Abstract
Objectives
To determine the value of conventional DWI, continuous-time random walk (CTRW), fractional order calculus (FROC), and stretched exponential model (SEM) in discriminating human epidermal growth factor receptor 2 (HER2) status of breast cancer (BC).
Methods
This prospective study included 158 women who underwent DWI, CTRW, FROC, and SEM and were pathologically categorized into the HER2-zero-expressing group (n = 10), HER2-low-expressing group (n = 86), and HER2-overexpressing group (n = 62). Nine diffusion parameters, namely ADC, αCTRW, βCTRW, DCTRW, βFROC, DFROC, μFROC, αSEM, and DDCSEM of the primary tumor, were derived from four diffusion models. These diffusion metrics and clinicopathologic features were compared between groups. Logistic regression was used to determine the optimal diffusion metrics and clinicopathologic variables for classifying the HER2-expressing statuses. Receiver operating characteristic (ROC) curves were used to evaluate their discriminative ability.
Results
The estrogen receptor (ER) status, progesterone receptor (PR) status, and tumor size differed between HER2-low-expressing and HER2-overexpressing groups (p < 0.001 to p = 0.009). The αCTRW, DCTRW, βFROC, DFROC, μFROC, αSEM, and DDCSEM were significantly lower in HER2-low-expressing BCs than those in HER2-overexpressing BCs (p < 0.001 to p = 0.01). Further multivariable logistic regression analysis showed that the αCTRW was the single best discriminative metric, with an area under the curve (AUC) being higher than that of ADC (0.802 vs. 0.610, p < 0.05); the addition of ER status, PR status, and tumor size to the αCTRW improved the AUC to 0.877.
Conclusions
The αCTRW could help discriminate the HER2-low-expressing and HER2-overexpressing BCs.
Clinical relevance statement
Human epidermal growth factor receptor 2 (HER2)-low-expressing breast cancer (BC) might also benefit from the HER2-targeted therapy. Prediction of HER2-low-expressing BC or HER2-overexpressing BC is crucial for appropriate management. Advanced continuous-time random walk diffusion MRI offers a solution to this clinical issue.
Key Points
• Human epidermal receptor 2 (HER2)-low-expressing BC had lower αCTRW, DCTRW, βFROC, DFROC, μFROC, αSEM, and DDCSEM values compared with HER2-overexpressing breast cancer.
• The αCTRW was the single best diffusion metric (AUC = 0.802) for discrimination between the HER2-low-expressing and HER2-overexpressing breast cancers.
• The addition of αCTRW to the clinicopathologic features (estrogen receptor status, progesterone receptor status, and tumor size) further improved the discriminative ability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Female breast cancer (BC) has been the most commonly diagnosed malignancy worldwide, the morbidity of which has surpassed lung cancer and colorectal cancer, accounting for 11.7% of new cancer cases and 6.9% of new cancer deaths annually [1]. According to the hormone receptor statuses, including estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression statuses, invasive BC is recapitulated into five main molecular subgroups with different biological features and treatment strategies [2]. Among them, HER2-overexpressing BC is a more biologically aggressive phenotype than HER2-negative ones [3]. Commonly, HER2-targeted therapies are suitable for BC with HER2-overexpressing status but not for those with HER2-negative status [4, 5]. Clinically, the HER2 expression status of primary BC is assessed by immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) analysis of specimens obtained from biopsy or surgery, based on which they are divided into HER2-overexpressing status (i.e., HER2 expression score 3+ on IHC or score 2+ on IHC with gene amplification on FISH) and HER2-negative status (i.e., HER2 expression score 0 or 1+ on IHC, or score 2+ on IHC without gene amplification on FISH) [6].
However, the current classification criteria of HER2-negative statuses were considered ambiguous for HER2-targeted therapies [7]. Notably, HER2-low-expressing BC with HER2 expression score 1+ on IHC or score 2+ on IHC without gene amplification on FISH has been proposed as a new subgroup, which should be separated from the HER2-negative BCs [7, 8]. Indeed, patients with HER2-low-expressing BC might also benefit from the HER2-targeted agents [7,8,9,10,11]. Thus, identifying this subgroup of BCs had the potential to optimize the treatment strategies for patients with traditionally HER2-negative BCs. Moreover, HER2 expression status in BC can change during the treatment process over time [12]. Biopsy or surgery is not always appropriate for dynamically monitoring of the HER2 status due to the invasive nature. Therefore, a noninvasive imaging method capable of discriminating the HER2-low-expressing from HER2-overexpressing BCs would be highly desirable.
Diffusion magnetic resonance imaging (MRI) could in vivo map the diffusion movement of water molecules in the microstructures of breast tissue. Previously, multiple distinct models of diffusion MRI, such as conventional diffusion-weighted imaging (DWI), diffusion tensor imaging (DTI), and diffusion kurtosis imaging, have been used for the diagnosis, differential diagnosis [13,14,15], and subtype classification [16] of BCs. In addition, neurite orientation dispersion and density imaging, mean apparent propagator, and diffusion spectrum imaging have been introduced to predict HER2 expression status [17]. However, these models exhibited a suboptimal diagnostic performance in predicting HER2-negative or HER2-positive statuses, with a reported AUC less than 0.800 [14, 16, 17]. Therefore, it is necessary to improve the predictive performance of diffusion MRI models, likely, using an advanced diffusion MRI technique, such as the q-space Cartesian grid sampling procedure [18, 19]. Notably, several diffusion models and metrics could be simultaneously obtained from the q-space Cartesian grid sampling method, which can reduce the scanning time and avoid the impact of different scanning parameters on the results and facilitate the comparison of diagnostic effectiveness of different parameters [18,19,20,21]. Continuous-time random walk (CTRW), fractional order calculus (FROC), and stretched exponential model (SEM) can be derived from the q-space Cartesian grid sampling method to reveal the diffusion heterogeneity of water molecules in non-Gaussian distribution [22]. Recently, these advanced diffusion models can provide a variety of diffusion metrics to measure the intravoxel heterogeneities of complex microstructures and have been increasingly applied to the central nervous system to diagnose Parkinson’s disease, grade gliomas, and differentiate pediatric brain tumors [22,23,24,25]. In addition, previous studies also showed that FROC and SEM were helpful in the discrimination between benign and malignant breast tumors and predicted the pathologic complete response to neoadjuvant chemotherapy for patients with BC [26,27,28,29]. However, whether CTRW, FROC, and SEM can be used to differentiate the HER2-expressing status of BC and which one is better remain unknown.
