Abstract
Objectives
Higher static magnetic field (SMF) enables higher imaging capability in magnetic resonance imaging (MRI), which encourages the development of ultra-high field MRIs above 20 T with a prerequisite for safety issues. However, animal tests of ≥ 20 T SMF exposure are very limited. The objective of the current study is to evaluate mice behaviour consequences of 3.5–23.0 T SMF exposure.
Methods
We systematically examined 112 mice for their short- and long-term behaviour responses to a 2-h exposure of 3.5–23.0 T SMFs. Locomotor activity and cognitive functions were measured by five behaviour tests, including balance beam, open field, elevated plus maze, three-chamber social recognition, and Morris water maze tests.
Results
Besides the transient short-term impairment of the sense of balance and locomotor activity, the 3.5–23.0 T SMFs did not have long-term negative effects on mice locomotion, anxiety level, social behaviour, or memory. In contrast, we observed anxiolytic effects and positive effects on social and spatial memory of SMFs, which is likely correlated with the significantly increased CaMKII level in the hippocampus region of high SMF-treated mice.
Conclusions
Our study showed that the short exposures to high-field SMFs up to 23.0 T have negligible side effects on healthy mice and may even have beneficial outcomes in mice mood and memory, which is pertinent to the future medical application of ultra-high field SMFs in MRIs and beyond.
Key Points
• Short-term exposure to magnetic fields up to 23.0 T is safe for mice.
• High-field static magnetic field exposure transiently reduced mice locomotion.
• High-field static magnetic field enhances memory while reduces the anxiety level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinical magnetic resonance imaging (MRI) scanners mainly use high static magnetic fields (SMFs) and radio waves to create images of the body parts. Since increased magnetic field flux density is directly associated with higher resolution, imaging capacity, and reduced imaging time, people are trying to push the upper limit of SMFs in the MRI scanners. Although most hospitals are still using 1.5 T or 3.0 T MRI, 7 T MRI has already been approved in 2017 to be used on humans. Ultra-high field MRI (UHF-MRI), defined as imaging with magnetic fields ≥ 7 T [1], is a potential future trend for clinical MRIs because it can achieve better resolution than 3 T. Successful applications have been reported for the human studies with 9.4 T MRI [2, 3]. UHF-MRI beyond 10 T has also been recently constructed and tested on humans, including whole-body 10.5 T or 11.7 T MRI, and a head-only 11.7 T MRI [4]. Efforts are now underway for a human 14 T and even for a potential 20 T human scanner [4]. A 21.1 T MRI has been tested on rodents and got superior images [5, 6]. The development of 14 T–20 T UHF-MRI is encouraged [7].
However, although it is clear that higher SMF could enable higher tissue resolution and imaging capability, safety issues need to be carefully addressed before launching any project of UHF-MRI for humans. Relevant studies about the safety of high SMF ≥ 20 T are very rare. We have previously conducted a pilot study using 16 tumor-bearing mice to test the biosafety and anti-tumor potential of 9-h exposure to 3.7–24.5 T ultra-high SMFs [8]. Our results showed that although 3.7–24.5 T SMF exposure for 9 h did not cause severe organ damage, the mice livers showed moderate abnormalities in these tumor-bearing mice. Then, we did two large-scale systematic studies using healthy mice to examine the consequence of 1–2 hour exposure to 3.5–33.0 T SMFs [9, 10]. We examined food and water intake, body weight gain, blood cell count, blood biochemistry, and organ immunohistochemistry a few weeks after the exposure, and did not find any severe long-term effects [9, 10].
In this study, we reported the behavioural analysis of the nervous system by monitoring the short- and long-term effects after the 2-h 3.5–23.0 T SMF exposure of these 112 healthy mice by using five different behaviour tests.
Materials and methods
Static magnetic field exposure
In the SMF exposure experiment, we used an upright magnet, which provides vertical magnetic field of 23.0 T SMF at the centre, and 3.5 T–21.9 T SMFs off the centre, as described in our previous study [10]. For the mice placed in the centre position, they were exposed to 23.0 T SMF with negligible gradient, while other mice were exposed to different SMF intensity and gradient depending on the location inside the magnet. The time of SMF exposure for each mouse was ~2 h (increasing field for 5 min, constant for 2 h, and reducing field for 5 min).
