Abstract
Objectives
This study was conducted to establish and validate a non-contrast T1 map–based radiomic nomogram for predicting major adverse cardiac events (MACEs) in patients with acute ST-segment elevation myocardial infarction (STEMI) undergoing percutaneous coronary intervention (PCI).
Methods
This retrospective study included 157 consecutive patients (training sets, 109 patients; test sets, 48 patients) with acute STEMI undergoing PCI. An open-source radiomics software was used to segment the myocardium on the non-contrast T1 mapping and extract features. A radiomic signature was constructed to predict MACEs using the least absolute shrinkage and selection operator method. The performance of the radiomic nomogram for predicting MACEs in both the training and test sets was evaluated by its discrimination, calibration, and clinical usefulness.
Results
The radiomic signature showed a good prognostic ability in the training sets with an AUC of 0.94 (95% CI, 0.86 to 1.00) and F1 score of 0.71, which was confirmed in the test sets with an AUC of 0.90 (95% CI, 0.74 to 1.00) and F1 score of 0.62. The nomogram consisting of the radiomic scores and cardiac troponin I showed good discrimination ability in the training and test sets with AUCs of 0.96 (95% CI, 0.91 to 1.00; F1 score, 0.71) and 0.94 (95% CI, 0.83 to 1.00; F1 score, 0.70), respectively.
Conclusions
The non-contrast T1 map–based radiomic nomogram is a useful tool for the prediction of MACEs in patients with acute STEMI undergoing PCI that can assist clinicians for optimised risk stratification of individual patients.
Key Points
• Radiomic signature improved MACE prediction in acute STEMI patients.
• T1 mapping–derived radiomic signature outperformed conventional cardiac MRI parameters in predicting MACEs in acute STEMI patients.
• The non-contrast T1 mapping–based radiomic nomogram can be used for prediction of MACEs and improvement of risk stratification in acute STEMI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cardiovascular mortality in the acute phase of ST-segment elevation myocardial infarction (STEMI) has progressively decreased over the past four decades, partly because of the prompt delivery of reperfusion treatment [1]. However, progressive left ventricular (LV) dysfunction and chronic heart failure have become the main aspects of mortality in STEMI [2]. Identifying high-risk patients after STEMI is critical for effective risk stratification, patient management, and efficient public health resource allocation.
Parameters obtained from early cardiac magnetic resonance (CMR), such as left ventricular ejection fraction (LVEF), infarction size (IS), and microvascular obstruction (MVO), were considered to be outstanding predictors of major adverse cardiovascular events (MACEs) during follow-up [3, 4]. LVEF was recommended as a decision-making marker for risk stratification in the latest American College of Cardiology/American Heart Association and European Society of Cardiology guidelines for STEMI [5, 6]. However, the influence of myocardial stunning and compensatory hyperkinesia in the non-infarcted segments limits the applicability of LVEF as a prognostic marker [7]. Non-contrast T1 mapping not only enables the evaluation of infarction severity but also allows the depiction of diffuse tissue anomalies in the remote non-infarcted myocardium in acute STEMI [8,9,10]. Previous studies suggested that evaluation of remote zone alterations in patients with STEMI undergoing percutaneous coronary intervention (PCI) by quantitative non-contrast T1 mapping had independent and greater prognostic value compared to that of established risk factors and traditional CMR outcome parameters [10, 11]. There is a demand for a contrast agent–avoiding and reliable approach incorporating information displayed in not only the infarcted tissue but also the remote tissue to predict MACEs.
Radiomics is a method that allows extracting quantitative information from imaging data using a high-dimensional feature space [12, 13]. Radiomics has exhibited great potential in neoplasm diagnosis, subtype classification, and prognosis and therapeutic response prediction [14,15,16]. The utility of radiomics in CMR has recently been outlined in the context of MI, showing the potential for texture analysis to detect small myocardial scars in cine images and to distinguish between acute and chronic MI [17, 18]. However, the role of radiomics derived from non-contrast native T1 images for the prediction of major clinical events remains uncertain, in particular, its potential greater prognostic value than that of traditional CMR markers.