In this prospective study, women with invasive BC underwent the conventional DWI, CTRW, FROC, and SEM MRI. Nine metrics, namely ADC, αCTRW, βCTRW, DCTRW, βFROC, DFROC, μFROC, αSEM, and DDCSEM, were measured from the four diffusion models. This study aims to determine the value of the conventional DWI, CTRW, FROC, and SEM in discriminating the HER2-low-expressing and HER2-overexpressing BCs.
Materials and methods
Study participants
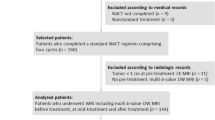
This prospective study was approved by the institutional review board, and all participants provided written informed consent. From January 2022 to July 2022, 176 women clinically suspected of having primary BC and with a solid breast lesion on ultrasound were enrolled. Participants were included if they had a BC confirmed by biopsy or surgical pathology. The exclusion criteria were as follows: (1) adjuvant chemotherapy or biopsy prior to MRI examination; (2) histologically confirmed other benign breast diseases; (3) poor quality of MRI images due to the artifacts; (4) claustrophobia; (5) previous history of allergic reaction to the contrast agents. Finally, 158 participants were included in our study. Per the pathologic-determined classification criteria of HER2 status [7], all participants were separated into the HER2-zero-expressing group, HER2-low-expressing group, and HER2-overexpressing group. The flow chart for the participants’ selection for this study is shown in Fig. 1.
MRI examination
All participants underwent breast MRI on a 3.0-T MR scanner (MAGNETOM Skyra, Siemens Healthcare) with a dedicated breast coil. The breast MRI sequences included axial three-dimensional T1-weighted imaging (T1WI), axial T2-weighted imaging (T2WI), axial dynamic contrast-enhanced (DCE)-MRI, and diffusion MRI including standard axial DWI, and axial spin-echo echo-planar imaging (SE-EPI). The radiofrequency field, field gradient, and magnetic field uniformity of the MR scanner were calibrated once a week using a commercial MR phantom of 5300 mL volume (SIMENS, Model 10606530) to ensure the stability of DCE-MRI and diffusion MRI signals. DCE-MRI was performed using the controlled aliasing in parallel imaging results in higher acceleration-volumetric interpolated breath-hold examination sequence, with 40 dynamic phases and a temporal resolution of 8 s. After the second phase of DCE-MRI acquisition, gadodiamide (Omniscan, GE Healthcare) at a dosage of 0.1 mmol/kg body weight was intravenously administrated with an injection rate of 3.5 mL/s. Before DCE-MRI scanning, the standard DWI and SE-EPI were performed. For DWI, the ADC maps were obtained using the b-values of 0 s/mm2 and 800 s/mm2. The SE-EPI sequence was acquired with an entire q-space Cartesian grid sampling procedure, which was a universal acquisition method for diffusion MRI, and acquired diffusion data with multiple b-values, multiple directions, and a fixed number of excitations [18, 19]. For SE-EPI, nine different b-values (0, 200, 450, 650, 900, 1100, 1350, 1800, and 2000 s/mm2) along with 100 directions (2, 6, 12, 8, 6, 24, 24, 12, and 6 directions for each b-value, respectively) and a number of excitation with one were applied. The acquisition time was 11 min 20 s for the SE-EPI sequence and about 6.9 s for each direction. The detailed acquisition parameters of the conventional MRI, DCE-MRI, and diffusion MRI are shown in Table 1.