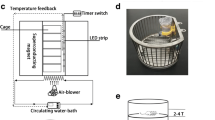
For each single experiment, there were eight mice placed inside the magnet, one in each chamber (Fig. 1a). We repeated the experiment in 6 independent days. Therefore, if we divide the subgroups strictly according to their magnetic field strength, gradient, and gradient direction, there were 6 mice per subgroup. However, if we only consider magnetic field strength and gradient, there were 12 mice at each condition, except for the 23 T and 3.5 T groups. In other words, for the mice other than 23 T and 3.5 T groups, there are two positions within the magnet that have identical magnetic field strength and gradient. The only difference is that the gradient direction is opposite in the upper and lower part of the magnet.
Experimental setup and design. a A total of 112 mice were divided into three groups: control, sham, and SMF exposure groups. Eight mice were housed in a column of individual chambers (38.5-mm diameter, 80 mm long) each time in an upright superconducting magnet. b The balance beam test and open field test were conducted within 30 min before and after magnetic field exposure experiment. The elevated plus maze test, three-chamber test, and Morris water maze test were carried out for animals from each group starting 20 days after SMF exposure experiment. T (unit of magnetic field intensity: Tesla)
The mice in the “Sham group” were also inserted into the water-cooled magnet device, which has water running to mimic the vibration and noise in the SMF exposure condition, but without electricity to produce magnetic field. The mice in the “Control” group were placed in the identical tube but left outside of the magnet. All three groups have the same air and temperature control conditions and were handled identically for the rest of the experiment.
Experimental design
A total of 112 wild-type 8-week-old C57BL/6 male healthy mice were divided into three groups: 16 mice in control, 48 mice in sham, and 48 mice in SMF-exposed group [10]. The allocation of mice to exposure groups was randomised. Due to the limitation of machine’s availability, we try to take advantage of all the spaces inside the magnet. Therefore, mice were in eight chambers in a column with different magnetic field flux density, gradient, as well as gradient direction. Since each mouse can be considered as a diamagnetic object as a whole, they are in “hypogravity-like conditions” in the upper part of the magnet and “hypergravity-like conditions” in the lower part of the magnet (Fig. 1a). “Hypogravity-like” and “hypergravity-like” refer to the conditions with altered forces in the upright direction caused by the gradient magnetic field. The exposure time was 2 h, and the short- and long-term effects of SMF exposure on mice were assessed using five behavioural tests. The balance beam test and the open field test were carried out both before and right after the SMF exposure experiments, which can reflect the “short-term” effects of SMF exposure. The elevated plus maze test, three-chamber test, and Morris water maze test were carried out between 20 and 40 days after the SMF exposure experiments, which can reflect the “long-term” effects of SMF exposure (Fig. 1b). The mice were sacrificed at the end of the whole experiment.
Due to the word limit, other materials and methods are included in the supplementary materials.
Results
Ultra-high field SMF causes transient impairment of balance and locomotion
The SMF exposure experimental design is shown in Fig. 1a and the timeline of the 5 behavioural tests is shown in Fig. 1b. The balance beam tests were performed both at 30 min before and after the SMF exposure experiment (Fig. 2a), in which the time for mice to traverse the beam was recorded. Not surprisingly, mice in the SMF-exposed group had significantly increased traverse time after exposure experiment, while both control and sham groups showed no differences (Fig. 2b). Among different SMF conditions, the increase in the traverse time of the hypergravity group and 23 T SMF-exposed group was statistically significant (Fig. 2c). Moreover, the traverse time increases seem to be more obvious in higher magnetic field (Fig. 2d and Supplementary Fig. 1), while no difference was observed in the individual group of sham condition before and after the experiment (Fig. 2e, f), which suggests that exposure to ultra-high SMF impaired the mice sense of balance.
