Abstract
Objectives
To compare the incidence of treatment-related necrosis between combination SRS+ICI therapy and SRS therapy alone in patients with brain metastases from melanoma and non-small cell lung cancer (NSCLC).
Methods
A systematic literature search of Ovid-MEDLINE and EMBASE was performed up to August 10, 2020. The difference in the pooled incidence of treatment-related necrosis after SRS+ICI or SRS alone was evaluated. The cumulative incidence of treatment-related necrosis at the specific time point after the treatment was calculated and plotted. Subgroup and meta-regression analyses were additionally performed.
Results
Sixteen studies (14 on melanoma, 2 on NSCLC) were included. In NSCLC brain metastasis, the reported incidences of treatment-related necrosis in SRS+ICI and SRS alone ranged 2.9–3.4% and 0–2.9%, respectively. Meta-analysis was conducted including 14 studies on melanoma brain metastasis. The incidence of treatment-related necrosis was higher in SRS+ICI than SRS alone (16.0% vs. 6.5%; p = 0.065; OR, 2.35). The incidence showed rapid increase until 12 months after the SRS when combined with ICI therapy (14%; 95% CI, 8–22%) and its pace of increase slowed thereafter. Histopathologic diagnosis as the reference standard for treatment-related necrosis and inclusion of only symptomatic cases were the source of heterogeneity in SRS+ICI.
Conclusions
Treatment-related necrosis tended to occur 2.4 times more frequently in the setting of combination SRS+ICI therapy compared with SRS alone in melanoma brain metastasis showing high cumulative incidence within the first year. Treatment-related necrosis should be considered when SRS+ICI combination therapy is used for melanoma brain metastasis, especially in the first year.
Key Points
• Treatment-related necrosis occurred 2.4 times more frequently in the setting of combination SRS+ICI therapy compared with SRS alone in melanoma brain metastasis.
• Treatment-related necrosis more frequently occurred in brain metastases from melanoma than NSCLC.
• Reference standard for treatment-related necrosis and inclusion of only symptomatic treatment-related necrosis were a significant source of heterogeneity, indicating varying definitions of treatment-related necrosis in the literature need to be unified.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Brain metastases develop in approximately 40% of adult cancer patients [1, 2]. The most common primary malignancies that result in brain metastases are lung cancer and melanoma [3]. Non-small cell lung cancer (NSCLC) has the higher prevalence of the two malignancies. At diagnosis, 16–27% of patients with NSCLC have brain metastases while the 5-year cumulative incidence of brain metastasis in patients with NSCLC has been reported at 20–25% [4, 5]. Melanoma has a high tendency to metastasize to the brain: 28% of patients with metastatic melanoma have brain metastases at the time of diagnosis [5] while the reported incidence in autopsy cases has been up to 75% [6,7,8]. In the USA, approximately 50,000 patients with NSCLC or melanoma develop brain metastases every year [9].
Radiation therapy is one of the most commonly used therapies for brain metastases, as recommended in National Comprehensive Cancer Network (NCCN) guidelines, in part because few systemic agents penetrate the blood-brain barrier [10]. The most significant toxicity of brain-directed stereotactic radiosurgery (SRS) is treatment-related necrosis. Approximately 30% of treated lesions increase in size and change their pattern of contrast enhancement as a response to SRS, with a peak at 12–18 months after radiation therapy [11]. Recently, several studies demonstrated the synergistic effect of systemic immune check point inhibitor (ICI) therapeutics in patients treated with SRS [12,13,14]. However, published literature have also suggested an association between the use of ICI and development of treatment-related necrosis [15, 16].
We performed a systematic review and meta-analysis to evaluate and compare the incidence of treatment-related necrosis between combination SRS+ICI therapy and SRS therapy alone in patients with brain metastases from NSCLC and melanoma.
Materials and methods
This study was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [17].
Search strategy and eligibility criteria
A literature search of the MEDLINE/PubMed and EMBASE databases was conducted using pertinent MeSH or EMTREE terms with common keywords for relevant articles until August 10, 2020. The search terms were as follows: ((brain metasta*) AND ((treatment-related necrosis) OR (radionecrosis)) AND ((immunotherapy) OR (immune checkpoint inhibitor) OR (immune checkpoint blockade) OR (CTLA4) OR (CTLA-4) OR (PD1) OR (PD-1) OR (PD-L1) OR (ipilimumab) OR (nivolumab) OR (pembrolizumab))). The search was not limited by language, human, animal study, or publication date.
