Abstract
Objectives
To evaluate the clinical usefulness of dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) in children and young adults with Crohn’s disease.
Methods
From August 2017 to October 2018, 30 patients with Crohn’s disease (21 males and 9 females; mean age 15.1 ± 2.5 years) underwent DCE-MRI with MRI enterography. We assessed the endoscopic finding, pediatric Crohn’s disease activity index (PCDAI), C-reactive protein (CRP) level (mg/dL), Crohn’s disease MR index (CDMI) score, and the perfusion parameters of DCE-MRI (Ktrans, Kep, and Ve) at the ileocecal region between the inactive and active groups based on the histopathologic status.
Results
The active Crohn’s disease group showed higher PCDAI, CRP, and CDMI scores than the inactive group (22.2 ± 18.8 vs. 6.3 ± 6.4, p = 0.027; 1.32 ± 1.79 vs. 0.10 ± 0.13, p = 0.005; 7.4 ± 3.9 vs. 4.5 ± 3.0, p = 0.047, respectively). The active Crohn’s disease group showed a higher Ktrans value than the inactive group (0.31 ± 0.12 vs. 0.16 ± 0.46 min−1, p = 0.002). Endoscopic finding; PCDAI, CRP, and CDMI scores; and Ktrans value were significant parameters in the identification of the active Crohn’s disease (p = 0.002, p < 0.001, p = 0.029, p = 0.006, and p < 0.001, respectively). Ktrans value was the most significant value for identifying active Crohn’s disease in the multivariate logistic regression analysis (p = 0.013).
Conclusion
Ktrans value could discriminate between inactive and active Crohn’s diseases. Ktrans value may have the potential to monitor the pediatric Crohn’s disease activity.
Key Points
• With dynamic contrast-enhanced MRI, we can quantitatively monitor the Crohn’s disease status in pediatric patients and provide proper management plans to clinicians.
• The K trans value of dynamic contrast-enhanced MRI perfusion parameter, as well as the clinical pediatric Crohn’s disease activity index, C-reactive protein level, the endoscopic score, and the Crohn’s disease MR index, was higher in the active Crohn’s disease than in the inactive group based on the histopathologic status.
• The K trans value among the dynamic contrast-enhanced MRI perfusion parameters was the most significant differentiating parameter for the active Crohn’s disease from inactive status among those parameters (p = 0.013).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Crohn’s disease is a chronic inflammatory disease that more commonly affects the ileocecal area in children [1]. Pediatric patients with Crohn’s disease are required to undergo endoscopy, magnetic resonance enterography (MRE), or clinical evaluations such as the pediatric Crohn’s disease activity index (PCDAI) score or C-reactive protein (CRP) level [2,3,4]. Conventional endoscopy for children can provide accurate mucosal information but is an invasive test [5]. MRE has been widely used to determine the disease extent such as structural changes and to have the ability to assess the whole abdomen and pelvis but has a limitation of physiologic evaluation of inflamed bowel [6, 7].
It has been known that dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) improves the ability to show the accompanying microscopic changes in early inflammation and fibrosis or evaluation of therapeutic response [2, 5, 8,9,10]. Quantitative analysis of DCE-MRI is used to estimate the gadolinium contrast agent concentration over time, so we can obtain quantitative perfusion parameters related to microvascular permeability of the inflamed bowel wall [2, 8,9,10,11]. However, few studies have evaluated the diagnostic performance of DCE-MRI with the histopathologic activity of pediatric Crohn’s disease patients as a standard reference.
Our hypotheses were that (a) DCE-MRI perfusion parameters can be used to quantitatively assess changes in the inflamed bowel wall and (b) it can reflect the histopathologic activity of Crohn’s disease. Therefore, the objective of this study was to evaluate the clinical usefulness of DCE-MRI in patients with pediatric Crohn’s disease.
Materials and methods
Our Institutional Review Board (IRB) approved this study. The requirement for informed consent was waived due to its retrospective nature.