This study aimed to investigate the role of a non-contrast T1 map–based radiomic nomogram for the prediction of MACEs in patients with acute STEMI treated with PCI.
Materials and methods
Study design, patients, and clinical endpoints
This retrospective, observational, cohort study was approved by the institutional review board of Shengjing Hospital of China Medical University (No. 2019PS071J) with a waiver of the need for written informed consent.
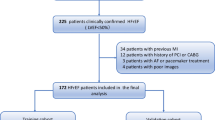
We retrospectively reviewed the records of patients who were first admitted to our hospital from April 2017 to December 2018 due to STEMI and underwent PCI. All patients underwent CMR at 3-7 days after PCI. The exclusion criteria included history of a previous MI or revascularisation, poor CMR image quality, and incomplete CMR imaging data. Relevant clinical history data were collected for all patients, including demographic characteristics (sex, age, and body mass index), cardiovascular risk factors (smoking, hypertension, diabetes mellitus, hyperlipidaemia, and family history of coronary artery disease), laboratory parameters, and electrocardiographic and angiographic information.
The clinical endpoint was the occurrence of MACEs, defined as a composite of death, occurrence of new congestive heart failure, and myocardial reinfarction within one year.
CMR imaging protocol
Imaging was performed using a Philips 3.0-T MR scanner (Ingenia, Philips Healthcare). To assess the left ventricular function, two-chamber, four-chamber, and left ventricular short-axis cine images were obtained using the balanced turbo field echo-breath hold (BTFE-BH) sequence. The left ventricular short-axis images covered the entire left ventricle. The scanning parameters were TE 1.45 ms, TR 2.89 ms, flip angle 45°, 30 phases, section thickness 8 mm, section space 2 mm, and FOV 340 mm × 340 mm. T2-weighted images were obtained using the spectral attenuated inversion recovery (SPAIR) sequence to assess for myocardial oedema. The scan parameters were TE 80 ms, TR 2250 ms, flip angle 90°, section thickness 8 mm, section space 2 mm, and FOV 340 mm × 340 mm. Native T1 mapping scan was performed before contrast administration, and the modified look-locker inversion recovery (MOLLI) sequence was used to cover the whole left ventricle on the short axis. The acquisition mode was 3–(3)–3–(3)–5. The scanning parameters were TE 1.05 ms, TR 2.30 ms, TI 150-3,287 ms, section thickness 8 mm, and FOV 320 mm × 320 mm. Late gadolinium enhancement (LGE) scan was performed 15-20 min after the first intravenous injection of 0.2 mmol/kg gadolinium-based contrast agent (GBCA; Omniscan, GE Healthcare). The phase-sensitive inversion recovery (PSIR) sequence was used to cover the entire left ventricle on the short axis. Scan parameters were TE 3.0 ms, TR 6.08 ms, TI 300 ms, section thickness 8 mm, and FOV 320 mm × 320 mm. Short-axis slices acquired with different sequences were matching covering the entire left ventricle.
CMR image analysis
Cardiac data were evaluated using CVI software (version 5.9.1, Circle Cardiovascular Imaging Inc.). The CMR images were analysed by two radiologists (reader 1, M.Q.M.; reader 2, Y.M.) with 8 and 12 years of cardiothoracic experience, respectively. Cardiac function was analysed in cine images, including LVEF, LV end-diastolic volume (LVEDV), and LV end-systolic volume (LVESV). The area at risk (AAR) was defined as the myocardium with a signal intensity more than two standard deviations (SDs) greater than the average signal intensity in the remote myocardium [19]. The infarcted myocardium was defined as the region with a signal intensity five SDs greater than the mean signal of the remote myocardial region of interest (ROI) on the LGE image [20]. The infarct size (IS) and transmurality were measured on the LGE image. MVO and intramyocardial haemorrhage were defined as the low-intensity core within an area of LGE or oedema, respectively. Native T1 values were obtained by T1 measurement within the remote myocardial ROI. The remote myocardium was defined as the region that did not display any LGE, oedema, or wall motion abnormalities [10]. Particular care was taken to perform adequate margin separation from the adjacent tissue (e.g., blood or lung) to avoid partial volume artefacts [21]. The relationship between MACE and traditional CMR parameters LVEF, LVEDV, LVESV, AAR, remote T1 value, IS, and MVO was evaluated.
