnancy (Fuchs 1895–1900).
In the 1st century A.D., Pliny the Elder mentions a plant called git in his “Natural History” (books XVII, XIX, XX; Von Jan 1857, 1859). He accredits various healing properties to the seeds of that plant which today is commonly interpreted as Nigella sativa. He recommends it as a digestive amarum, as an ingredient of antidotes curing snake bites and scorpion stings, as a fumigant for driving away snakes, and also as a condiment in bread.
Pliny’s contemporary Dioscorides illustrates similar uses of git, adding those of an emmenagogue, galactagogue, vermifuge, and disinfectant (Aufmesser 2002). It should however be mentioned that he warns against possibly fatal effects of an overdose of git. Consequently it should be considered that Dioscorides might have confused Nigella with a poisonous plant: indeed, it is commonly accepted today that in classical and medieval literature Nigella (and N. sativa in particular) has often been confused with corn cockle, Agrostemma githago (Caryophyllaceae). Though quite dissimilar in growth habit and flowers, this once very common crop weed shares Nigella‘s characteristic of black seeds. This has also caused it share the same name in history. The species epithet of A. githago refers to this ancient connexion: “(seeds) resembling git(h)”, that is Nigella (Flückiger 1871).
Medieval documents on useful plants and healing herbs unfortunately challenge the reader with the same inaccuracies:
-
Albertus Magnus mentions a plant named nigella, but is obviously writing about corn cockle (Flückiger 1871; Fischer-Benzon 1894).
-
In the 9th century A.D. the famous decree “Capitulare de villis vel curtis imperialibus” by Charlemagne, an assemblage of rules and guidelines for agriculture, lists git among desirable garden herbs (Fischer-Benzon 1894). No description of the plant is added.
-
In her “Physica”, well-known 12th century abbess Hildegard von Bingen refers to the healing herb (githerum) ratden and warns of harmful effects when eaten (Riethe 1989). The translator interprets that plant as being either Nigella sativa or Lolium temulentum L. (darnel ryegrass).
Yet in the Renaissance, Fuchs (1543) clearly differentiates and describes the Nigella species known at that time. He assigns the same healing properties to N. damascena (“schwartz coriander”) as to the other species he mentions (N. arvensis, N. sativa). He recommends Nigella seeds for chiefly the same uses as Dioscorides and Pliny the Elder did, furthermore against ulcers, various pains, pulmonary diseases, and as a carminative agent. Nota bene that Fuchs, too, warns of fatal overdoses.
Today N. damascena and N. sativa seeds are widely used in folk medicine in Arabian countries from Morocco to Pakistan, as well as in southern Europe. N. sativa is regarded a panacea (universal remedy) in Islamic folk medicine and used for mostly the same purposes as passed down from Pliny and Dioscorides (Ali and Blunden 2003). N. damascena seeds are used for example in Sicilian folk medicine as an emmenagogue and diuretic (Ballero and Fresu 1993). Modern analyses show oestrogenic activity (Agradi et al. 2002) and analgesic properties (Bekemeier et al. 1967). Apart from medical uses, Nigella seeds are still used as spices today. Both N. sativa and N. damascena are used in bread and cheese, while N. damascena also serves as a condiment in sweets (D’Antuono et al. 2002). N. damascena also has some horticultural importance as it is cultivated in many varieties as an ornamental plant.
Discussion and conclusions
The Nigella damascena seed that has been retrieved from the Bronze Age slag-washing site near Schwaz is definitely a remarkable find. As far as we currently know about the history of natural distribution of N. damascena, it can be assumed that the plant had been imported to the area by humans. Considering the archaeological context of an ore processing site, the seed had obviously been brought to its place of discovery by someone involved in mining, ore processing, or metal trade.
However, we cannot reliably assign any ethnobotanical relevance to the Nigella seed. Due to poor documentation of historical use of N. damascena as a spice and as a remedy, many questions are left unanswered. The seed may have been part of a Bronze Age miner’s diet, flavouring his millet gruel. Only the single millet caryopsis retrieved from the same place supports this theory. The plant may also have been an ephemeral ruderal plant in the area of the Inntal, imported from the south together with agricultural crops, or spices. In this connexion the presence of other ruderal plants at the mining site (Daucus carota, Stellaria media agg. and Urtica dioica) should be emphasised once again.
At any rate, the find of the N. damascena seed in the mining site near Schwaz is clear evidence of the association between Alpine copper ore mining and the Mediterranean during the middle to late Bronze Age. Effectively, the find points to either trade or migration between southern or south-eastern regions and central Europe. These large-scale bonds have been documented before by artefact finds and by evidence of the spread of cultural and technological innovations. It is plausible to assume that N. damascena had been brought to the Alps by the same routes.