Clinical severity scoring
All included patients were assessed using the PCDAI score based on clinical status [12]. The PCDAI score included subjective reporting of the patient’s symptom, the presence of extra-intestinal manifestations, physical examination findings, and laboratory findings [12]. The PCDAI score could be range from 0 to 100, with higher scores indicating more active disease. The CRP level was also assessed in all patients as a secondary clinical severity standard [10]. Clinical characteristics in each patient were evaluated by a pediatric gastroenterologist (J.S.M., with 15 years of clinical experience in pediatric gastroenterology). The PCDAI score and CRP level were assessed within 2 days prior to the MRI examination (0.8 ± 0.5 days).
Reference standard examination
Ileocolonoscopy was performed using standard bowel preparation by a pediatric gastroenterologist (J.S.M., with 15 years of clinical experience in pediatric gastroenterology). The gastroenterologist performed the biopsy at six bowel segments, namely the ileocecal region, ascending colon, transverse colon, descending colon, sigmoid colon, and rectum, within 3 days of the MRI examination. The severity of inflammatory lesions was graded on a scale from 0 to 3: grade 0, absence of lesions; grade 1, presence of inflammatory lesions without ulceration, including erythema, edema, pseudopolyps, and aphthae; and grade 3, presence of superficial or deep ulcerations [13]. The average value of endoscopy score was evaluated at the six bowel segments. The endoscopy with obtaining a biopsy specimen was performed before and after 2 days at the MRI examination (1.2 ± 0.3 days).
A pathologist (G.H.K., over 20 years of clinical experience in pediatric gastrointestinal pathology) had recorded the disease activity such as an active or inactive state of Crohn’s disease at each biopsy specimen, who was unaware of the clinical activity or MRE findings. The definition of the active Crohn’s disease was the presence of neutrophil infiltrations in the cryptal/surface epithelium or lamina propria of the biopsy specimen [14]. The inactive Crohn’s disease was the presence of crypt architectural distortion or crypt atrophy at the biopsy specimen without neutrophil infiltration. We set the histopathologic diagnosis as the reference standard of disease activity by reviewing the electronic medical record for the biopsy specimen.
Image acquisition
MRI examinations were performed using a 1.5-T MRI unit (MAGNETOM Avanto; Siemens Healthineers) with an 8-channel body coil. Supine images were acquired, and then, anti-peristaltic agents (Buscopan; Boehringer Ingelheim) were administered intravenously slowly over 2 min at a dose of 0.3 mg/kg. For patients with pediatric Crohn’s disease, the following sequences were obtained based on our institution’s standard protocol including axial/coronal T2-weighted single-shot fast spin-echo (SSFSE) sequences with and without fat suppression (Table 1).
In order to perform DCE-MRI, the enhancement of the dynamic MR signal must be converted to a change in the tissue relaxation rate R1 (1/T1) and adjusted to the contrast agent concentration of the tissue. Therefore, unenhanced T1-weighted volumetric interpolated breath-hold examination (VIBE) images were obtained at the center of an ileocecal area with flip angles of 2°, 5°, 8°, and 15° for T1 mapping [15,16,17]. The center of the ileocecal region was defined at the location of visibly inflamed bowel (on SSFSE images), or the ileocecal valve when absent of visible bowel wall thickening, and then the coverage expanded with the 4-mm slice thickness and the 24 number of slices on the T1 map images. DCE-MRI performed at the same slices of the T1 map images.
DCE-MRI examinations were performed at the center of the ileocecal area by using a bolus injection of 0.1 mmol/kg of gadoterate meglumine (Dotarem; Guerbet) with a rate of 2.0 mL/s, followed by 25 mL of normal saline by using an MR-compatible power injector (Spectris Solaris; Medrad). All patients were instructed to breathe quietly during the examination. The total scan time of the DCE-MRI sequence was 3 min 25 s including 80 phases, at a temporal resolution of 2.5 s.
Conventional MR severity index
On a picture archiving and communication system workstation, we assessed the mural thickness (mm) and mural signal intensity at the most thickened ileocecal region on the fat-suppressed axial T2 SSFSE sequence using a previously detailed four-grade system [18, 19]. A model for the Crohn’s disease MR index (CDMI) score calculation was used, using the following equation calculated by a pediatric radiologist (S.L., with 6 years of clinical experience in pediatric radiology): CDMI score = mural thickness grade + mural T2 signal grade + perimural T2 signal grade + contrast enhancement grade [10, 20, 21].