Radiomic feature extraction and radiomic signature construction
Myocardial segmentation and feature extraction were conducted using a free and open-source package (MaZda, version 4.6; Institute of Electronics, Technical University of Lodz) [22, 23]. The texture feature extraction formula, which was included in this analysis, is shown in the Supplementary Materials. The automated T1 mapping images created by the Philips 3.0-T MR scanner were exported for further analysis as single digital imaging and communications in medicine images, and the section with the greatest extent of LGE was chosen for radiomic feature extraction. ROIs were delineated twice in a subset of 30 subjects by reader 1 to evaluate intra-observer variability of texture features and by reader 2 to assess inter-observer variability. To prevent partial volume effects, the contours were drawn carefully to avoid involving the trabeculated layer and epicardial boundary. Figure 1 shows the workflow of myocardial segmentation and feature extraction.
Work flow of myocardial segmentation and feature extraction. a A short-axis late gadolinium enhancement (LGE) image shows the large extent of the infarction zone (white arrow). b A short-axis T1 map matching the LGE showed increased T1 value in the infarction zone (black ROI) compared to that in the remote zone (white ROI), 1498 ms vs. 1159 ms, respectively. c Myocardial segmentation. The edge of the lesion is drawn carefully not involving adjacent blood or lung tissue to avoid partial volume artefacts. The red area represents the selected region on the T1 map matching the LGE image. d Radiomic feature of skewness extracted from the T1 map. ROI, region of interest
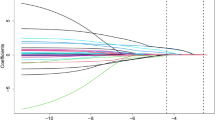
Patients were randomly divided into training and test sets in a ratio of 7:3. Intra-class correlation coefficients (ICCs) were calculated to assess intra- and inter-observer reproducibility, and features with an ICC < 0.75 were excluded. The remaining features were analysed using Student’s t test or Mann–Whitney’s nonparametric test, as appropriate, and the least absolute shrinkage and selection operator (LASSO) regression algorithm was used to select those with the greatest predicting ability. The best predicting features were obtained at the tuning parameter (λ) with the minimum binomial deviance. These were used to construct the radiomic signature with multivariable logistic regression analysis in the training set. A linear combination of the selected features weighted by their LASSO coefficients was used to calculate the radiomic score (Rad-score) for each patient.
Radiomic nomogram construction
Clinical data, including demographic features, cardiovascular risk factors, myocardial enzymes, CMR parameters, and Rad-score, were evaluated using univariate logistic regression in the training set. Variables with p < 0.1 in the univariate analysis were included for further multivariate logistic analysis and radiomic nomogram construction. Receiver operator characteristic (ROC) curve analysis was used to compare the prediction performance of the clinical factors, radiomic signature, and radiomic nomogram for MACEs in both the training and test sets. A calibration curve was used to evaluate the calibration of the nomogram, and its goodness of fit was assessed using the Hosmer–Lemeshow test. A radiomic nomogram score (Nomo-score) was calculated for each patient in the training and test sets. Decision curve analysis (DCA) was performed to assess the clinical usefulness of the radiomic nomogram in the whole cohort.
Statistical analysis
Statistical analysis was performed using R (version 3.5.1; R Foundation for Statistical Computing) with RStudio (version 1.0.136; RStudio). R packages used in this study are listed in the Supplementary Materials. The p values of multiple comparisons were corrected using the Benjamini–Hochberg method [24]. The LASSO regression analysis was used with a 10-fold cross-validation tuning of the penalty parameter on the basis of the minimum criteria. Differences in the area under the ROC curve (AUC) values between different models were analysed using the Delong test. Categorical variables were compared using the chi-square test or Fisher’s test if the expected cell count was < 5. Student’s t test was used to compare normally distributed continuous variables. Otherwise, Mann–Whitney’s U test was used. Categorical variables are presented as frequencies with percentages, normally distributed continuous variables as means ± SD, and other data as medians with interquartile ranges (IQRs). The significance level was set at 0.05, and two-sided tests were used.