High SMF induced transiently impaired sense of balance. a Experimental design for the balance beam test. b Traverse time of mice in control, sham, and SMF-exposed group before (“Before exposure”) and after (“After exposure”) experiment. c Traverse time of mice grouped in “hypogravity-like”, “hypergravity-like”, and 23 T conditions from the SMF-exposed group. d Traverse time of mice grouped in different magnetic intensities. e, f Traverse time of mice in the sham group. Animals were grouped according to the chambers with corresponding SMF magnetic intensity. Data are represented as mean ± SEM (Supplementary Table 1)
After the balance beam test, we immediately measured their locomotion activity with open field test (OFT) (Fig. 3a). The locomotion velocity of mice was significantly reduced by the SMF-exposure (Fig. 3b, c), especially in the hypogravity group (Fig. 3d, e). We also observed a reduction for the time spent in the centre in both the sham and SMF groups (Fig. 3f–h and Supplementary Fig. 2), suggesting that the operation vibration of the water-cooled magnet in the sham and SMF groups may have contributed to the increased anxiety level of mice.
High-field SMF exposure transiently reduced mice locomotor activity and increased anxiety-like behaviour. a Experimental design for the open field test. b Examples of mice trajectories in the open field box from control, sham, and SMF-exposed groups. c Velocity (cm/s) of mice in the open field over a 5-min session in control, sham, and SMF-exposed group before (“Before exposure”) and after (“After exposure”) experiment. d Velocity (cm/s) of mice in the open field over a 5-min session from the SMF-exposed group. e Velocity (cm/s) of mice in the open field over a 5-min session from the sham group. f Time spent in the centre arena over a 5-min session of mice in the control, sham, and SMF-exposed group before and after exposure experiment. g Time spent in the centre arena over a 5-min session from the SMF-exposed group. h Time spent in the centre arena over a 5-min session from the sham group. Data are represented as mean ± SEM (Supplementary Table 2)
Magnetic field exposure reduces the anxiety level of mice
After the above experiments, all mice in the control, sham, and SMF groups were housed in the normal condition. After 20 days, we started three behaviour experiments to evaluate their mood and cognitive function. We first used the elevated plus maze test to detect their anxiety-like behaviour (Fig. 4a). In normal conditions, mice prefer to stay in the two closed arms and will only explore the open arms occasionally. The time spent in the open arms and the number of entries into the open arms indicate the level of exploration activities, which will be suppressed when the anxiety level of mice increased [11]. However, it is very interesting that we observed the SMF-exposed mice spent more time in the open arms than the control and sham groups (Fig. 4b, c and Supplementary Fig. 3a), and their entry numbers in the open arms were also significantly higher than those of the control and the sham groups (Fig. 4d and Supplementary Fig. 3b), which indicates that SMF-exposed mice have reduced anxiety level. The mice in the hypergravity-like group have a more obvious change than the hypogravity group (Fig. 4e, f).
High-field SMF exposure reduced mice anxiety-like behaviour 20 days after SMF exposure. a A diagram of the elevated plus maze. b Representative trajectories on the maze of a control, a sham, and a SMF-exposed mouse. c Averaged time spent in open arms from all mice in each group. d The average number of entries in open arms from all mice in each group. e Averaged time spent in open arms for mice grouped in different gravity conditions of SMF-exposed group. f The number of entries in open arms for mice grouped in different “gravity” conditions of the SMF-exposed group. Data are represented as mean ± SEM (Supplementary Table 3, EPM)
Magnetic field exposure enhances mice social memory
To assess the role of ultra-high SMFs on social behaviour, we performed the three-chamber test [11,12,13,14,15] (Fig. 5a). At stage 1, all mice spent more time in the cage with a stranger mouse than in the empty cage, indicating a normal sociability (Fig. 5b). We observed longer total sniffing time for mice in the sham and SMF-exposed groups compared to the control group (Fig. 5c), indicating higher interests of interaction. However, there was no difference between the sham and SMF-exposed groups, indicating that these behaviour changes were caused by the sham condition, but not SMF exposure per se.
Improved social interests and social memory 25 days after SMF exposure. a Diagrams of the three-chamber test. b Time spent in each chamber in stage 1, the sociability test. c Time spent sniffing each cage in stage 1. d Time spent in each chamber in stage 2, the novelty test. e Time spent sniffing each cage with familiar or novel juvenile mouse. Data are represented as mean ± SEM (Supplementary Table 4)
At stage 2, all mice tested preferred the novel mouse than the familiar one, which also indicated a normal social novelty preference since mice generally prefer to sniff novel conspecific target (Fig. 5d). The difference between the time spent sniffing different target mice was most dramatic in the SMF-exposed group (Fig. 5e), indicating an enhanced social novelty preference, a consequence of enhanced social memory to discriminate novel and familiar mice.