The mural thickness was graded on a scale from 0 to 3: grade 0, less than 3 mm; grade 1, 3–5 mm; grade 2, 5–7 mm; and grade 3, over than 7 mm [21]. The grading of the mural signal intensity was graded on a scale from 0 to 3: grade 0, equivalent to normal bowel wall; grade 1, bowel wall appears dark gray on fat-saturated images; grade 2, bowel wall appears light gray on fat-saturated images; and grade 3, bowel wall contains areas of white high signal approaching that of luminal content [18]. The grading of the perimural T2 signal was graded on a scale from 0 to 3: grade 0, equivalent to normal mesentery; grade 1, increase in mesenteric signal but no fluid; grade 2, small fluid rim (≤ 2 mm); and grade 3, large fluid rim (> 2 mm) [18]. The grading of contrast enhancement was graded on a scale from 0 to 3: grade 0, equivalent to normal bowel wall; grade 1, bowel wall signal greater than normal small bowel but significantly less then nearby vascular structures; grade 2, bowel wall signal increased but somewhat less than then nearby vascular structures; and grade 3, bowel wall signal approaches that of nearby vascular structures [18].
Quantitative analysis of DCE-MRI
Voxel-wise perfusion maps were generated by using a dedicated post-processing software program for DCE-MRI (Tissue 4D; Siemens Healthineers), into which the volume transfer coefficient (Ktrans), extracellular extravascular volume fraction (Ve), and rate constant (Kep) were input. We performed the motion correction using the Tissue 4D program, and the motion-corrected images were registered on the T1 map, the signal intensity was converted to a gadolinium concentration, and the time-intensity curve (TIC) was calculated using the Tissue 4D program. Perfusion parameter calculation was based on the Tofts model [22, 23] with the arterial input function (AIF) chosen according to the population-based intermediate sampling method [24].
Two authors (S.L and Y.H.C., with 6 years and 14 years of clinical experience in pediatric radiology, respectively), who were blinded to the patients’ clinical information, performed region of interest (ROI) analysis of the image sets. ROIs were drawn at the three times over the most thickened ileocecal region on the axial images. Circular ROI area ranged from 0.3 to 0.5 cm2. The average value of the perfusion parameters was accepted as the final result for the quantitative perfusion parameter values.
Statistical analysis
All statistical analyses were performed with SPSS version 21.0 (SPSS Inc.).
The PCDAI score, CRP level, endoscopy score, the mural thickness, CDMI score, and DCE-MRI parameters were assessed using the unpaired t test to compare significant differences between the inactive Crohn’s disease group and active group. Grading of the mural thickness, mural signal intensity, perimural signal intensity, and contrast enhancement were compared using Fisher’s exact test. To assess the reproducibility of the DCE-MRI parameter measurements, the intra-class correlation coefficient (ICC) with a two-way random model was calculated between the two observers. The agreement was classified as excellent (ICC > 0.75), moderate (ICC = 0.40–0.75), or poor (ICC < 0.40).
DCE-MRI parameters were compared with the PCDAI score, CRP level, endoscopy score, and the CDMI score, using Pearson’s correlation test. Subsequently, receiver operating characteristic (ROC) curve analysis was used to assess the diagnostic performance of each parameter for active Crohn’s disease patients. The area under the ROC curve (AUC) was used to determine the best cutoff values for the perfusion parameters that proved to be substantial variables in differentiating inactive from active Crohn’s disease by using the method of DeLong et al [25]. Subsequently, multivariate stepwise logistic regression analysis was performed to identify the significant parameters to distinguish the inactive and active groups using the forward stepwise selection method.
Variables with a p value of < 0.05 according to the univariate analysis were used as input variables for the multivariable linear regression analysis, with an iterative entry of variables on the basis of the test results (p values of < 0.05).