Results
Clinical characteristics
A total of 157 patients with native T1-mapping were included in the study. Among them, 17 patients (10.3%) experienced a MACE during the 1-year follow-up period: five patients (3.2%) died, three (1.9%) had reinfarction, and nine (5.7%) had a newly developed congestive heart failure.
The median remote zone native T1 value of the entire study cohort was 1327 ms (IQR 1302-1369). The remote zone native T1 values were significantly higher in patients with MACEs than in those without MACEs (1369 ms [IQR 1347-1392] vs. 1321 ms [IQR 1298-1358], p = 0.002). Based on the AUCs, remote zone native T1 values > 1345 ms best predicted MACEs (AUCs 0.73, 95% CI 0.66-0.80, p < 0.001).
Patients underwent CMR at a median of 3.2 days (IQR 2.1-4.3 days) after PCI. The following conventional CMR parameters were significantly associated with the occurrence of MACEs in the univariate analysis in the training set: anterior MI, cardiac troponin I (cTnI), MVO, EF, IS, AAR, and remote myocardial T1 values. The patients’ characteristics and CMR data are presented in Table 1.
Radiomic signature construction and evaluation
Of the total 157 patients, 109 (mean age 56.4 ± 10.5 years) were allocated into the training set and 48 (mean age 56.2 ± 10.3 years) were allocated into the test set.
A total of 279 radiomic features of six subsets were extracted from the native T1 map ROIs (Table 2, as described in detail in the Supplementary Materials). Of those, 198 features demonstrated a good inter- and intra-observer agreement, with ICCs ranging from 0.75 to 0.98. Among these, 66 radiomic features showing significant differences between patients with MACEs and patients without MACEs (p = 0.001–0.050) by multiple-comparison Student’s t test/Mann–Whitney’s U test were included in the univariate logistic analysis. A total of 56 features were included into the LASSO logistic regression model to select those with the greatest predicting ability (Supplementary Fig. S1). Subsequently, three features were selected to construct a radiomic signature. The Rad-score was calculated using the following formula:
Here, S(5,5)Contrast was the measure of contrast of intensity between a pixel and its neighbourhood, Vertl_LngREmph was a measure that emphasises the long runs of a grey-level image, and WavEnLH_s-3 was a class of functions used to localise a given function in both space and scaling. The Rad-score in patients with MACEs showed higher values than those in patients without MACEs in both the training (p < 0.001) and test (p = 0.002) sets. The violin plot of the Rad-score in both sets is shown in Fig. 2a. The radiomic model showed a favourable discriminatory ability in the training set (AUC 0.94 [95% CI 0.86-1.00], F1 score 0.71), which was confirmed in the test set (AUC 0.90 [95% CI 0.74-1.00], F1 score 0.62) (Fig. 2b). There was no significant difference in the ROCs between the two sets (DeLong test, p = 0.798). The accuracy of the radiomic model was 93% (sensitivity 94%, specificity 83%) in the training set and 90% (sensitivity 91%, specificity 80%) in the test set. The radiomic model outperformed the conventional clinical and CMR markers (cTnI, EF, IS, AAR, remote myocardial T1 values) in the prediction of MACEs (Fig. 3).