SMF-exposed mice show improved spatial memory
We used the Morris water maze (MWM), one of the most frequently used methods to evaluate the spatial learning and memory for mice (Fig. 6a). All animals showed normal learning curve for finding the hidden platform. However, mice in the sham and SMF groups learned faster than mice in the control group (Fig. 6b, Supplementary Fig. 4). After recognising the existence and spatial location of the hidden platform (at target quadrant), we removed the platform on the probe day, and mice were released to the water maze from the opposite of the target quadrant. The latency that mice swam to the location where the platform used to be is an important indicator for spatial memory. Our results show that the SMF-exposed mice had shorter latency than mice in the sham group (Fig. 6c) while their swimming velocity had no difference (Fig. 6d).
Improved spatial memory and increased CaMKII expression in mouse hippocampus 32 days after SMF exposure. a A diagram of the Morris water maze test. b Escape latency in learning trails for 5 days with a hidden platform. c Escape latency of locating the target quadrant in the probe day without the hidden platform. d Swimming velocity of mice on the probe day. e Western blot of mice hippocampus from the control, sham, and SMF group for NR1, CaMKII, Arc, and GAPDH. f Quantification of CaMKII, Arc, and NR1 expression level normalised to GAPDH. Data are represented as mean ± SEM (Supplementary Table 3, MWM)
Since spatial memory replies on hippocampus function, we next tested several key proteins important for hippocampal function after the behaviour tests. Western blot analysis showed that the CaMKII (calcium/calmodulin-dependent protein kinase II), a key protein kinase in neural plasticity and memory [16, 17], was significantly increased in SMF-exposed mice, while there were no significant changes for NMDAR1 (NMDA receptor subunit1) and ARC (activity-regulated cytoskeleton-associated protein) (Fig. 6e, f).
It should be mentioned that in contrast to the impairment of locomotion immediately after SMF exposure, as shown by the balance beam (Fig. 2) and open field experiments (Fig. 3), we did not spot any abnormality in the locomotion 20 days after SMF exposure in the Morris water maze test (Fig. 6d, Supplementary Fig. 4). Moreover, the moving distance and velocity between control and SMF-exposed animals did not show differences in the three-chamber test either (Supplementary Fig. 5).
Discussion
Our study shows that 2-h exposure of 3.5–23.0 T SMFs caused a transient reduction in the sense of balance and suppression of locomotor activity. However, they did not cause long-term damage in locomotor activity, social behaviour, learning, or memory. On the contrary, we observed reduced anxiety and enhanced social and spatial memory.
Side effects of high static magnetic field
The temporary balance impairment we observed is consistent with the well-known acute side effects of MRI on human bodies, such as dizziness and nausea [18,19,20]. This is common for people subjected to high-field MRI and the symptoms usually disappear a few hours after MRI examination [18], which is largely because magnetic field stimulates the vestibular sensors in the inner ear [21,22,23]. Houpt et al. compared different head orientations of mice in a 14.1 T magnet for 15 min and found that the SMF exposure–induced circular swimming is head orientation-dependent, which had mechanistic implications for modelling SMF interactions with the vestibular apparatus of the inner ear [24]. The transient reduction in locomotion we observed after SMF exposure is possibly due to the interaction between magnetic field and the vestibular system.
It should be mentioned that both magnetic field flux density and exposure time are key factors that determine whether the ultra-high field exposure will cause side effects. A recent work by Tkáč et al shows that C57BL/6 mice will have long-term impaired vestibular system after 48-h exposure to 16.4 T (3 h/each time, 2 times/week, 8 weeks in total), but not 24-h exposure to 10.5 T (3 h/each time, 2 times/week, 4 weeks in total), which is evidenced by locomotor behaviour changes [25]. We did not observe such consequences in our study, which is likely because our exposure time is limited to 2 h, which is already much longer than the regular MRI examination time.