Image analysis
The raw data of diffusion MRI was transformed to the NIfTI-1.1 format with the MRIcron (https://www.nitrc.org/projects/mricron), and then processed with NeuDiLab software rooted in DIPY (https://dipy.org/). For the conventional DWI, its quantitative metric ADC is generated with a monoexponential fitting using the following formula [30]:
where S(0) and S(b) represent the signal intensity of water molecule movement in the absence and presence of diffusion sensitization, respectively, and b is a diffusion sensitization factor that decides the degree of diffusion weighting in the signal intensity. CTRW, FROC, and SEM metrics were derived from the SE-EPI sequence. For the CTRW model, three diffusion metrics (αCTRW, βCTRW, and DCTRW) were calculated by a simplified mathematical formula [23]:
where Eα represents the Mittag-Leffler function of α order. In this model, D represents an anomalous diffusion coefficient, and α and β represent diffusion metrics correlated with the temporal diffusion heterogeneity and spatial diffusion heterogeneity, respectively. α and β both range from 0 to 1 (i.e., in a homogeneous medium). For the FROC model, three diffusion metrics (βFROC, DFROC, and μFROC) were calculated according to a complicated equation [24]:
where Gd and δ are the amplitude and pulse width of diffusion gradient, respectively, and Δ represents the gradient lobe separation. In this model, β and D describe spatial diffusion heterogeneity of water molecules, and μ is a spatial diffusion parameter for preserving the nominal units of D in mm2/s. For the SEM, two diffusion metrics (DDCSEM and αSEM) are figured by the following equation [25]:
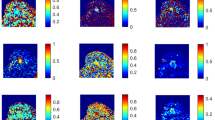
where DDC means distributed diffusion coefficient and α stands for diffusion non-Gaussianity empirical parameter. Maps of 9 diffusion metrics were obtained according to the above equations with BoDiLab, which is based on Python 3.7, and then showed by ITK-SNAP (http://www.itksnap.org/pmwiki/pmwiki.php). All diffusion metrics and tumor size were measured independently by two radiologists (X.Z. and C.M., who had 10 and 8 years of experience in breast MRI, respectively) in a blinded fashion. In brief, DCE-MRI images acquired at 88 s (the 11th dynamic phase) after injection of the contrast agent that showed the maximum tumor dimension were selected to identify the largest dimension of breast tumor because DCE-MRI images in the 90-s phase were considered to have optimal image contrast [31]. A free-hand region of interest (ROI) was drawn along the tumor contour on DCE-MRI images, and the maximal diameter of the tumor (tumor size) was measured. To measure the diffusion metrics, the ROI defined on DCE-MRI was used as a reference for placing ROI on the quantitative map of conventional DWI (i.e., ADC) and eight quantitative maps (i.e., αCTRW, βCTRW, DCTRW, βFROC, DFROC, μFROC, αSEM, and DDCSEM) derived from the SE-EPI sequence. The ROIs on the ADC map and the quantitative maps from SE-EPI sequence were delineated separately and were placed along the border of the tumor on the largest dimension and excluded adjacent normal mammary glands and fat tissue. The eight quantitative maps from the SE-EPI sequence were obtained from the same diffusion sequence. Thus, only one ROI was delineated on one quantitative map (e.g., αCTRW) and automatically copied to other seven quantitative maps using the copy-to-paste method on the ITK-SNAP (Figs. 2, 3, and 4).
Human epidermal growth factor receptor 2 (HER2)-zero-expressing breast cancer confirmed by surgical pathology in the right breast of a 43-year-old woman. A DCE-MRI showed a solid mass (arrow). B–I Pseudo-colorized images showed the ADC (B), αCTRW (C), DCTRW (D), βFROC (E), DFROC (F), μFROC (G), αSEM (H), and DDCSEM (I) maps derived from the conventional DWI, continuous-time random walk (CTRW), fractional order calculus (FROC), and stretched exponential model (SEM), respectively
Human epidermal growth factor receptor 2 (HER2)-low-expressing breast cancer confirmed by surgical pathology in the left breast of a 48-year-old woman. A DCE-MRI showed a solid mass (arrow). B–I Pseudo-colorized images showed the ADC (B), αCTRW (C), DCTRW (D), βFROC (E), DFROC (F), μFROC (G), αSEM (H), and DDCSEM (I) maps derived from the conventional DWI, continuous-time random walk (CTRW), fractional order calculus (FROC), and stretched exponential model (SEM), respectively
Human epidermal growth factor receptor 2 (HER2)-overexpressing breast cancer confirmed by surgical pathology in the left breast of a 49-year-old woman. A DCE-MRI showed a solid mass (arrow). B–I Pseudo-colorized images showed the ADC (B), αCTRW (C), DCTRW (D), βFROC (E), DFROC (F), μFROC (G), αSEM (H), and DDCSEM (I) maps derived from the conventional DWI, continuous-time random walk (CTRW), fractional order calculus (FROC), and stretched exponential model (SEM), respectively
Pathological evaluation
All participants underwent a biopsy or surgery. Specimens of breast tumor from each participant were processed to determine the HER2 expression status by IHC and FISH. The pathological type, histologic grade, HER2-expressing status, ER status, PR status, and Ki-67 index were assessed by a senior pathologist (Y.L., who had 11 years of experience in pathologic diagnosis of BC). The HER2-zero-expressing BC was defined as the HER2 expression score 0 on IHC; The HER2-low-expressing BC was defined as the HER2 expression score 1+ on IHC or score 2+ on IHC with FISH negative; The HER2-overexpressing status was defined as the HER2 expression score 3+ on IHC or score 2+ on IHC with FISH positive, as previously described [6,7,8,9,10]. In addition, the ER and PR statuses and Ki-67 index were assessed as previously reported [32, 33]. The cut-off values for ER and PR were 1% (negative, ≤ 1%; positive, > 1%), and the cut-off value of Ki-67 was 14% (low proliferation, < 14%; high proliferation, ≥ 14%).
Statistical analysis
Continuous variables were expressed as means ± standard deviation. The inter-class correlation coefficients (ICCs) were calculated and used for assessing the inter-observer and test-retest reliabilities in measuring those nine diffusion metrics. ICC more than 0.9 and between 0.75 and 0.9 indicated excellent and good performance, respectively. The mean values of tumor size and diffusion MRI metrics calculated by two radiologists (X.Z. and C.M., who had 10 and 8 years of experience in breast MRI, respectively) were used for analysis. As appropriate, the comparisons of diffusion metrics and clinicopathologic variables among the three groups were tested with the ANOVA test (with Bonferroni correction) and the chi-square test. Univariable and multivariable logistic regression analyses were used to select the optimal diffusion metrics for discriminating the HER2-zero-expressing, HER2-low-expressing, and HER2-overexpressing BCs. A combined model incorporating the optimal diffusion metric and significant clinicopathologic variables was established using multivariable logistic regression analysis. Receiver operating characteristic (ROC) curves were plotted, and the area under the curves (AUCs) were obtained to quantify the discriminant ability. The ROC curves were calculated and presented with two-sided 95% confidence intervals (CIs). The AUCs were compared using the DeLong test. A two-sided p < 0.05 was considered statistically significant. An adjusted p < 0.017 for pairwise comparisons among HER2-zero-expressing, HER2-low-expressing, and HER2-overexpressing groups was considered statistically significant. Statistical analyses were conducted with SPSS 26.0 and MedCalc (version 20, https://www.medcalc.org).