After eliminating duplicates, articles were screened on the basis of the title and abstract. Full-text articles were assessed according to the following eligibility criteria: (a) population: patients with brain metastasis from melanoma or NSCLC; (b) intervention: SRS with ICI; (c) comparator(s)/control: SRS alone; (d) outcomes: incidence of treatment-related necrosis; (e) study design: not limited. We excluded studies that met any of the following criteria: (a) review; (b) case reports or case series including fewer than 10 patients; (c) conference abstracts; (d) letters, editorials, reply, and comments; (e) animal studies; (f) studies on pediatric patients; (g) studies with a partially overlapping patient cohort (for studies with an overlapping study population, the study with the largest population was selected). The literature search and criteria application were conducted independently by two authors (P.H.K. and C.H.S., with 3 and 7 years of experience in performing systematic reviews and meta-analyses, respectively). Any discrepancies were resolved via consensus meeting.
Data extraction and quality assessment
A standardized extraction form was used to obtain the following information from the selected studies: (a) study characteristics: institution and nation, recruitment period, study design (retrospective vs. prospective); (b) demographic and clinical characteristics: primary malignancy (melanoma vs. NSCLC), number of treated patients/lesions, age and sex distribution of the study population, follow-up period; (c) characteristics associated with the diagnosis of treatment-related necrosis: reference standard (clinical and radiologic diagnosis vs. histopathologic diagnosis), clinicoradiologic definition of treatment-related necrosis, presence vs. absence of associated symptoms; (d) characteristics associated with treatment: treatment used (SRS+ICI vs. SRS), ICI used (e.g., ipilimumab, pembrolizumab, and nivolumab), dose of ICI, timing of ICI (e.g., before SRS, after SRS, or concurrent use), mode of RT, dose, fraction, and gross target volume; (e) characteristics associated with outcome: incidence of treatment-related necrosis, interval from the treatment to the diagnosis of treatment-related necrosis if available. The quality of evidence in the included studies was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system [18, 19]. The GRADE system rates the quality of evidence from very low to high based on study design, risk of bias, imprecision, inconsistency, indirectness, magnitude of effect, dose-response relationship, and consideration of all plausible residual confounders. The data extraction and quality assessment were conducted independently by two authors (P.H.K. and C.H.S., with 3 and 7 years of experience in performing systematic reviews and meta-analyses, respectively). Any discrepancies were resolved via consensus meeting.
Data synthesis and analysis
The primary study outcome of this meta-analysis was the difference in the incidence of treatment-related necrosis after the use of combination SRS+ICI therapy vs. SRS therapy alone. The incidences of treatment-related necrosis reported in each included study were pooled separately based upon the treatment groups. Meta-analytic pooling was based on the inverse variance method for calculating weights so as to give more importance to larger studies, and the pooled estimates with their 95% confidence intervals (CI) were determined using DerSimonian–Laird random-effects modeling. In addition, between-study heterogeneity was assessed using the Q test and I2 statistic, with I2 > 50%, indicating the presence of heterogeneity [20,21,22]. Publication bias was evaluated using the funnel plot and Egger’s test [23, 24]. Publication bias-adjusted estimates were also obtained using the trim and fill method if significant publication bias was noted. Indirect comparisons were then made between the treatment groups, and odds ratio (OR) with 95% CI was additionally calculated. Direct comparison, which only included studies providing direction comparison between SRS+ICI and SRS groups, was also performed, also expressed as ORs with 95% CI.
In addition, the cumulative incidence of treatment-related necrosis at the specific time point after the treatment (including 95% CIs) was calculated and plotted. For this, Engauge Digitizer (Version 10.7; http://markummitchell.github.io/engauge-digitizer) was used to extract cumulative incidence of treatment-related necrosis at the specific time point based on the Kaplan-Meier (KM) curves provided in the study if available. Then, the cumulative incidence at each specific time point was meta-analytically pooled using the restricted maximum-likelihood estimation of random-effects model.
Subgroup and meta-regression analyses were additionally performed to identify the source of heterogeneity and to demonstrate the incidence of treatment-related necrosis in each subgroup. In the analysis, the study in which both clinical and radiologic and histopathologic diagnoses were used as the reference standard was categorized into clinical and radiologic diagnosis subgroups.
Statistical analyses were performed using R software (version 3.1.2; R Foundation for Statistical Computing) with the “meta” and the “metafor” packages, and line graphs were drawn with the “ggplot2” package. In the indirect comparison and meta-regression analysis, p < 0.05 was considered statistically significant.