Beneficial effects of high static magnetic field
TMS (transcranial magnetic stimulation), a non-invasive procedure that uses non-static magnetic fields to stimulate nerve cells in the brain, has therapeutic effects in anxiety and depression [26,27,28,29,30,31], as well as affecting human decisions of using left- or right-hand choices [32]. However, the beneficial effects of SMFs in reducing anxiety and promoting social and spatial memory have never been reported before until recently [33]. Although our analysis shows that CaMKII is significantly elevated in SMF-treated mice, indicating the possible molecular mechanism for the observed phenotypes, more investigation in depth on the cellular and circuit level changes after SMF exposure need to be carried out, and people could investigate the effect of high SMFs on mice with mood or memory disorders to get comprehensive understanding.
Sham condition–induced effects
The sham condition we used completely mimicked the noise and subtle vibration environment in the SMF group. In fact, we think the noise and subtle vibration of the magnet are similar to the clinical MRI environment that patients also experience. For the short-term effects, we found that this environment did not affect the balance or mobility, but reduced the time that mice stayed at the centre in the open field test, indicating a slightly higher level of anxiety. However, in the elevated plus maze experiment performed 20 days after SMF exposure, the SMF-exposed group showed reduced anxiety compared to the sham group. For the long-term effects, it is interesting that this special environment also slightly increased CaMKII level in mice hippocampus and their social preference. In fact, it has been reported previously that whole-body vibration can improve brain function [34], which is consistent with the phenomenon that the sham group mice showed some beneficial long-term effects. For example, in the first five training days of the Morris maze test, both sham and SMF group mice learned faster than the control group. In the three-chamber test, the mice in the sham group sniffed the stranger mouse more than the control, indicating better sociability. On the other hand, in the open field test right after the experiment, the sham group mice showed transiently reduced locomotor activity and increased anxiety-like behaviours. For now, we still do not know the exact reason for these observations, which deserves more investigation, including the vibration and noises caused by machine operation.
Study limitations
The major limitation of our study is the small sample size. However, although there are very limited reports about mice behaviour studies of high SMFs above 10 T, all of them have used very small sample size. This is a common limitation for these explorative mice experiments for all high magnetic field labs, which is mainly due to the high cost, limited bore size, and limited machine time available for the ultra-high magnets. For example, in 2009, Cason et al. also used only 6 mice in each group to test the effect of 14.1 T SMFs for their effect on female rats [35]. In 2013, Houpt et al. tested mice swimming after exposure to 14.1 T SMF at the United States National High Magnetic Field Laboratory at Florida State University. They used 30 mice in total, 6 mice in each group for different exposure angles [24]. In 2019, Wang et al. also placed 6 mice at each magnetic field exposure condition [36].
It should also be mentioned that for the difference between the upper part and the lower part of the magnet, the magnetic field gradient directions are opposite, which produce “hypogravity-like” and “hypergravity-like” conditions, respectively. However, we found that among all five studies, only the balance beam test gave significant difference between these two conditions. None of the long-term behaviour tests showed significant differences between them. However, this point needs to be further validated with more experiments in the future.
In addition, although our results provide important information for the development of UHF-MRI, we did not address the effect of radiofrequency waves, which is another key aspect for the biosafety of UHF-MRI. At last, another limitation of our study is that we used an upright magnet, which provides vertical magnetic field, but not horizontal magnetic field as in most clinical MRIs.
In conclusion, the absence of long-term damage in our study indicates that high SMFs of 3.5–23 T within this range are relatively safe for healthy mice. Our results indicate that short exposure to ultra-high SMF may even have beneficial effects on mice mood and memory, which is likely relevant to the elevated level of CaMKII in the mice hippocampus in SMF-treated mice. Although mice studies do not guarantee the same results will appear on human bodies, and more investigations are needed to evaluate the safety issues comprehensively, our current study provides important references for the future development of UHF-MRI above 20 T.