Results
Clinicopathologic characteristics of participants
A total of 158 women with breast invasive ductal carcinoma were included. Their clinicopathologic characteristics are shown in Table 2. Among them, 10 participants (mean age ± standard deviation, 47.5 years ± 9.5) had HER2-zero-expressing tumors, 86 participants (mean age ± standard deviation, 50.7 years ± 10.4) had HER2-low-expressing tumors, and 62 participants (mean age ± standard deviation, 47.1 years ± 11.6) had HER2-overexpressing tumors. The ER status (p < 0.001), PR status (p < 0.001), and tumor size (p = 0.01) showed statistical differences between the HER2-low-expressing and HER2-overexpressing groups, while the ER status, PR status, and tumor size showed no statistical difference between the HER2-zero-expressing and HER2-low-expressing groups (p = 0.04, Bonferroni correction; p = 0.69; p > 0.99), as well as between the HER2-zero-expressing and HER2-overexpressing groups (p = 0.74; p = 0.10; p > 0.99). No difference was found in the age (p = 0.17), menarche age (p = 0.23), menstrual history (p = 0.15), reproductive history (p = 0.21), family history (p = 0.33), lesion location (p = 0.91), histologic grade (p = 0.93), and Ki-67 index (p = 0.81) among the HER2-zero-expressing, HER2-low-expressing, and HER2-overexpressing groups.
Comparison of diffusion metrics among HER2-zero-expressing, HER2-low-expressing, and HER2-overexpressing groups
The ICCs of diffusion metrics and tumor size ranged from 0.876 to 0.960, which indicated good to excellent agreements. The comparisons of quantitative diffusion metrics among the HER2-zero-expressing, HER2-low-expressing, and HER2-overexpressing groups are shown in Table 3 and Fig. 5. The ADC (1.16 ± 0.37 × 10−3 mm2/s vs. 1.04 ± 0.26 × 10−3 mm2/s vs. 1.10 ± 0.28 × 10−3 mm2/s; p = 0.01), αCTRW (0.82 ± 0.10 vs. 0.78 ± 0.11 vs. 0.88 ± 0.06; p < 0.001), DCTRW (1.12 ± 0.32 × 10−3 mm2/s vs. 0.99 ± 0.19 × 10−3 mm2/s vs.1.16 ± 0.20 × 10−3 mm2/s; p < 0.001), βFROC (0.72 ± 0.09 vs. 0.71 ± 0.05 vs. 0.74 ± 0.04; p = 0.004), DFROC (0.79 ± 0.24 × 10−3 mm2/s vs. 0.68 ± 0.13 × 10−3 mm2/s vs. 0.82 ± 0.15 × 10−3 mm2/s; p < 0.001), μFROC (3.75 ± 0.33 mm vs. 3.82 ± 0.27 mm vs. 3.90 ± 0.21 mm; p = 0.01), αSEM (0.71 ± 0.09 vs. 0.70 ± 0.06 vs. 0.73 ± 0.04; p = 0.003), and DDCSEM (1.09 ± 0.36 × 10−3 mm2/s vs. 0.93 ± 0.22 × 10−3 mm2/s vs. 1.15 ± 0.24 × 10−3 mm2/s; p < 0.001) were significantly different among HER2-zero-expressing, HER2-low-expressing, and HER2-overexpressing groups, and no difference was found in βCTRW among them (0.78 ± 0.09 vs. 0.78 ± 0.05 vs. 0.78 ± 0.04; p = 0.73). The αCTRW, DCTRW, βFROC, DFROC, μFROC, αSEM, and DDCSEM were significantly lower in the HER2-low-expressing group than their counterparts in the HER2-overexpressing group (p < 0.001 to p = 0.01). Among nine quantitative diffusion metrics, there was no significant difference between the HER2-zero-expressing and HER2-low-expressing groups (p = 0.09 to p > 0.99), as well as between the HER2-zero-expressing and HER2-overexpressing groups (p = 0.17 to p > 0.99).