A violin plot of the radiomic scores in the training and test sets. a Wider sections of the plot mean a higher probability that patients of the group would take on the given value; thinner sections represent a lower probability. The median value is defined by the red lines, and the interquartile values are defined by the blue lines. The difference in the radiomic scores between the two sets was compared using Student’s t test. b Receiver operating characteristic curves of the radiomic signature for MACE prediction in the training and test sets. MACE, major adverse cardiovascular events
Graph showing the receiver operating characteristic (ROC) curve analyses for predicting MACEs in the training (a) and test (b) sets. The ROC analysis indicates that the radiomic signature had the highest accuracy for predicting MACEs compared to the traditional clinical and CMR factors (cTnI, LVEF, IS, AAR, and remote myocardial T1 values) in the training or test sets. MACE, major adverse cardiovascular events; cTnI, cardiac troponin I; LVEF, left ventricular ejection fraction; IS, infarct size; AAR, area at risk
Radiomic nomogram construction and evaluation
The radiomic nomogram for prediction of MACEs was constructed with the cTnI and the Rad-score (Fig. 4a). The nomogram showed good performance for predicting MACEs over a period of 1 year and in the training (AUC 0.96 [95% CI 0.91-1.00], F1 score 0.71) and test (AUC 0.94 [95% CI 0.83-1.00], F1 score 0.70) sets, respectively (Fig. 4b). The calibration curves of the nomogram in both sets are displayed in Fig. S2a, b. The calibration curve and Hosmer–Lemeshow’s test showed good calibration in the training (p = 0.289) and test (p = 0.524) sets, indicating that the nomogram was acceptable.
The radiomic nomogram and its predictive performance for MACEs. a The radiomic nomogram combining cTnI and radiomic score developed in the training set. b The ROC analysis indicates that the radiomic nomogram had an excellent performance for predicting MACEs in the training or test datasets. MACE, major adverse cardiovascular events; ROC, receiver operating characteristics; cTnI, cardiac troponin I
The Nomo-score for each patient is shown in Fig. 5. The Nomo-score was calculated using the following formula:
Here, Rad-score was computed through a linear combination of selected features weighted by their respective coefficients and cTnI was referred to cardiac troponin I. The diagnostic performance for each model is summarised in Table 3. The AUC value of the radiomic nomogram was higher than that of the clinical and conventional CMR factor (cTnI and IS) combination model both in the training (p = 0.030) and the test (p = 0.013) sets.
The DCA for the three models is shown in Fig. 6. The DCA showed that the radiomic nomogram had a higher overall net benefit in predicting MACEs in STEMI than those of the traditional CMR model and the radiomic signature across the majority of the range of reasonable threshold probabilities.
Decision curve analysis for the three models in the training set. The y-axis indicates the net benefit; the x-axis indicates threshold probability. The grey line represents the assumption that all patients have MACE. The black line represents the assumption that all patients have negative MACE. The red line, blue line, and green line represent the net benefit of the radiomic nomogram, the radiomic signature, and the traditional clinical factor (cTnI + IS) model, respectively. The radiomic nomogram model and radiomic signature had a higher net benefit compared with the conventional clinical factor model. MACE, major adverse cardiovascular events; IS, infarct size
Discussion
In this study, we investigated the utility of a non-contrast T1 mapping–based radiomic nomogram for prediction of MACEs over a period of 1 year in patients with STEMI undergoing PCI. The radiomic signature consisted of three robust features and successfully stratified patients according to their statistical risk of MACE occurrence. An easy-to-use radiomic nomogram that incorporates the cTnI level and the radiomic signature demonstrated favourable discrimination in both the training (AUC 0.96, F1 score 0.71) and test (AUC 0.94, F1 score 0.70) sets, outperforming the traditional clinical factors and remote myocardial T1 values.
CMR provides not only a comprehensive evaluation of the left ventricular structure and function but also a multi-parameter assessment of the infarcted myocardium and microvascular injury, with strong evidence for its utility in risk stratification after STEMI [25]. However, its clinical applicability remains controversial to date due to insufficient evidence that CMR-based parameters would effectively improve long-term risk stratification of patients with STEMI [7]. Various studies have shown that the IS in STEMI survivors was strongly related to cardiovascular outcomes [26, 27]. Recent advances in MRI technology and parallel imaging techniques have allowed T1 parametric mapping to investigate the myocardial tissue characterisation [28]. Carrick et al. found a significant association between the remote zone native T1 value and the IS [10]. They demonstrated that the remote zone native T1 value is independently associated with LV remodelling, as measured by CMR at 6 months after STEMI. In addition, they also identified an association between the native remote zone T1 value alterations and MACE occurrence. Reinstadler et al. demonstrated that native T1 value measured in the remote myocardium was a strong independent predictor of MACEs post-STEMI [11]. Therefore, the radiomic approach incorporating not only the extent of infarcted tissue but also the diffuse tissue abnormalities in the non-ischaemic areas would be more beneficial for risk stratification. The radiomics-based nomogram in the present study showed higher AUCs of 0.96 and 0.94 in the training and test sets than those of the other models. The excellent performance of the nomogram was presumably attributable to the inclusion of the Rad-score, which incorporated numerous quantitative features extracted from the infarcted tissue and remote myocardium, particularly parameters not easily visible by simple visual observation or analysed by regular imaging tools.