Results
Literature search
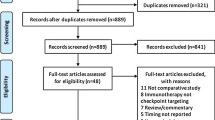
A flowchart of the publication selection process is summarized in Fig. 1. A total of 159 non-duplicated studies were identified. Of these, 124 articles were excluded on the basis of their titles and abstract because of the following reasons: (a) conference abstract (n = 83); (b) not in the field of interest (n = 67); (c) reviews (n = 41); (d) case reports (n = 5); (e) editorials (n = 5); (f) animal studies (n = 3); (g) reply (n = 2); and (h) studies on pediatric population (n = 1). Subsequently, 35 potentially eligible articles were assessed according to the eligibility criteria, and 19 additional studies were excluded because of the following reasons: (a) articles reporting incidence of treatment-related necrosis but including target primary tumor (i.e., melanoma and NSCLC) and non-target tumor in an inseparable way (n = 8); (b) articles not reporting the incidence of treatment-related necrosis (n = 6); (c) studies with a partially overlapping patient cohort (n = 4); and (d) articles reporting the incidence of treatment-related necrosis but including SRS+ICI and SRS-alone groups in an inseparable way (n = 1). Consequently, a total of 16 studies including 16 cohorts using SRS+ICI [12,13,14, 16, 25,26,27,28,29,30,31,32,33,34,35,36] and 8 cohorts using SRS [12, 14, 16, 25, 26, 29, 31, 35] met the eligibility criteria and were included in the analysis.
Characteristics of the included studies
The detailed study and patient characteristics are summarized in Table 1. All included studies were conducted as a retrospective design, and two studies were conducted as multicenter studies [13, 28]. All but three studies were conducted in the USA [12,13,14, 16, 25,26,27, 29, 31, 33,34,35,36]. Primary tumors of the brain metastases were melanoma in 14 studies [13, 14, 16, 25,26,27,28,29,30,31,32,33,34, 36], and NSCLC in the other two studies [12, 35]. Fourteen of 16 studies conducted the per-patient analysis [12, 13, 16, 25,26,27,28,29,30, 32,33,34,35,36], and the other two studies conducted the per-lesion analysis [14, 31].
The detailed characteristics associated with the diagnosis of treatment-related necrosis are summarized in Table 2. Reference standards for the diagnosis of treatment-related necrosis were variable and variably reported. The histopathologic diagnosis was used as the reference standard in four studies [14, 33,34,35], and the clinical and radiologic diagnosis was used as the reference standard in seven studies [12, 13, 16, 27, 29, 30, 32]. In four studies, both histopathologic diagnosis and the clinical and radiologic diagnosis was used as the reference standard [16, 28, 31, 36]. The clinical and radiologic definition of treatment-related necrosis is highly variable across the studies. Ten studies defined clinicoradiologic treatment-related necrosis [12, 13, 16, 27,28,29,30,31,32, 36], and the most common element for the diagnosis of treatment-related necrosis was stable or improved radiographic findings during the follow-up, which was used in seven studies [13, 16, 27, 28, 30, 31, 36] (Table 2). However, only two studies elucidated the exact follow-up period for the diagnosis (“6 months” in Minniti et al [13] and “two consecutive scans” in Pires da Silva I et al [28]). Furthermore, only two studies specified in the definition that the location of the treatment-related necrosis was the same location previously irradiated [13, 29]. No studies specified the minimum interval between the radiation and necrosis for the diagnosis. Two studies mentioned conventional MR features indicating necrosis in their definitions (e.g., enhancing lesion with a central area of necrosis, peripheral enhancement, and central hypointensity) [28, 30]. Six studies [27,28,29,30,31,32] used advanced MRI techniques for the diagnosis, including perfusion imaging [27, 29,30,31,32] and MR spectroscopy [29, 32]. Two studies used FDG-PET for the diagnosis of the treatment-related necrosis [28, 31]. The information regarding symptomatic vs. asymptomatic treatment-related necrosis were available in 13 studies [12,13,14, 16, 25, 27,28,29,30, 32, 33, 35, 36] and the proportion of symptomatic treatment-related necrosis ranged 25–100%, and seven studies only included symptomatic treatment-related necrosis [12,13,14, 25, 32, 33, 35]. The median follow-up period ranged 5–31.6 months.