References
Agradi E, Fico G, Cillo F, Francisci C, Tomè F (2002) Estrogenic activity of Nigella damascena extracts, evaluated using a recombinant yeast screen. Phytother Res 16:414–416
Ali BH, Blunden G (2003) Pharmacological and Toxicological Properties of Nigella sativa. Phytother Res 17:299–305
Aufmesser M (transl) (2002) Pedanius Dioscorides De materia medica [Fünf Bücher über die Heilkunde]. Altertumswissenschaftliche Texte und Studien, vol 37. Olms-Weidmann, Hildesheim
Ballero M, Fresu I (1993) Le piante di uso officinale nella Barbagia di Seui (Sardegna Centrale). Fitoterapia 64:141–150
Bekemeier H, Leuschner G, Schmollack W (1967) Antipyretische, antiödematöse und analgetische Wirkung von Damascenin im Vergleich mit Acetylsalicylsäure und Phenylbutazon. Archives Internationales de Pharmacodynamie et de Thérapie 168:199–211
Biertümpfel A, Vetter A, Zirr K (2001) Ergebnisse der Evaluierung von Schwarzkümmelarten und -herkünften (Nigella sativa L.) hinsichtlich Ertrag, Inhaltsstoffen und Nutzungsmöglichkeiten. Zeitschrift für Arznei- und Gewürzpflanzen 3/2001:161–164
Bronk Ramsey C (1994) Analysis of chronological information and radiocarbon calibration: the program OxCal. Archaeolog Comput Newsletter 41:11–16
D’Antuono LF, Moretti A, Lovato AFS (2002) Seed yield, yield components, oil content and essential oil content and composition of Nigella sativa L. and Nigella damascena L. Industr Crops Prod 15:59–69
Della Casa PH, Primas M (1998) La production et la circulation des métaux du IIIe au Ier millénaire sur la côte est de l’Adriatique d’après les analyses métallurgiques de Velika Gruda (Monténégro). In: Mordant, C., Pernot, M., Rychner, V. (eds), L’Atelier du bronzier en Europe du XXe au VIIIe siècle avant notre ère. Actes du colloque international Bronze ‘96, Neuchâtel et Dijon. Tome I. Paris, CTHS, pp 239–245
Ellenberg H (1996) Vegetation Mitteleuropas mit den Alpen. In ökologischer, dynamischer und historischer Sicht. UTB, Stuttgart
Fischer-Benzon R (1894) Altdeutsche Gartenflora. Lipsius & Tischer, Kiel/Leipzig. Reprinted 1991 by Sändig Reprint, Vaduz
Flückiger FA (1871) Nigella Seeds, or Black Cummin. Am J Pharm 43:1871. In: Kress H (ed) (2000–2004) Henriette’s herbal homepage. Classical texts: journals and periodicals. http://www.ibiblio.org/herbmed/eclectic/journals/ajp-1871.html
Frohne D, Jensen U (1998) Systematik des Pflanzenreichs – Unter besonderer Berücksichtigung chemischer Merkmale und pflanzlicher Drogen, 5th edn. Wissenschaftliche Verlagsgesellschaft, Stuttgart
Fuchs L (1543) New Kreüterbuch [The New Herbal of 1543]. Annotated reprint 2001. Taschen, Köln
Fuchs R (ed) (1895–1900) Hippokrates Corpus Hippocraticum [Sämmtliche Werke]. 3 volumes. Lüneburg, München
Gebhard R (1999) Der Goldfund von Bernstorf bei Kranzberg. Bayerische Vorgeschichtsblätter 64:1–18
Gerlach D (1969) Botanische Mikrotechnik – Eine Einführung. Thieme, Stuttgart
Giardino C (1998) Tyrrhenian Italy and Sicily in the protohistoric metal trade across the Mediterranean: An archaeometallurgical outline. In: Mordant C, Pernot M, Rychner V (eds) L’Atelier du bronzier en Europe du XXe au VIIIe siècle avant notre ère. Actes du colloque international Bronze ‘96, Neuchâtel et Dijon. Tome II. Paris, CTHS, pp 157–168
Goldenberg G (1998) L’exploitation du cuivre dans les Alpes Autrichiennes à l’âge du Bronze. In: Mordant C, Pernot M, Rychner V (eds), L’Atelier du bronzier en Europe du XXe au VIIIe siècle avant notre ère. Actes du colloque international Bronze ‘96, Neuchâtel et Dijon. Tome II. Paris, CTHS, pp 9–24
Goldenberg G (2001) Bronzezeitlicher Kupferbergbau in Nordtirol. In: Brunn A, Steinacker C, Jordan T (eds), Archäologie Online. http://www.archaeologie-online.de/thema/2001/02/
Hegi G (ed) (1975). Illustrierte Flora von Mitteleuropa. vol. III/3, 2nd edn., Parey, Berlin
Heiss AG (2001). Anthrakologische und paläoethnobotanische Untersuchungen im bronzezeitlichen Bergbaugebiet Schwaz – Brixlegg (Tirol). Unpublished diploma thesis. University of Innsbruck