This study explored alternative approaches to radiomic analysis to improve MACE prediction without LGE in patients with STEMI undergoing PCI. The three dominant radiomic features (S(5,5)Contrast, Vertl_LngREmph, and WavEnLH_s-3) selected to construct the radiomic signature were of three different kinds (grey-level co-occurrence matrix, grey-level run-length matrix, and wavelets). We did not directly associate our findings with a histopathological analysis in this study; therefore, we cannot define the specific association between the radiomic features and histopathological changes. The histopathological correlation of myocardial fibrosis and T1 mapping is well established [29, 30]. Radiomic features are mathematical parameters derived from the pixel distribution that characterises the structure underlying the image objects, providing important complementary data on the imaging phenotype that may include a wealth of information [31, 32]. MRI T1 images do not present microscopic details, but changes in histology may cause changes in texture that are suitable for texture analysis.
We constructed an easy-to-use radiomic nomogram allowing clinicians to obtain results quickly and reliably by simply drawing the ROI in the myocardium and several lines in the nomogram. The nomogram is of great clinical significance in that it assists clinicians for optimised risk stratification of individual patients. Both the radiomic model and the nomogram in the current study yielded excellent performance for prediction of MACEs and could offer net benefits over the “treat-all” or “treat-none” strategy within a certain threshold probability. Furthermore, the avoidance of an exogenous GBCA has important clinical implications. In a previous study, the authors described an association between the occurrence of nephrogenic systemic fibrosis and GBCA use in patients with severe renal dysfunction, as well as accumulation of GBCA in multiple tissues (including bone, kidneys, and brain) despite intact renal function [33, 34]. Therefore, native T1 mapping applies to a broader range of patients with STEMI, including those with severe renal impairment and those in need of more aggressive treatment [35].
The present study had several limitations. First, the study only included a small number of patients and the prevalence of events was low. Overall, a smaller sample size leads to higher report classification accuracy [36]. The existence of low-prevalence binary predictors can cause the problem of complete or quasi-complete separation in logistic regression [37]. Thus, future studies with a larger population and external validation are warranted. Second, the study focused only on binary classification of patients (negative or positive for MACEs). The role of radiomics in the prediction of detailed time-dependent MACEs (death, occurrence of new congestive heart failure, and myocardial reinfarction) merits further studies. Third, as previous studies have shown that different T1 sequences may result in T1 measurement variability [38], additional research should be performed to further clarify the impact of technical variables (including the physiological factors, tissue-specific properties, field strength, and sequence parameters) on tissue inhomogeneity-related markers from radiomic analysis of T1 mapping. Finally, radiomic analysis should also be compared to other non-contrast approaches, such as MR cine or T2 mapping images in future work, and the predictive performance of different combined techniques deserves further investigation.
In conclusion, the non-contrast radiomic nomogram can effectively predict MACEs in patients with STEMI undergoing PCI. This simple-to-use tool can assist clinicians for optimised risk stratification of individual patients. Furthermore, because it is based on non-contrast T1 mapping, this approach applies to a broader range of patients with STEMI, including those with severe renal impairment and those in need of more aggressive treatment.