The treatment characteristics are summarized in Table 3. Maximum interval between SRS and ICI to define SRS+ICI combination therapy was described in seven studies, ranging from 2 to 3 days to 1 year [12, 13, 28,29,30, 33, 36]. Among the included studies, eight studies used only SRS [12,13,14, 26, 29, 30, 33, 35], and the other eight studies used also whole-brain radiation therapy or fractionated stereotactic radiotherapy for their study population [16, 25, 27, 28, 31, 32, 34, 36]. The types of ICI used were available in all except one study. Ipilimumab was only used in six studies [14, 25,26,27, 29, 34], and pembrolizumab was only used in two studies [30, 32]. The other six studies used two or more ICIs for their study population [12, 13, 16, 28, 35, 36].
Quality assessment
Since all studies were conducted as a retrospective fashion, all were initially rated with a low certainty rate. In the risk of bias domain, two studies were downrated as they performed per-lesion analysis [14, 31]. In the inconsistency domain, one study was downrated because of a large difference of reported incidence compared to other studies [29]. Consequently, the quality of evidence was low in 13 [12, 13, 16, 25,26,27,28, 30, 32,33,34,35,36], and very low in three studies [14, 29, 31].
Incidence of treatment-related necrosis
In melanoma brain metastasis including 14 studies [13, 14, 16, 25,26,27,28,29,30,31,32,33,34, 36], the incidences of treatment-related necrosis in SRS and SRS+ICI group were 6.5% (95% CI, 2.4–16.1%) and 16.0% (95% CI, 11.2–22.4%), respectively. In NSCLC brain metastasis including two studies [12, 35], the incidences of treatment-related necrosis in SRS and SRS+ICI group ranged 2.9–3.4% and 0–2.9%, respectively. Meta-analytic pooling was not conducted because of the small number of included studies.
Considering the difference of incidence of treatment-related necrosis and only two studies available on NSCLC brain metastasis, further analyses were performed only for 14 studies on melanoma brain metastasis. In melanoma brain metastasis, the incidence of treatment-related necrosis based on random-effects modeling was higher in the SRS+ICI group compared to SRS, with borderline significance (16.0% vs. 6.5%; p = 0.065; OR, 2.35 [95% CI, 0.948–5.818]; Fig. 2). There was no significant publication bias noted in the SRS group (p = 0.918), but significant publication bias was noted in the SRS+ICI group (p = 0.010). After adjusting for publication bias using the trim and fill method, the pooled incidence of treatment-related necrosis in the SRS+ICI group increased moderately to 21.3% (95% CI, 14.8–29.6%). There was substantial heterogeneity (I2 > 50%) observed in both treatment groups. The difference was not demonstrated in the direct comparison (OR, 0.84; 95% CI, 0.20–3.58; p = 0.816; Fig. 3).
In addition, the incidences of treatment-related necrosis at the specific time points were pooled including the four studies which provided the KM curves demonstrating the cumulative incidence of treatment-related necrosis across the follow-up [16, 28, 29, 36]. All four studies assessed the incidence of treatment-related necrosis in patients with melanoma brain metastasis with the clinical and radiologic diagnosis as the reference standard. The proportion of symptomatic treatment-related necrosis ranged from 54 to 75%. The median follow-up period of four studies ranged 7.3 to 26.3 months. Consequently, cumulative incidences of treatment-related necrosis 3 and 6 months and 1, 2, and 3 years after the use of SRS+ICI in patients with melanoma brain metastasis were 3% (95% CI, 1–6%), 6% (95% CI, 2–12%), 14% (95% CI, 8–22%), 19% (95% CI, 15–24%), and 22% (95% CI, 17–26%) (Fig. 4). The pooled incidence was slightly higher at the first year, but did not reach statistical significance compared to that at the second (p = 0.268) or third year (p = 0.137). One study provided the KM curves demonstrating cumulative incidence of treatment-related necrosis after SRS [29]; the 6-month cumulative incidence was not statistically different between the SRS+ICI and SRS groups (6% [95% CI, 2–12%] vs. 5.9% [95% CI, 2–21%]; p = 0.888).
Subgroup and meta-regression analysis
The results of the subgroup and meta-regression analyses are summarized in Table 4. Of note, reference standard for treatment-related necrosis was identified as the source of heterogeneity in the SRS+ICI group. The incidence of treatment-related necrosis was significantly higher when clinical and radiologic diagnosis was used as the reference standard compared to histopathologic diagnosis as the reference standard (19.3% vs. 5.2%; p = 0.014). When histopathologic diagnosis was used as the reference standard, the incidence of treatment-related necrosis was not statistically different between the SRS+ICI and SRS groups (5.2% vs. 2.3%; p = 0.408). The incidence of treatment-related necrosis was higher in the SRS+ICI group when the clinical and radiologic diagnosis was used as the reference standard, but did not reach statistical significance (19.3% vs. 8.3%; p = 0.115).