Abbreviations
- Arc:
-
Apoptosis repressor with CARD
- CaMKII:
-
Ca2+/calmodulin-dependent protein kinase II
- cm/sec:
-
Average velocity
- EPM:
-
Elevated plus maze
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- IOD:
-
Integrated optical density
- MRI:
-
Magnetic resonance imaging
- MWM:
-
Morris water maze
- NMDAR1:
-
N-Methyl-d-aspartate receptor subunit 1
- OFT:
-
Open field test
- SDS:
-
Sodium dodecyl sulfate
- SMF:
-
Static magnetic field
- T:
-
Unit of magnetic field intensity: Tesla
- TBST:
-
Tris-buffered saline with Tween
- TMS:
-
Transcranial magnetic stimulation
- UHF-MRI:
-
Ultra-high field MRI
- USTC:
-
University of Science and Technology of China
References
Polimeni JR, Uludağ K (2018) Neuroimaging with ultra-high field MRI: present and future. Neuroimage 168:1–6
Ugurbil K, Garwood M, Moortele PF et al (2006) 9.4 T human MRI: preliminary results. Magn Reson Med 56(6):1274–1282
Henning A, Graaf R, Feyter H et al (2021) Deuterium metabolic imaging in the human brain at 9.4 Tesla with high spatial and temporal resolution. Neuroimage 244(1):118639
Nowogrodzki A (2018) The world’s strongest MRI machines are pushing human imaging to new limits. Nature 563(7729):24–26
Nagel AM, Umathum R, Rösler MB et al (2016) (39) K and (23) Na relaxation times and MRI of rat head at 21.1 T. NMR Biomed 29(6):759–766
Schepkin VD, Bejarano FC, Morgan T, Gower-Winter S, Ozambela M Jr, Levenson CW (2012) In vivo magnetic resonance imaging of sodium and diffusion in rat glioma at 21.1 T. Magn Reson Med 67(4):1159–1166
Budinger TF, Bird MD (2018) MRI and MRS of the human brain at magnetic fields of 14T to 20T: technical feasibility, safety, and neuroscience horizons. Neuroimage 168:509–531
Tian X, Wang Z, Zhang L et al (2018) Effects of 3.7 T-24.5 T high magnetic fields on tumor-bearing mice. Chinese Phys B 27(11):118703
Tian X, Lv Y, Fan Y, et al (2020) Safety evaluation of mice exposed to 7.0–33.0 T high static magnetic fields. J Magn Reson Imaging. https://doi.org/10.1002/jmri.27496.
Tian X, Wang D, Feng S et al (2019) Effects of 3.5-23.0 T static magnetic fields on mice: a safety study. Neuroimage 199:273–280
Silverman JL, Yang M, Lord C, Crawley JN (2010) Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci 11(7):490–502
Hsiao EY, McBride SW, Hsien S et al (2013) Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155(7):1451–1463
Semple BD, Canchola SA, Noble-Haeusslein LJ (2012) Deficits in social behavior emerge during development after pediatric traumatic brain injury in mice. J Neurot 29(17):2672–2683
Crawley JN (2004) Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment Retard Dev D R 10(4):248–258
Gonzales EL, Yang SM, Choi CS et al (2015) Repeated neonatal propofol administration induces sex-dependent long-term impairments on spatial and recognition memory in rats. Biomol Ther 23(3):251–260
Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER (1996) Control of memory formation through regulated expression of a CaMKII transgene. Science 274(5293):1678–1683
Zalcman G, Federman N, Romano A (2018) CaMKII isoforms in learning and memory: localization and function. Front Mol Neurosci 11:445
Ward B, Zee D (2016) Dizziness and vertigo during MRI. New Engl J Med 375(21):e44
Schaap K, Christopher-de Vries Y, Mason CK, de Vocht F, Portengen L, Kromhout H (2014) Occupational exposure of healthcare and research staff to static magnetic stray fields from 1.5-7 Tesla MRI scanners is associated with reporting of transient symptoms. Occup Environ Med 71(6):423–429
Schaap K, Portengen L, Kromhout H (2016) Exposure to MRI-related magnetic fields and vertigo in MRI workers. Occup Environ Med 73(3):161–166
Pais-Roldán P, Singh AP, Schulz H, Yu X (2016) High magnetic field induced otolith fusion in the zebrafish larvae. Sci Rep 6:24151
Roberts DC, Marcelli V, Gillen JS, Carey JP, Della Santina CC, Zee DS (2011) MRI magnetic field stimulates rotational sensors of the brain. Curr Biol 21(19):1635–1640