Abbreviations
- AAR:
-
Area at risk
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- CMR:
-
Cardiac magnetic resonance
- cTnI:
-
Cardiac troponin I
- DCA:
-
Decision curve analysis
- GBCA:
-
Gadolinium-based contrast agent
- IQR:
-
Interquartile range
- IS:
-
Infarct size
- LASSO:
-
Least absolute shrinkage and selection operator
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricle
- LVEF:
-
Left ventricular ejection fraction
- MACE:
-
Major adverse cardiovascular event
- MI:
-
Myocardial infarction
- MOLLI:
-
Modified look-locker inversion recovery
- MVO:
-
Microvascular obstruction
- PCI:
-
Percutaneous coronary intervention
- Rad-score:
-
Radiomic score
- ROC:
-
Receiver operator characteristic
- ROI:
-
Region of interest
- STEMI:
-
ST-segment elevation myocardial infarction
References
Rodriguez-Palomares JF, Gavara J, Ferreira-González I et al (2019) Prognostic value of initial left ventricular remodeling in patients with reperfused STEMI. JACC Cardiovasc Imaging 12:2445–2456
Roger VL, Go AS, Lloyd-Jones DM et al (2011) Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation 123:e18–e209
Izquierdo M, Ruiz-Granell R, Bonanad C et al (2013) Value of early cardiovascular magnetic resonance for the prediction of adverse arrhythmic cardiac events after a first noncomplicated ST-segment-elevation myocardial infarction. Circ Cardiovasc Imaging 6:755–761
Eitel I, de Waha S, Wöhrle J et al (2014) Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J Am Coll Cardiol 64:1217–1226
Steg PG, James SK, Atar D et al (2012) ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 33:2569–2619
O’Gara PT, Kushner FG, Ascheim DD et al (2013) 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 61:e78–e140
Symons R, Pontone G, Schwitter J et al (2018) Long-term incremental prognostic value of cardiovascular magnetic resonance after ST-segment elevation myocardial infarction: a study of the collaborative registry on CMR in STEMI. JACC Cardiovasc Imaging 11:813–825
Carrick D, Haig C, Rauhalammi S et al (2016) Prognostic significance of infarct core pathology revealed by quantitative non-contrast in comparison with contrast cardiac magnetic resonance imaging in reperfused ST-elevation myocardial infarction survivors. Eur Heart J 37:1044–1059
Ugander M, Oki AJ, Hsu L-Y et al (2012) Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. Eur Heart J 33:1268–1278
Carrick D, Haig C, Rauhalammi S et al (2015) Pathophysiology of LV remodeling in survivors of STEMI: inflammation, remote myocardium, and prognosis. JACC Cardiovasc Imaging 8:779–789
Reinstadler SJ, Stiermaier T, Liebetrau J et al (2018) Prognostic significance of remote myocardium alterations assessed by quantitative noncontrast T1 mapping in ST-segment elevation myocardial infarction. JACC Cardiovasc Imaging 11:411–419
Yip SS, Aerts HJWL (2016) Applications and limitations of radiomics. Phys Med Biol 61:R150–R166
Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14:749–762
Nie P, Yang G, Wang Z et al (2019) A CT-based radiomics nomogram for differentiation of renal angiomyolipoma without visible fat from homogeneous clear cell renal cell carcinoma. Eur Radiol 30:1274–1284
Ji G-W, Zhu F-P, Zhang Y-D et al (2019) A radiomics approach to predict lymph node metastasis and clinical outcome of intrahepatic cholangiocarcinoma. Eur Radiol 29:3725–3735
Wang Y, Liu W, Yu Y et al (2019) CT radiomics nomogram for the preoperative prediction of lymph node metastasis in gastric cancer. Eur Radiol 30:976–986
Larroza A, Materka A, López-Lereu MP, Monmeneu JV, Bodí V, Moratal D (2017) Differentiation between acute and chronic myocardial infarction by means of texture analysis of late gadolinium enhancement and cine cardiac magnetic resonance imaging. Eur J Radiol 92:78–83
Baessler B, Mannil M, Oebel S, Maintz D, Alkadhi H, Manka R (2018) Subacute and chronic left ventricular myocardial scar: accuracy of texture analysis on nonenhanced cine MR images. Radiology 286:103–112