Symptomatic vs. asymptomatic treatment-related necrosis was also identified as the source of heterogeneity in the SRS+ICI group. The incidence of treatment-related necrosis was significantly lower when analysis was restricted to the studies only including symptomatic treatment-related necrosis compared to that restricted to the studies including both symptomatic and asymptomatic (8.9% vs. 27.9%; p < 0.001). The trend was also observed in the SRS group, but did not reach statistical significance (5.5% vs. 10.1%; p = 0.603). Publication year and method of analysis were also identified as the source of heterogeneity in the SRS+ICI group, but the higher incidence of treatment-related necrosis in the SRS+ICI group remained in each subgroup except in the subgroup using per-lesion analysis. Other covariates, including mode (SRS alone vs. SRS combined with whole brain or fractionated stereotactic radiotherapy) of SRS, were not identified as a significant source of heterogeneity.
Discussion
This meta-analysis demonstrated that the incidence of treatment-related necrosis was higher with the borderline significance when using SRS plus ICI combination therapy than SRS therapy alone for the treatment of melanoma brain metastases (16.0% vs. 6.5%; p = 0.065; OR, 2.35). Treatment-related necrosis more frequently occurred with melanoma than NSCLC.
The incidence of treatment-related necrosis showed rapid increase until 12 months after the SRS treatment when combined with ICI therapy (14%; 95% CI, 8–22%) and its pace of increase slowed thereafter. In the subgroup and meta-regression analysis, the reported incidence of treatment-related necrosis was lower when histologic diagnosis was used as the reference standard compared to clinical and radiologic diagnosis and when only symptomatic treatment-related necrosis was included in the SRS+ICI group. The incidence of treatment-related necrosis in the SRS+ICI group remained higher when stratified by publication year and method of analysis, except in the subgroup using per-lesion analysis, which were identified as sources of data heterogeneity.
SRS+ICI combination therapy has been increasingly adopted since several studies demonstrated a high local efficacy for the treatment of brain metastasis [12, 25, 30, 35, 37,38,39]. However, it has remained controversial whether treatment-related necrosis, one of the most important toxicities of SRS, occurs more frequently when combined with ICI therapy [40]. Our study showed that treatment-related necrosis more frequently developed in patients who received SRS+ICI therapy than in those treated with SRS alone. The higher incidence of treatment-related necrosis in the SRS+ICI group was observed in all subgroup analyses, indicating that these results are robust. This higher incidence could be explained by the hypothesis that radiation induces immune activation targeting not only tumor but also peritumoral brain tissue [41,42,43]. In particular, for melanoma brain metastases, treatment-related necrosis based on clinical and radiologic diagnosis developed in a considerable portion of patients, approximately 20% at the first year after the treatment.
However, it should be emphasized that diagnostic criteria of and reference standard for treatment-related necrosis varied across the studies. Moreover, the studies varied on whether asymptomatic necrosis was included. These two factors may be correlated because symptomatic treatment-related necrosis requires further treatment such as surgery; thus, there may be a bias toward having histopathological confirmation in patients with symptomatic treatment-related necrosis. While histopathologic diagnosis is the gold standard for the diagnosis of treatment-related necrosis, brain biopsy and surgery are non-trivial, and it is thus somewhat inevitable and clinically necessary to define treatment-related necrosis with clinical and radiologic information. However, in that setting, it is difficult to differentiate treatment-related necrosis from true tumor progression [44]. The RANO brain metastasis working group recommends follow-up imaging 4 to 6 weeks after assessment in equivocal cases following SRS [45] and follow-up imaging at 3-month intervals during immunotherapy [46, 47]. The majority of the included studies utilized this follow-up strategy, but the exact follow-up period was often unreported. Furthermore, some studies used advanced imaging and/or FDG-PET for the diagnosis, but some studies did not. There is currently no specific clinicoradiologic criteria for the diagnosis of treatment-related necrosis in the setting of SRS+ICI combination therapy. Standards should be developed to improve clinical practice and focus future research.