Results
We performed DCE-MRI on patients under age 20 having Crohn’s disease between August 2017 and October 2018. The inclusion criteria were as follows: (a) DCE-MRI was performed with an adequate scan range including the ileocecal area; (b) the kinetic model was successfully generated on the TIC of the DCE-MRI; (c) MRI was of good bowel distension; (d) pediatric Crohn’s disease activity and CRP evaluation had been simultaneously obtained by pediatric clinicians within 2 days at the DCE-MRI examination; and (e) the histopathologic evaluation had performed before and after 2 days at the DCE-MRI examination. A total of 30 patients were included in this study (mean age, 15.1 years; age range, 10–20 years; 21 boys and 9 girls). An excluded 31 patients were of which eight patients did not show with an adequate scan range. The TIC did not successfully generate on the DCE-MRI analysis at seven patients. The proper bowel distension had failed at four patients. PCDAI score and CRP evaluation had not obtained at two patients, and the histopathologic evaluation had not performed before the DCE-MRI examination at ten patients.
The number of patients in the inactive and active groups was 8 and 22 patients, respectively (Table 2). There was no difference in age and sex between the inactive and active groups. The active group showed a higher PCDAI score than the inactive group (22.2 ± 18.8 vs. 6.3 ± 6.4, p = 0.027). The active group also showed a higher CRP (mg/dL) level than the inactive group (1.32 ± 1.79 vs. 0.10 ± 0.13, p = 0.005) (Table 2).
In terms of endoscopic findings, the scores of six bowel segments were averaged and then regarded as the total endoscopy score. The endoscopy score in the active group was higher than that in the inactive group, but there was no statistical significance (0.84 ± 0.54 vs. 0.54 ± 0.55, p = 0.160). The endoscopic score at the ileocecal region was a higher score than that of the inactive group (1.55 ± 0.74 vs. 0.88 ± 0.83, p = 0.042) (Table 2).
The mural thickness of the ileocecal area in the active group was higher than that in the inactive group without significance (p = 0.056). Mural thickness grade and mural signal grading did not show any significant difference between the active group and the inactive group (p = 0.309 and p = 0.195). However, there was a significant difference of perimural T2 signal grade and contrast enhancement of the bowel wall between the active and inactive groups (p = 0.021 and p = 0.024). There was a significantly higher CDMI score in the active group than in the inactive group (p = 0.047) (Table 3).
For DCE-MRI perfusion parameters, the Ktrans value was significantly higher in the active Crohn’s disease group than in the inactive group (0.31 ± 0.12 vs. 0.16 ± 0.46, p = 0.002) (Table 4). However, Kep and Ve values did not show any significant difference between the active and inactive groups (Table 4). The perfusion parameters (Ktrans, Kep, and Ve) had ICC values of 0.912 (95% confidence interval [CI], 0.886–0.946), 0.876 (95% CI, 0.843–0.941) and 0.861 (95% CI, 0.813–0.937), respectively.
There was a significant correlation between Ktrans and other perfusion parameters, clinical scores, endoscopic scores (except ileocecal region), or CDMI (Table S1). The PCDAI score, CRP level, and CDMI score were also correlated with other parameters (Table S1).
The ROC analysis for PCDAI score, CRP level, endoscopy finding, CDMI score, and DCE-MRI perfusion parameters for distinguishing the active Crohn’s disease group is shown in Table 5. In the multivariate logistic regression analysis, we identified the Ktrans value (p = 0.013) as a significant variable in the differentiation of the active Crohn’s disease group from the inactive group, whereas PCDAI score, CRP level, endoscopy finding of ileocecal region, and CDMI score were not included in the logistic regression equation.
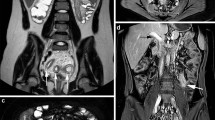
Figures 1 and 2 show representative active and inactive Crohn’s disease cases, respectively.