Schulz-Menger J, Bluemke DA, Bremerich J et al (2013) Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson 15:35
Bondarenko O, Beek AM, Hofman MBM et al (2005) Standardizing the definition of hyperenhancement in the quantitative assessment of infarct size and myocardial viability using delayed contrast-enhanced CMR. J Cardiovasc Magn Reson 7:481–485
Puntmann VO, Peker E, Chandrashekhar Y, Nagel E (2016) T1 Mapping in characterizing myocardial disease: a comprehensive review. Circ Res 119:277–299
Szczypinski PM, Strzelecki M, Materka A, Klepaczko A (2009) MaZda--a software package for image texture analysis. Comput Methods Programs Biomed 94:66–76
Strzelecki M, Szczypinski P, Materka A, Klepaczko A (2013) A software tool for automatic classification and segmentation of 2D/3D medical images. Nucl Instrum Methods Phys Res Sect A 702:137–140
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 57:289–300
Dastidar AG, Rodrigues JCL, Baritussio A, Bucciarelli-Ducci C (2016) MRI in the assessment of ischaemic heart disease. Heart Br Card Soc 102:239–252
Stone GW, Selker HP, Thiele H et al (2016) Relationship between infarct size and outcomes following primary PCI: patient-level analysis from 10 randomized trials. J Am Coll Cardiol 67:1674–1683
Reinstadler SJ, Thiele H, Eitel I (2015) Risk stratification by cardiac magnetic resonance imaging after ST-elevation myocardial infarction. Curr Opin Cardiol 30:681–689
Messroghli DR, Moon JC, Ferreira VM et al (2017) Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 19:75
Ide S, Riesenkampff E, Chiasson DA et al (2017) Histological validation of cardiovascular magnetic resonance T1 mapping markers of myocardial fibrosis in paediatric heart transplant recipients. J Cardiovasc Magn Reson 19:10
Diao KY, Yang ZG, Xu HY et al (2016) Histologic validation of myocardial fibrosis measured by T1 mapping: a systematic review and meta-analysis. J Cardiovasc Magn Reson 18:92
Aerts HJWL (2016) The potential of radiomic-based phenotyping in precision medicine: a review. JAMA Oncol 2:1636–1642
Castellano G, Bonilha L, Li LM, Cendes F (2004) Texture analysis of medical images. Clin Radiol 59:1061–1069
Kanda T, Fukusato T, Matsuda M et al (2015) Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 276:228–232
Grobner T, Prischl FC (2007) Gadolinium and nephrogenic systemic fibrosis. Kidney Int 72:260–264
Hammer-Hansen S, Ugander M, Hsu LY et al (2014) Distinction of salvaged and infarcted myocardium within the ischaemic area-at-risk with T2 mapping. Eur Heart J Cardiovasc Imaging 15:1048–1053
Vabalas A, Gowen E, Poliakoff E, Casson AJ (2019) Machine learning algorithm validation with a limited sample size. PLoS One 14:e0224365
Ogundimu EO, Altman DG, Collins GS (2016) Adequate sample size for developing prediction models is not simply related to events per variable. J Clin Epidemiol 76:175–182
Teixeira T, Hafyane T, Stikov N, Greiser A, Friedrich MG, Akdeniz C (2016) Comparison of different cardiovascular magnetic resonance sequences for native myocardial T1 mapping at 3T. J Cardiovasc Magn Reson 18:65
Acknowledgements
We thank Yan Guo for her expert opinion and helpful comments.
Funding
This study has received funding from the 345 Talent Project in Shengjing Hospital of China Medical University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Yang Hou.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Yan Guo kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was waived by the institutional review board.
Ethical approval
Institutional review board approval was obtained.
Methodology
• retrospective
• Diagnostic or prognostic study / observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 487 kb)
Rights and permissions
About this article
Cite this article
Ma, Q., Ma, Y., Wang, X. et al. A radiomic nomogram for prediction of major adverse cardiac events in ST-segment elevation myocardial infarction. Eur Radiol 31, 1140–1150 (2021). https://doi.org/10.1007/s00330-020-07176-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07176-y