In this context, issues regarding pseudoprogression should be further discussed. Treatment-related necrosis refers to a late-delayed necrosis as a consequence of radiation with or without chemotherapy, characterized by irreversible damage to blood vessels leading to ischemic necrosis, demyelination, and hemorrhage [44, 48]. In contrast, pseudoprogression refers to a unique, transient, predominantly radiologic phenomenon after the treatment, usually used in patients with gliomas especially within 3 months after the treatment [44]. Advanced MR techniques such as spectroscopy, perfusion imaging, and amino acid PET have been reported to be helpful for discriminating pseudoprogression and true progression [49, 50]. Recently, pseudoprogression is also used to indicate the radiologic change of other cancers after immunotherapy [51]. The pooled incidence of pseudoprogression seems to be much higher in patients with glioblastoma (36%; 95% CI, 33–40%) [52] than in patients with metastasis from other solid tumors (6%; 95% CI, 5–7%) [53]. Indeed, pseudoprogression as a consequence of ICI therapy in the early time frame of treatment might be misinterpreted to be treatment-related necrosis leading to a bias, but discrimination between pseudoprogression and treatment-related necrosis was not clear in the included studies, which remains to be a diagnostic challenge.
In this meta-analysis, the incidence of treatment-related necrosis was much higher in melanoma than NSCLC. This finding might be due to the small number of NSCLC studies included, which may have led to inaccuracy of the pooled estimate. However, the possibility of an intrinsic difference in the sensitivity to radiation and/or ICI therapy between melanoma and NSCLC cannot be excluded and has been previously reported with a treatment-related necrosis hazard ratio in melanoma of 4.02 and in NSCLC of 2.56 compared also with renal cell carcinoma [15]. Indeed, melanoma has been known to be radioresistant thereby requiring a higher radiation dose to control [54]. Although it was not possible to compare radiation dose between NSCLC and melanoma brain metastasis in our study because information regarding radiation dose was not reported in two studies on NSCLC, it might be assumed that a higher radiation dose might be prescribed during SRS for treating melanoma brain metastasis, and this also could be a reason to explain the difference of the incidence between melanoma and NSCLC.
There are several notable limitations to this study. First, all included studies were retrospective and assessment time for the diagnosis of treatment-related necrosis was not clear in most of the studies. Second, definitions of treatment-related necrosis and follow-up time were inconsistent across the included studies. Furthermore, only two studies specified in the definition that the location of the treatment-related necrosis was the same location previously irradiated. These would impose the risk of inaccuracy of the pooled estimates. Third, the number of included studies is modest, and in particular, there were only eight studies providing information for the direct comparison. Fourth, multicollinearity between the identified sources of heterogeneity could not be assessed based on the study-level data. Fifth, it was not possible to evaluate the association between the development of treatment-related necrosis and what and how ICI was used (e.g., monotherapy vs. combination therapy, ipilimumab vs. nivolumab vs. pembrolizumab). Finally, we could not perform meta-regression analysis to evaluate whether the timing in regard to ICI (before SRS vs. after SRS vs. concurrent use), mode, and radiation fractions of SRS would be the source of heterogeneity. Further studies are required to clarify this issue.
In conclusion, treatment-related necrosis tended to occur 2.4 times more frequently in the setting of SRS+ICI combination therapy compared with SRS alone in melanoma brain metastasis showing high cumulative incidence within the first year. Treatment-related necrosis should be considered when SRS+ICI therapy combination is used for melanoma brain metastasis, especially in the first year.