Dynamic contrast-enhanced perfusion MRI images of active Crohn’s disease. The histopathologic diagnosis of this 16-year-old female patient was active in Crohn’s disease. Her pediatric Crohn’s disease activity (PCDAI) score and C-reactive protein level were 47.5 and 1.1 (mg/dL), respectively. a The T2-weighted axial image showed the thickened bowel wall at the ileocecal valve (arrow), which appeared light gray on fat-saturated image. b The T1-weighted contrast-enhanced axial image showed the bowel wall signal increased (arrow) similar to that of nearby vascular structures. Thus, the Crohn’s disease magnetic resonance imaging index (CDMI) score was 10 with a marked thickness of mural bowel wall and enhancement. c Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) color map images were generated through the time-intensity curve (TIC) using the Tissue 4D program. d The region of interest (circle) was drawn at the most thickened bowel wall without invading the bowel lumen. The yellowish area in the volume transfer coefficient (Ktrans) map indicates the increase of the Ktrans value. The abnormal bowel segment showed an increase in the Ktrans value (0.428). e The reddish area in the rate constant (Kep) map indicates the increase of the Kep value. There was a mild increase in the Kep value in the abnormal bowel segment (Kep = 1.21). f The orange-greenish area in the extracellular extravascular volume fraction (Ve) map indicates the increase of the Ve value. There was also an increase in the Ve value in the abnormal bowel segment (Ve = 0.353)
Dynamic contrast-enhanced perfusion MRI images of inactive Crohn’s disease. The histopathologic diagnosis of this 14-year-old male patient was inactive in Crohn’s disease. His pediatric Crohn’s disease activity (PCDAI) score and C-reactive protein level were 5.0 and 0.01 (mg/dL), respectively. a The T2-weighted axial image showed the mildly thickened bowel wall at the ileocecal valve (arrow), which appeared light gray on fat-saturated image. b The T1-weighted contrast-enhanced axial image showed the bowel wall signal was higher than normal small bowel (arrow) but significantly less than nearby vascular structures. Thus, the Crohn’s disease magnetic resonance imaging index (CDMI) score was 5 with a mild thickness of mural bowel wall and mild enhancement. c Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) color map images were generated through the time-intensity curve (TIC) using the Tissue 4D program. d The region of interest (circle) was drawn at the most thickened bowel wall without invading the bowel lumen. The yellowish area in the volume transfer coefficient (Ktrans) map indicates the increase of the Ktrans value. The abnormal bowel segment showed a rise increase in the Ktrans value (0.214). e The reddish area in the rate constant (Kep) map indicates the increase of the Kep value. There was a mildly increase in the Kep value in the abnormal bowel segment (Kep = 1.305). f The orange-greenish area in the extracellular extravascular volume fraction (Ve) map indicates the increase of the Ve value. There was no increase in the Ve value in the abnormal bowel segment (Ve = 0.164)
Discussion
This study assessed whether DCE-MRI can identify changes in the inflamed bowel wall and evaluate clinical or histopathologic disease severity in pediatric patients with Crohn’s disease. Our study demonstrated that the Ktrans value of DCE-MRI can be used to quantify perfusion changes in pediatric Crohn’s disease patients.
DCE-MRI has been used for perfusion status assessment during the disease process [26]. In terms of Crohn’s disease pathogenesis, it is known that disease progression induces microvascular remodeling, increases the permeability of blood vessel walls, and increases exudation from blood vessels to extracellular space [27]. Oto et al [8] demonstrated that DCE-MRI perfusion parameters were a more useful diagnostic tool than conventional MRE in adult patients. Ziech et al [28] also reported that changes in Ktrans might be more indicative of changes in the inflamed bowel wall. However, Taylor et al [29] reported that the Ktrans value did not show any correlation with histologic and clinical markers of inflammation, despite a correlation with disease chronicity and angiogenesis. Despite previous controversial studies [28, 29], there are few studies in pediatric Crohn’s disease patients that performed DCE-MRI. Our study showed that the perfusion parameter of DCE-MRI might be higher in the active Crohn’s disease group than in the inactive group, according to the histopathologic activity. These results imply that perfusion changes in patients who had an active histopathologic result could be successfully monitored by DCE-MRI.
Crohn’s disease is a chronic inflammatory bowel disease, the severity of which should be evaluated using various imaging modalities. The objective assessment of disease severity is important for therapeutic decisions; therefore, clinical practice may require a scoring system with clinical, endoscopic, radiological, and combined indices [21]. Among the clinical scoring systems, the PCDAI score and CRP level are known to be valid indicators of disease activity in pediatric Crohn’s disease patients [4, 12, 30]. Our study demonstrated the Ktrans value had a significant correlation with the PCDAI score and CRP level. PCDAI score was based on the clinician’s opinion regarding disease activity in the patient. It was expected to increase Ktrans value because the increased permeability is a hallmark of active inflammation, although this factor could not represent whole clinical spectrums.