Violin graphs showing the significant quantitative metrics from four MRI diffusion models among the HER2-zero-expressing, HER2-low-expressing, and HER2-overexpressing groups (A–I). The statistically significant level, *p < 0.05. Abbreviations: CTRW, continuous-time random walk; FROC, fractional order calculus; SEM, stretched exponential model; DWI, diffusion-weighted imaging; ADC, apparent diffusion coefficient; HER2, human epidermal growth factor receptor 2; ns, no significant
Diagnostic performances of diffusion metrics and clinicopathologic features in discriminating HER2-low-expressing and HER2-overexpressing groups
ROC analyses of diffusion metrics and clinicopathologic features are shown in Table 4 and Fig. 6. Univariable logistic regression analysis showed that αCTRW, DCTRW, βFROC, DFROC, μFROC, αSEM, and DDCSEM were associated with discrimination between the HER2-low-expressing and HER2-overexpression BCs (Table 5). For the seven diffusion metrics, including αCTRW, DCTRW, βFROC, DFROC, μFROC, αSEM, and DDCSEM, the AUCs ranged from 0.610 to 0.802, with the sensitivities ranging from 53.5% (46/86) to 76.7% (66/86), the specificity ranging from 54.8% (34/62) to 83.9% (52/62), and the accuracy ranging from 61.5% (91/148) to 70.9% (105/148). Among these seven metrics, αCTRW had the highest AUC of 0.802. The AUC of αCTRW was higher than that of conventional ADC (0.802 vs. 0.610, p = 0.001). Multivariable logistic regression analysis demonstrated that the αCTRW was the single best quantitative parameter (Table 5). In addition, the combined model incorporating αCTRW and three clinicopathologic variables (ER, PR, and tumor size) achieved an AUC of 0.877 in differentiating the HER2-low-expressing and HER2-overexpressing BCs, which was higher than that of each diffusion metric alone (AUC ranged from 0.610 to 0.802, p < 0.001 to p = 0.007) or each clinicopathologic variable alone (AUC ranged from 0.643 to 0.694, all p < 0.001). The combined model had a sensitivity of 88.3% (76/86), a specificity of 80.6% (50/62), and an accuracy of 83.8% (124/148). The AUCs of ER status, PR status, and tumor size were 0.684, 0.694, and 0.643, respectively, with the sensitivities ranging from 79.1% (68/86) to 90.7% (78/86), the specificities ranging from 35.5% (22/62) to 59.7% (37/62), and the accuracies ranging from 67.6% (100/148) to 71.6% (106/148) (Table 4).
Receiver operating characteristic (ROC) curves of single diffusion metric and the combined model in discriminating human epidermal growth factor receptor 2 (HER2)-low-expressing and the HER2-overexpressing breast cancers. The combined model comprises estrogen receptor status, progesterone receptor status, tumor size, and αCTRW
Discussion
Our study demonstrated that eight diffusion metrics derived from conventional DWI, CTRW, FROC, and SEM differed between the HER2-low-expressing and the HER2-overexpressing BCs. Among these diffusion metrics, the αCTRW from the CTRW model had the highest discriminative performance (AUC = 0.802) and outperformed the ADC from conventional DWI (AUC = 0.610, p = 0.007). In addition, the combined model incorporating αCTRW and ER status, PR status, and tumor size achieved an AUC of 0.877, which was higher than that of the αCTRW (p = 0.007), ER status (AUC = 0.684, p < 0.001), PR status (AUC = 0.694, p < 0.001), or tumor size (AUC = 0.643, p < 0.001) alone.
So far, HER2-targeted therapy is the leading treatment for patients with HER2-overexpressing BC, but not for those with HER2-negative BC [34]. However, the HER2-low-expressing BC accounting for 40–50% of all kinds of BCs, which is a large subset of HER2-negative BC, might also benefit from the HER2-targeted therapy in recent views [9, 10]. For instance, a phase Ib study showed that a new HER2-targeted antibody-drug conjugate, such as the trastuzumab deruxtecan, exhibited antitumor effect in patients with HER2-low-expressing BCs, with an objective response rate of 37.0% and a median duration of response of 10.4 months [9]. Previously, T2WI and DWI have been used to determine the HER2-expressing status in BCs, yet no particular signal intensity pattern of T2WI or DWI was found to be associated with HER2-positive status [35]. Besides, the texture features of DCE-MRI identified by three machine learning classifiers have been reported to be helpful in predicting the HER2-positive status with an AUC ranging from 0.808 to 0.865 [36].
Diffusion MRI is a powerful tool for exploring biological microstructures [13,14,15,16,17]. Compared with conventional MRI, diffusion MRI models are capable of revealing intravoxel tissue cellularity, extracellular matrix, vascularity, and microstructures of tumors [37]. Four studies have shown that single or combined metrics from DWI, DTI, and diffusion spectrum imaging can predict HER2-negative and HER2-positive statuses in BCs with an AUC varying from 0.501 to 0.800 [14, 16, 17, 38]. However, it has also been demonstrated that diffusion metrics of DWI and DTI cannot be used to predict HER2 status in BCs [13, 39]. Our study showed that the HER2-low-expressing BC had a comparable ADC value compared with the HER2-overexpressing BC and the AUC of ADC was moderate (0.610) in discriminating the HER2-low-expressing and HER2-overexpressing BCs. This finding was different from the study by Park et al, where HER2-positive BC was reported to have significantly higher ADC values than HER2-negative BC [40]. In contrast, our finding was consistent with another study by Roknsharifi et al, where the ADC values were comparable in the HER2-negative and HER2-positive BCs [39]. Conventional DWI is a monoexponential model, and its quantitative parameter, the ADC value, could be affected by the highly intratumoral tissue heterogeneity [37, 41]. Collectively, the robustness of ADC derived from the conventional DWI in predicting the HER2 expression status is suboptimal, which might be due to the intratumoral tissue heterogeneity.