Abbreviations
- CI:
-
Confidence interval
- ICI:
-
Immune checkpoint inhibitor
- KM curve:
-
Kaplan-Meier curve
- NSCLC:
-
Non-small cell lung cancer
- SRS:
-
Stereotactic radiosurgery
References
Gavrilovic IT, Posner JB (2005) Brain metastases: epidemiology and pathophysiology. J Neurooncol 75:5–14
Nayak L, Lee EQ, Wen PY (2012) Epidemiology of brain metastases. Curr Oncol Rep 14:48–54
Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE (2004) Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 22:2865–2872
Schouten LJ, Rutten J, Huveneers HA, Twijnstra A (2002) Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 94:2698–2705
Cagney DN, Martin AM, Catalano PJ et al (2017) Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol 19:1511–1521
Sampson JH, Carter JH Jr, Friedman AH, Seigler HF (1998) Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg 88:11–20
Amer MH, Al-Sarraf M, Vaitkevicius VK (1979) Clinical presentation, natural history and prognostic factors in advanced malignant melanoma. Surg Gynecol Obstet 149:687–692
Budman DR, Camacho E, Wittes RE (1978) The current causes of death in patients with malignant melanoma. Eur J Cancer 14:327–330
Davis FG, Dolecek TA, McCarthy BJ, Villano JL (2012) Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol 14:1171–1177
Giglio P, Gilbert MR (2003) Cerebral radiation necrosis. Neurologist 9:180–188
Patel TR, McHugh BJ, Bi WL, Minja FJ, Knisely JP, Chiang VL (2011) A comprehensive review of MR imaging changes following radiosurgery to 500 brain metastases. AJNR Am J Neuroradiol 32:1885–1892
Shepard MJ, Xu Z, Donahue J et al (2019) Stereotactic radiosurgery with and without checkpoint inhibition for patients with metastatic non-small cell lung cancer to the brain: a matched cohort study. J Neurosurg. https://doi.org/10.3171/2019.4.Jns19822:1-8
Minniti G, Anzellini D, Reverberi C et al (2019) Stereotactic radiosurgery combined with nivolumab or ipilimumab for patients with melanoma brain metastases: evaluation of brain control and toxicity. J Immunother Cancer 7:102
Diao K, Bian SX, Routman DM et al (2018) Combination ipilimumab and radiosurgery for brain metastases: tumor, edema, and adverse radiation effects. J Neurosurg 129:1397–1406
Martin AM, Cagney DN, Catalano PJ et al (2018) Immunotherapy and symptomatic radiation necrosis in patients with brain metastases treated with stereotactic radiation. JAMA Oncol 4:1123–1124
Kaidar-Person O, Zagar TM, Deal A et al (2017) The incidence of radiation necrosis following stereotactic radiotherapy for melanoma brain metastases: the potential impact of immunotherapy. Anticancer Drugs 28:669–675
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151:W65–W94
Atkins D, Eccles M, Flottorp S et al (2004) Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res 4:38
Guyatt G, Oxman AD, Akl EA et al (2011) GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64:383–394
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Kim KW, Lee J, Choi SH, Huh J, Park SH (2015) Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-part I. General guidance and tips. Korean J Radiol 16:1175–1187
Lee J, Kim KW, Choi SH, Huh J, Park SH (2015) Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-part II. Statistical methods of meta-analysis. Korean J Radiol 16:1188–1196
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Sterne JA, Egger M, Smith GD (2001) Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ 323:101–105
Silk AW, Bassetti MF, West BT, Tsien CI, Lao CD (2013) Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med 2:899–906
Knisely JP, Yu JB, Flanigan J, Sznol M, Kluger HM, Chiang VL (2012) Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg 117:227–233
Skrepnik T, Sundararajan S, Cui H, Stea B (2017) Improved time to disease progression in the brain in patients with melanoma brain metastases treated with concurrent delivery of radiosurgery and ipilimumab. Oncoimmunology 6:e1283461
Pires da Silva I, Glitza IC, Haydu LE et al (2019) Incidence, features and management of radionecrosis in melanoma patients treated with cerebral radiotherapy and anti-PD-1 antibodies. Pigment Cell Melanoma Res 32:553–563
Patel KR, Shoukat S, Oliver DE et al (2017) Ipilimumab and stereotactic radiosurgery versus stereotactic radiosurgery alone for newly diagnosed melanoma brain metastases. Am J Clin Oncol 40:444–450
Nardin C, Mateus C, Texier M et al (2018) Tolerance and outcomes of stereotactic radiosurgery combined with anti-programmed cell death-1 (pembrolizumab) for melanoma brain metastases. Melanoma Res 28:111–119
Kotecha R, Miller JA, Venur VA et al (2018) Melanoma brain metastasis: the impact of stereotactic radiosurgery, BRAF mutational status, and targeted and/or immune-based therapies on treatment outcome. J Neurosurg 129:50–59