Straumann D, Bockisch CJ (2011) Neurophysiology: vertigo in MRI machines. Curr Biol 21(19):R806–R807
Houpt TA, Kwon B, Houpt CE, Neth B, Smith JC (2013) Orientation within a high magnetic field determines swimming direction and laterality of c-Fos induction in mice. Am J Phys Regul Integr Comp Phys 305(7):R793–R803
Tkáč I, Benneyworth MA, Nichols-Meade T et al (2021) Long-term behavioral effects observed in mice chronically exposed to static ultra-high magnetic fields. Magn Reson Med 86(3):1544–1559
Reardon J, Solvason BH, Janicak PG et al (2007) Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry 62(11):1208–1216
Zwanzger P, Steinberg C, Rehbein MA et al (2014) Inhibitory repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex modulates early affective processing. Neuroimage 101:193–203
Luber BM, Davis S, Bernhardt E et al (2017) Using neuroimaging to individualize TMS treatment for depression: toward a new paradigm for imaging-guided intervention. Neuroimage 148:1–7
Pascual-Leone A, Rubio B, Pallardo F, Catala MD (1996) Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet 348(9022):233–237
George MS, Wassermann EM, Williams WA et al (1995) Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport 6(14):1853–1856
Pirmoradi M, Dolatshahi B, Rostami R, Mohammadkhani P, Dadkhah A (2017) The changes of social performance with transcranial magnetic stimulation (TMS) in depressed patients. Eur Psychiatry 41(S1):S375–S376
Oliveira FT, Diedrichsen J, Verstynen T, Duque J, Ivry RB (2010) Transcranial magnetic stimulation of posterior parietal cortex affects decisions of hand choice. Proc Natl Acad Sci U S A 107(41):17751–17756
Lv Y, Fan Y, Tian X, et al (2021). A 33 T The anti-depressive effects of ultra-high static magnetic field. J Magn Reson Imaging. https://doi.org/10.1002/jmri.28035
Boerema AS, Heesterbeek M, Boersma SA et al (2018) Beneficial effects of whole body vibration on brain functions in mice and humans. Dose Response 16(4):1559325818811756
Cason AM, Kwon B, Smith JC, Houpt, T. A. (2009) Labyrinthectomy abolishes the behavioral and neural response of rats to a high-strength static magnetic field. Physiol Behav 97(1): 36–43
Wang S, Luo J, Lv H et al (2019) Safety of exposure to high static magnetic fields (2 T-12 T): a study on mice. Eur Radiol 29(11):6029–6037
Acknowledgements
We would like to thank the staff members in the High Magnetic Field Laboratory, Chinese Academy of Sciences, for their technical assistance, and Shu-tong Maggie Wang for cartoon illustration.
Funding
This study has received funding from the National Key R&D Program of China (2016YFA0400900) to Xin Zhang and Tian Xue; National Natural Science Foundation of China (U21A20148), the CASHIPS Director’s Fund (BJPY2021A06, 2021YZGH04) to Xin Zhang; National Natural Science Foundation of China (52007185) to Xiaofei Tian; National Natural Science Foundation of China (U20A2017 and 32071020), Guangdong Basic and Applied Basic Research Foundation (2020B1515120014), and Shenzhen Key Laboratory of Drug Addiction (ZDSYS20190902093601675) to Jin Bao; and CAS Project for Young Scientists in Basic Research (YSBR-013) to Tian Xue and international collaboration grant (211134KYSB20190122) to Jin Bao.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Xin Zhang.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Approval from the institutional animal care committee was obtained.
Ethics approval
Institutional review board approval was obtained.
Study subjects or cohorts overlap
Some study subjects have been previously reported in Neuroimage. 2019. 199. 273-280.
Methodology
• experimental
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 8660 kb)
Rights and permissions
About this article
Cite this article
Khan, M.H., Huang, X., Tian, X. et al. Short- and long-term effects of 3.5–23.0 Tesla ultra-high magnetic fields on mice behaviour. Eur Radiol 32, 5596–5605 (2022). https://doi.org/10.1007/s00330-022-08677-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08677-8