Unlike conventional DWI, the CTRW, FROC, and SEM can reveal the distributed diffusion effect of water molecules in the tumor and identify the presence of intravoxel heterogeneity of water molecule diffusion in space and time [26,27,28]. Suo et al showed that the αSEM was associated with tumor size and Ki-67 index in patients with BC, and DDCSEM was associated with ER status [28]. Jin et al reported that αSEM was lower in malignant breast lesions than in benign breast lesions [29]. To the best of our knowledge, there was a lack of study on the potential of the CTRW, FROC, and SEM metrics in the discrimination of the HER2-expressing status. In our study, the αCTRW, DCTRW, βFROC, DFROC, μFROC, αSEM, and DDCSEM were lower in the HER2-low-expressing BC than those in the HER2-overexpressing BC. The reduced DCTRW, DFROC, μFROC, and DDCSEM values might indicate more limited diffusion of the water molecule in the HER2-low-expressing BC than in the HER2-overexpressing BC. As the lower αCTRW, βFROC, and αSEM values indicate a less homogeneous microenvironment that water molecules diffuse through [22,23,24,25], our findings suggest more markable intratumoral heterogeneity in the HER2-low-expressing BC than in the HER2-overexpressing BC. A recent study also showed that radial diffusivity and mean diffusivity from DTI, and mean squared diffusion and q-space inverse variance from mean apparent propagator were lower in HER2-negative BC than in HER2-positive BC [17]. Comparatively, diffusion coefficients from CTRW, FROC, and SEM can better describe the diffusion movement of the water molecule in heterogeneous tumor tissue [37]. Further ROC analyses of the single metrics showed that the αCTRW had the highest performance (AUC = 0.802) in discriminating the HER2-low-expressing from the HER2-overexpressing BCs, which outperformed ADC from conventional DWI. This might be explained by the theory that αCTRW is characteristic of non-Gaussian distribution and directly reflects the underlying structural complexity and spatial heterogeneity of tumor tissue, while the DWI model was constructed according to the Gaussian distribution. Taken together, the lower values of the αCTRW, DCTRW, βFROC, DFROC, μFROC, αSEM, and DDCSEM in the HER2-low-expressing BC reflect higher intratumoral heterogeneity and restricted water molecular diffusion in the HER2-low-expressing than in the HER2-overexpressing BC. The single diffusion metric, αCTRW, can be an imaging biomarker for discriminating HER2-low-expressing or HER2-overexpressing statuses in BC patients.
In addition, three clinicopathologic variables, ER status, PR status, and tumor size, were found to be different between the HER2-low-expressing and the HER2-overexpressing BCs in our study. ROC analyses showed that these three clinicopathologic variables only have a modest discriminative ability (AUC = 0.643–0.694). Notably, when combined with αCTRW, these three clinicopathologic variables attained a higher AUC of 0.877. This finding indicates the addition of αCTRW to clinicopathologic variables can improve discriminative performance. Clinically, the HER2-low-expressing and the HER2-overexpressing statues are usually determined by biopsy. The biopsy is invasive, and there is a potential risk of inadequate sampling. Diffusion MRI is a noninvasive procedure and can provide a holistic vision of the entire tumor mass. Thus, the predictive diffusion metric, αCTRW, might be a surrogate marker for patients eligible for invasive biopsy. More importantly, these advanced diffusion models could be used to guide the proper site of biopsy to improve the accuracy of the histologic evaluation of HER2-expressing status.
This study had several limitations. First, our study was a single-center study. Further multi-center studies with a large cohort are needed to validate the discriminative performance of these diffusion-based metrics. Second, the test-retest procedure for diffusion MRI was not attempted in the same participant. According to recommendations of the Quantitative Imaging Biomarkers Alliance (QIBA), this test-retest procedure is preferred for determining the repeatability and reproducibility of quantitative MRI technology when providing biomarkers in imaging oncology [42]. Nonetheless, the test-retest procedure in real practice was limited in terms of time, cost, patient tolerance, and compliance, which were not easy to achieve. Besides, previous studies have already shown the reliability of these diffusion MRI models [18, 19, 23,24,25]. Third, a two-dimensional ROI was applied during the measurements of diffusion metrics, while the three-dimensional volume of interest (VOI) might provide more comprehensive information about the intravoxel structural heterogeneity in BC. In comparison, the delineation of a VOI was laborious and time-consuming. Drawing the VOI with artificial intelligence might help to overcome this problem. Fourth, the sample size of the HER2-zero-expressing BC was small. In this prospective study, there were only 10 participants with a HER2-zero-expressing BC and no difference was found in all the diffusion metrics from conventional DWI, CTRW, FROC, and SEM between the HER2-zero-expressing and HER2-low-expressing BCs as well as between the HER2-zero-expressing and HER2-overexpressing BCs. Such a small sample size may decreases statistical power. Future studies with a large sample size of HER2-zero-expressing BC are needed to validate the role of DWI, CTRW, FROC, and SEM in differentiating HER2-zero-expressing BCs from HER2-low-expressing and HER2-overexpressing BCs.