Du Four S, Janssen Y, Michotte A et al (2018) Focal radiation necrosis of the brain in patients with melanoma brain metastases treated with pembrolizumab. Cancer Med 7:4870–4879
Robin TP, Breeze RE, Smith DE et al (2018) Immune checkpoint inhibitors and radiosurgery for newly diagnosed melanoma brain metastases. J Neurooncol 140:55–62
Olson AC, Thomas S, Qin R et al (2016) Outcomes and toxicity of stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab. Melanoma Manag 3:177–186
Hubbeling HG, Schapira EF, Horick NK et al (2018) Safety of combined PD-1 pathway inhibition and intracranial radiation therapy in non–small cell lung cancer. J Thorac Oncol 13:550–558
Fang P, Jiang W, Allen P et al (2017) Radiation necrosis with stereotactic radiosurgery combined with CTLA-4 blockade and PD-1 inhibition for treatment of intracranial disease in metastatic melanoma. J Neurooncol 133:595–602
Yang L, Liu L, Wu X, Guo X, Yang Y, Wang T (2020) Hypofractionated radiation therapy with versus without immune checkpoint inhibitors in patients with brain metastases: a meta-analysis. Int Immunopharmacol 80:106148
Anderson ES, Postow MA, Wolchok JD et al (2017) Melanoma brain metastases treated with stereotactic radiosurgery and concurrent pembrolizumab display marked regression; efficacy and safety of combined treatment. J Immunother Cancer 5:76
Trommer-Nestler M, Marnitz S, Kocher M et al (2018) Robotic stereotactic radiosurgery in melanoma patients with brain metastases under simultaneous anti-PD-1 treatment. Int J Mol Sci 19:2653
Galldiks N, Kocher M, Ceccon G et al (2020) Imaging challenges of immunotherapy and targeted therapy in patients with brain metastases: response, progression, and pseudoprogression. Neuro Oncol 22:17–30
Alomari AK, Cohen J, Vortmeyer AO et al (2016) Possible interaction of Anti-PD-1 therapy with the effects of radiosurgery on brain metastases. Cancer Immunol Res 4:481–487
Rauch PJ, Park HS, Knisely JP, Chiang VL, Vortmeyer AO (2012) Delayed radiation-induced vasculitic leukoencephalopathy. Int J Radiat Oncol Biol Phys 83:369–375
Alomari A, Rauch PJ, Orsaria M, Minja FJ, Chiang VL, Vortmeyer AO (2014) Radiologic and histologic consequences of radiosurgery for brain tumors. J Neurooncol 117:33–42
Winter SF, Loebel F, Loeffler J et al (2019) Treatment-induced brain tissue necrosis: a clinical challenge in neuro-oncology. Neuro Oncol 21:1118–1130
Alexander BM, Brown PD, Ahluwalia MS et al (2018) Clinical trial design for local therapies for brain metastases: a guideline by the Response Assessment in Neuro-Oncology Brain Metastases working group. Lancet Oncol 19:e33–e42
Camidge DR, Lee EQ, Lin NU et al (2018) Clinical trial design for systemic agents in patients with brain metastases from solid tumours: a guideline by the Response Assessment in Neuro-Oncology Brain Metastases working group. Lancet Oncol 19:e20–e32
Okada H, Weller M, Huang R et al (2015) Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol 16:e534–e542
Na A, Haghigi N, Drummond KJ (2014) Cerebral radiation necrosis. Asia Pac J Clin Oncol 10:11–21
van Dijken BRJ, van Laar PJ, Holtman GA, van der Hoorn A (2017) Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high-grade glioma, a systematic review and meta-analysis. Eur Radiol 27:4129–4144
Kim SJ, Ryul Shim S (2019) Diagnostic value of radiolabeled amino acid PET for detection of pseudoprogression of brain tumor after treatment: a meta-analysis. Nucl Med Commun 40:965–972
Nishino M, Hatabu H, Hodi FS (2019) Imaging of cancer immunotherapy: current approaches and future directions. Radiology 290:9–22
Abbasi AW, Westerlaan HE, Holtman GA, Aden KM, van Laar PJ, van der Hoorn A (2018) Incidence of tumour progression and pseudoprogression in high-grade gliomas: a systematic review and meta-analysis. Clin Neuroradiol 28:401–411
Park HJ, Kim KW, Pyo J et al (2020) Incidence of pseudoprogression during immune checkpoint inhibitor therapy for solid tumors: a systematic review and meta-analysis. Radiology 297:87–96
Goyal S, Silk AW, Tian S et al (2015) Clinical management of multiple melanoma brain metastases: a systematic review. JAMA Oncol 1:668–676
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Chong Hyun Suh.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study because this study is a systematic review and meta-analysis.
Ethical approval
Ethical approval was not required for this study because this study is a systematic review and meta-analysis.
Methodology
• Meta-analysis
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, P.H., Suh, C.H., Kim, H.S. et al. Immune checkpoint inhibitor therapy may increase the incidence of treatment-related necrosis after stereotactic radiosurgery for brain metastases: a systematic review and meta-analysis. Eur Radiol 31, 4114–4129 (2021). https://doi.org/10.1007/s00330-020-07514-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07514-0