Hooker JD, Jackson DB (eds) (1893 et seq) Index Kewensis Plantarum Phanerogamarum, vol 2, 19 supplement. Clarendon Press, Oxford, pp 1901–1991
Hort A (ed) (1916) Theophrastos Πɛρί ϕυτικών ίστοριών [Peri phytikon historion, Enquiry into plants] vol 2 reprinted 1961. Harvard University Press, Cambridge Massachusetts
Jacomet S, Kreuz A (1999) Archäobotanik. Ulmer, Stuttgart
Von Jan L (ed) (1857) C. Plini Secundi Naturalis historiae libri XXXVII. Libb. XVI–XXII. Teubner, Leipzig
Von Jan L (ed). (1859) C. Plini Secundi Naturalis historiae libri XXXVII. Libb. XXIII—XXXII. Teubner, Leipzig
Kästner A, Jäger EJ, Schubert R (2001) Handbuch der Segetalpflanzen Mitteleuropas. Springer, Wien
Krause R (2004) Eliten der frühen Bronzezeit zwischen Alpen und Donau. In: Krieger, Fürsten und Helden. Katalog Trento 2004 (in press)
Kroll H (2004) Literature on archaeological remains of cultivated plants 1981–2004. http://www.archaeobotany.de/database.html
Matuschik I (1997) Der neue Werkstoff - Metall. In: Planck D (ed) ALManach 2, Archaeologisches Landesmuseum Baden-Württemberg. Theiss, Stuttgart, pp 16–25
Oeggl K (1992) Botanische Untersuchungen zur menschlichen Besiedlung im mittleren Alpenraum während der Bronze- und Eisenzeit. In: Metzger IR, Gleirscher P (eds) Die Räter—I Reti. Athesia, Bozen, pp 709–721
Pabst G (ed) (1887, 1889) Köhler’s Medizinal-Pflanzen in naturgetreuen Abbildungen mit kurz erläuterndem Texte. Gera-Untermhaus, Köhler
Pernicka E (1990) Gewinnung und Verbreitung der Metalle in prähistorischer Zeit. Jahrbuch Römisch Germanisches Zentralmuseum 37:21–129
Riethe P (ed) (1989) Hildegard von Bingen Liber subtilitatum diversarum naturarum creaturarum [Naturkunde, Physica]. 4th edn. Müller, Salzburg
Schwenzer S (2004) Frühbronzezeitliche Vollgriffdolche: typologische, chronologische und technische Studien auf der Grundlage einer Materialaufnahme von Hans-Jürgen Hundt. Kataloge Vor- u. Frühgeschichtlicher Altertümer, 36, Mainz
Skamen J (ed) (2002 onwards) Tiroler Raumordnungs-Informationssystem (tiris), Kartengalerie. http://tiris.tirol.gv.at/kartengal/karteng.cfm
Spiess E (ed) (2002) Schweizer Weltatlas. Neuausgabe 2002. Lehrmittelverlag des Kantons Zürich, Zürich. http://www.educeth.ch/geographie/weltatlas/
Stevens PF (2001 onwards) Angiosperm Phylogeny Website. http://www.mobot.org/MOBOT/research/APweb/
Strahm C (1994) Die Anfänge der Metallurgie in Mitteleuropa. Helv Archaeol 25:2–39
Swidrak I, Oeggl K (1998) Palaeoethnobotanische Untersuchungen von Bodenproben aus der bronzezeitlichen Siedlung von Sotćiastel. In: Tecchiati U (ed) Sotćiastel—Un abitato fortificato dell’età del bronzo in Val Badia. Istitut Cultural Ladin “Micurà de Rü”, Soprintendenza Provinciale ai Beni Culturali di Bolzano, pp 335–346
Tutin TG et al (1964–80) Flora Europea, vol 1 (1964, 2nd edn. 1993). Cambridge University Press, Cambridge
Zohary M (1983) The genus Nigella (Ranunculaceae)—a taxonomic revision. Plant Systematics and Evolution 142:71–107
Acknowledgements
The current work is basing on data from the FWF project P12049 supported by the Austrian Science Fund (“Fonds zur Förderung der wissenschaftlichen Forschung”). We thank H. Kroll (Kiel) for his investigations on archaeological finds of N. damascena, and R. Krause (Landesdenkmalamt Baden-Württemberg) for the additional information on archaeometallurgy and artefact finds in the Alps during the Bronze Age. We also thank A. Weber (Wien) for the valuable information on differentiating between N. damascena and N. elata. Further thanks go to H.-P. Stika (Hohenheim) for providing us with seeds of N. integrifolia
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heiss, A.G., Oeggl, K. The oldest evidence of Nigella damascena L. (Ranunculaceae) and its possible introduction to central Europe. Veget Hist Archaeobot 14, 562–570 (2005). https://doi.org/10.1007/s00334-005-0060-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00334-005-0060-4