In conclusion, our preliminary study showed that multiple diffusion metrics derived from CTRW, FROC, and SEM MRI differed between the HER2-low-expressing and HER2-overexpressing BCs. The αCTRW from CTRW was the single best diffusion parameter in discriminating the HER2-low-expressing and HER2-overexpressing BCs. The αCTRW derived from the CTRW model could be helpful for discrimination between the HER2-low-expressing and HER2-overexpressing BCs. The addition of αCTRW to the clinicopathologic features further improves the discriminative performance. The αCTRW holds potential as an imaging biomarker to identify the HER2 expression status in BCs, which was worthy of further validation.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Area under the curve
- BC:
-
Breast cancer
- CI:
-
Confidence interval
- CTRW:
-
Continuous-time random walk
- DCE:
-
Dynamic contrast-enhanced
- DTI:
-
Diffusion tensor imaging
- DWI:
-
Diffusion-weighted imaging
- ER:
-
Estrogen receptor
- FISH:
-
Fluorescence in situ hybridization
- FOV:
-
Field of view
- FROC:
-
Fractional order calculus
- HER2:
-
Human epidermal growth factor receptor 2
- ICC:
-
Inter-class correlation coefficient
- IHC:
-
Immunohistochemistry
- MRI:
-
Magnetic resonance imaging
- PR:
-
Progesterone receptor
- QIBA:
-
Quantitative Imaging Biomarkers Alliance
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- SE-EPI:
-
Spin-echo echo-planar imaging
- SEM:
-
Stretched exponential model
- T1WI:
-
T1-weighted imaging
- T2WI:
-
T2-weighted imaging
- TE:
-
Echo time
- TR:
-
Repetition time
- VOI:
-
Volume of interest
References
Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Harbeck N, Penault-Llorca F, Cortes J et al (2019) Breast cancer. Nat Rev Dis Primers 5:66. https://doi.org/10.1038/s41572-019-0111-2
Horvat JV, Bernard-Davila B, Helbich TH et al (2019) Diffusion-weighted imaging (DWI) with apparent diffusion coefficient (ADC) mapping as a quantitative imaging biomarker for prediction of immunohistochemical receptor status, proliferation rate, and molecular subtypes of breast cancer. J Magn Reson Imaging 50:836–846. https://doi.org/10.1002/jmri.26697
Gingras I, Gebhart G, de Azambuja E, Piccart-Gebhart M (2017) HER2-positive breast cancer is lost in translation: time for patient-centered research. Nat Rev Clin Oncol 14:669–681. https://doi.org/10.1038/nrclinonc.2017.96
Modi S, Saura C, Yamashita T et al (2020) Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med 382:610–621. https://doi.org/10.1056/NEJMoa1914510
Wolff AC, Hammond ME, Hicks DG et al (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31:3997–4013. https://doi.org/10.1200/JCO.2013.50.9984
Tarantino P, Hamilton E, Tolaney SM et al (2020) HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol 38:1951–1962. https://doi.org/10.1200/JCO.19.02488
Denkert C, Seither F, Schneeweiss A et al (2021) Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol 22:1151–1161. https://doi.org/10.1016/S1470-2045(21)00301-6
Modi S, Park H, Murthy RK et al (2020) Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol 38:1887–1896. https://doi.org/10.1200/JCO.19.02318
Eiger D, Agostinetto E, Saúde-Conde R, de Azambuja E (2021) The exciting new field of HER2-low breast cancer treatment. Cancers (Basel) 13:1015. https://doi.org/10.3390/cancers13051015
Modi S, Jacot W, Yamashita T et al (2022) Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med 387:9–20. https://doi.org/10.1056/NEJMoa2203690
Jordan NV, Bardia A, Wittner BS et al (2016) HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature 537:102–106. https://doi.org/10.1038/nature19328
Onaygil C, Kaya H, Ugurlu MU, Aribal E (2017) Diagnostic performance of diffusion tensor imaging parameters in breast cancer and correlation with the prognostic factors. J Magn Reson Imaging 45:660–672. https://doi.org/10.1002/jmri.25481
Meng N, Wang X, Sun J et al (2021) A comparative study of the value of amide proton transfer-weighted imaging and diffusion kurtosis imaging in the diagnosis and evaluation of breast cancer. Eur Radiol 31:1707–1717. https://doi.org/10.1007/s00330-020-07169-x
Andreassen MMS, Rodríguez-Soto AE, Conlin CC et al (2021) Discrimination of breast cancer from healthy breast tissue using a three-component diffusion-weighted MRI model. Clin Cancer Res 27:1094–1104. https://doi.org/10.1158/1078-0432.CCR-20-2017
Vidić I, Egnell L, Jerome NP et al (2018) Support vector machine for breast cancer classification using diffusion-weighted MRI histogram features: preliminary study. J Magn Reson Imaging 47:1205–1216. https://doi.org/10.1002/jmri.25873
Mao C, Jiang W, Huang J et al (2022) Quantitative parameters of diffusion spectrum imaging: HER2 status prediction in patients with breast cancer. Front Oncol 12:817070. https://doi.org/10.3389/fonc.2022.817070
Gao A, Zhang H, Yan X et al (2022) Whole-tumor histogram analysis of multiple diffusion metrics for glioma genotyping. Radiology 302:652–661. https://doi.org/10.1148/radiol.210820
Mao J, Zeng W, Zhang Q et al (2020) Differentiation between high-grade gliomas and solitary brain metastases: a comparison of five diffusion-weighted MRI models. BMC Med Imaging 20:124. https://doi.org/10.1186/s12880-020-00524-w
Vidić I, Egnell L, Jerome NP et al (2020) Modeling the diffusion-weighted imaging signal for breast lesions in the b = 200 to 3000 s/mm2 range: quality of fit and classification accuracy for different representations. Magn Reson Med 84:1011–1023. https://doi.org/10.1002/mrm.28161
You C, Li J, Zhi W et al (2019) The volumetric-tumour histogram-based analysis of intravoxel incoherent motion and non-Gaussian diffusion MRI: association with prognostic factors in HER2-positive breast cancer. J Transl Med 17:182. https://doi.org/10.1186/s12967-019-1911-6
Karaman MM, Zhang J, Xie KL, Zhu W, Zhou XJ (2021) Quartile histogram assessment of glioma malignancy using high b-value diffusion MRI with a continuous-time random-walk model. NMR Biomed 34:e4485. https://doi.org/10.1002/nbm.4485
Zhong Z, Merkitch D, Karaman MM et al (2019) High-spatial-resolution diffusion MRI in Parkinson disease: lateral asymmetry of the substantia nigra. Radiology 291:149–157. https://doi.org/10.1148/radiol.2019181042
Sui Y, Wang H, Liu G et al (2015) Differentiation of low- and high-grade pediatric brain tumors with high b-value diffusion-weighted MR imaging and a fractional order calculus model. Radiology 277:489–496. https://doi.org/10.1148/radiol.2015142156
Bai Y, Lin Y, Tian J et al (2016) Grading of gliomas by using monoexponential, biexponential, and stretched exponential diffusion-weighted MR imaging and diffusion kurtosis MR imaging. Radiology 278:496–504. https://doi.org/10.1148/radiol.2015142173
Bickelhaupt S, Steudle F, Paech D et al (2017) On a fractional order calculus model in diffusion weighted breast imaging to differentiate between malignant and benign breast lesions detected on X-ray screening mammography. PLoS One 12:e0176077. https://doi.org/10.1371/journal.pone.0176077
Suo S, Yin Y, Geng X et al (2021) Diffusion-weighted MRI for predicting pathologic response to neoadjuvant chemotherapy in breast cancer: evaluation with mono-, bi-, and stretched-exponential models. J Transl Med 19:236. https://doi.org/10.1186/s12967-021-02886-3
Suo S, Cheng F, Cao M et al (2017) Multiparametric diffusion-weighted imaging in breast lesions: association with pathologic diagnosis and prognostic factors. J Magn Reson Imaging 46:740–750. https://doi.org/10.1002/jmri.25612
Jin YN, Zhang Y, Cheng JL, Zheng DD, Hu Y (2019) Monoexponential, Biexponential, and stretched-exponential models using diffusion-weighted imaging: a quantitative differentiation of breast lesions at 3.0T. J Magn Reson Imaging 50:1461–1467. https://doi.org/10.1002/jmri.26729
Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M (1988) Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168:497–505. https://doi.org/10.1148/radiology.168.2.3393671
Rahbar H, Partridge SC, Demartini WB et al (2012) In vivo assessment of ductal carcinoma in situ grade: a model incorporating dynamic contrast-enhanced and diffusion-weighted breast MR imaging parameters. Radiology 263:374–382. https://doi.org/10.1148/radiol.12111368
Hammond ME, Hayes DF, Dowsett M et al (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28:2784–2795. https://doi.org/10.1200/JCO.2009.25.6529
Dowsett M, Nielsen TO, A’Hern J et al (2011) Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst 103:1656–1664. https://doi.org/10.1093/jnci/djr393
Fehrenbacher L, Cecchini RS, Geyer CE Jr et al (2020) NSABP B-47/NRG oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2. J Clin Oncol 38:444–453. https://doi.org/10.1200/JCO.19.01455
Yuen S, Monzawa S, Yanai S et al (2020) The association between MRI findings and breast cancer subtypes: focused on the combination patterns on diffusion-weighted and T2-weighted images. Breast Cancer 27:1029–1037. https://doi.org/10.1007/s12282-020-01105-z
Jiang Z, Song L, Lu H, Yin J (2019) The potential use of DCE-MRI texture analysis to predict HER2 2+ status. Front Oncol 9:242. https://doi.org/10.3389/fonc.2019.00242
Tang L, Zhou XJ (2019) Diffusion MRI of cancer: from low to high b-values. J Magn Reson Imaging 49:23–40. https://doi.org/10.1002/jmri.26293
Du S, Gao S, Zhang L, Yang X, Qi X, Li S (2021) Improved discrimination of molecular subtypes in invasive breast cancer: comparison of multiple quantitative parameters from breast MRI. Magn Reson Imaging 77:148–158. https://doi.org/10.1016/j.mri.2020.12.001
Roknsharifi S, Fishman MDC, Agarwal MD, Brook A, Kharbanda V, Dialani V (2019) The role of diffusion weighted imaging as supplement to dynamic contrast enhanced breast MRI: can it help predict malignancy, histologic grade and recurrence? Acad Radiol 26:923–929. https://doi.org/10.1016/j.acra.2018.09.003
Park SH, Choi HY, Hahn SY (2015) Correlations between apparent diffusion coefficient values of invasive ductal carcinoma and pathologic factors on diffusion-weighted MRI at 3.0 Tesla. J Magn Reson Imaging 41:175–182. https://doi.org/10.1002/jmri.24519
Kim JJ, Kim JY, Suh HB et al (2022) Characterization of breast cancer subtypes based on quantitative assessment of intratumoral heterogeneity using dynamic contrast-enhanced and diffusion-weighted magnetic resonance imaging. Eur Radiol 32:822–833. https://doi.org/10.1007/s00330-021-08166-4
Shukla-Dave A, Obuchowski NA, Chenevert TL et al (2019) Quantitative imaging biomarkers alliance (QIBA) recommendations for improved precision of DWI and DCE-MRI derived biomarkers in multicenter oncology trials. J Magn Reson Imaging 49:e101–e121. https://doi.org/10.1002/jmri.26518
Funding
This study was supported by the National Natural Science Foundation of China (82102130, 12126610), Guangdong Basic and Applied Basic Research Foundation (2021A1515010385, 2023A1515011305), Guangzhou Basic and Applied Basic Research Foundation (2023A04J2112), and SKY Imaging Research Fund Project of China International Medical Foundation (Z-2014-07-1912-21).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Jun Shen.
Conflict of interest
Two of the authors (Mengzhu Wang and Xu Yan) are employees of Siemens Healthcare.
The other authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
All participants provided written informed consent.
Ethical approval
Institutional Review Board approval was obtained from the Institutional Review Board of Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University (Guangzhou, China) (SYSEC-KY-KS-2022-027).
Study subjects or cohorts overlap
No study subject or cohort has been previously reported in this study.
Methodology
• prospective
• diagnostic study
• single-center study
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mao, C., Hu, L., Jiang, W. et al. Discrimination between human epidermal growth factor receptor 2 (HER2)-low-expressing and HER2-overexpressing breast cancers: a comparative study of four MRI diffusion models. Eur Radiol 34, 2546–2559 (2024). https://doi.org/10.1007/s00330-023-10198-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10198-x