Crohn’s disease has a characteristic that can affect all layers of the intestinal wall. The endoscopic finding from the mucosal surface may not detect the disease activity in the deep layers of the affected bowel segment [13]. We evaluated the simple grading of endoscopy lesion (absence, presence, and presence with ulceration) activity associated with Crohn’s disease. There was a significant correlation between the sum of endoscopy abnormality and Ktrans, CDMI score, or clinical index such as PCDAI and CRP level. However, we could not demonstrate the validity of our simplified endoscopic scoring system at the ileocecal region; therefore, the further prospective design would need for the objective endoscopic grading in the affected bowel segments.
Several previous studies have examined the ability of MRE to monitor disease severity in relation to disease activity status in adult patients [11, 13, 19, 31]. In terms of pediatric patients with Crohn’s disease, Pomerri et al [32] have reported that the Magnetic Resonance Index of Activity (MaRIA) based on MRE findings, such as wall thickness (mm), relative contrast enhancement, and presence of edema and ulcers, was correlated with clinical validity. However, the MaRIA score has a potential limitation to reproducibility because of subjective factors [33]. Steward et al [19] have presented the CDMI scoring system using the weighted sum of mural thickness and mural signal change as predictive histopathological findings, although this study was conducted in adult patients. Our study demonstrated that both the CDMI score and the Ktrans value showed a significant difference between the active and inactive groups. In the multivariate logistic regression model, the Ktrans value was the most significant variable in predicting active Crohn’s disease patients. The CDMI index has been shown to be accurate for the diagnosis of disease activity but not for quantification of severity [34]. Ziech et al [28] demonstrated that the bowel wall thickness, the critical objective score in the CDMI system, did not linearly increase with disease activity due to the fibrous component or previous inflammation sequel. Therefore, the Ktrans value might have a further advantage of the quantification of the disease activity or the assessment of the treatment response.
However, some practical limitations of the Ktrans value have to be acknowledged. Our study showed that there was a difference in the perfusion parameter values reported in previous studies. Our mean Ktrans values in the inactive and active Crohn’s disease groups were 0.16 min−1 and 0.31 min−1, respectively. The results described herein are similar to those described by Taylor et al [29], but these values are very low compared with previously reported Ktrans values [8, 10]. Another explanation of these results might be the lower MR field strength resulting in a temporal resolution of 2.5 s. The different temporal resolutions could affect the AIF measurements, and we believe that the most important factor might be the image acquisition method such as AIF [35]. We used the population-based AIF method that is provided in the Tissue 4D software, similar to Taylor et al’s study. On the other hand, other studies that found higher Ktrans values used a customized AIF method [8, 10, 36]. In addition, the different temporal resolutions could impact the incorrect AIF measurement and ultimately lower Ktrans values [8]. The main advantage of this study, however, is that our DCE-MRI results were obtained uniformly and a standard histopathologic evaluation was used as a reference.
Our study has several limitations that need to be taken into account when interpreting the data. First, the perfusion parameters estimated by segmental ROIs of DCE-MRI images might have a weak correlation with biopsy specimen of whole bowel segments. However, it is also important to focus on quantitative changes of perfusion parameters in each pediatric patient depending on their histopathologic result because endoscopy or histopathologic result may also have any segmental sampling problem. Second, it is up to the observer to choose the ROI in the inflamed bowel wall. Previous DCE-MRI studies had remarked that the thin diameter of the normally distended bowel wall can introduce inappropriate ROI placement [2, 29]. Thus, we used an inter-observer reliability test to assess perfusion parameters; there was an excellent agreement in measurements of the inflamed bowel wall. Third, we allowed the free breathing quietly during the DCE-MRI examination because many pediatric patients could not endure the breath hold during the image scan. Even a quiet breath could affect the correct evaluation of DCE-MRI perfusion data. However, there was no breathing-related artifact because a mean age of the patient was adolescent age. Finally, we did not compare the perfusion parameters to the enhancement pattern or the presence of diffusion restriction. Previous studies [31, 37] had demonstrated that delayed contrast enhancement or diffusion-weighted imaging technique could be a useful imaging technique to differentiate the fibrosis and inflammatory components in Crohn’s disease. Hectors et al [38] had demonstrated that the combination of diffusion-weighted imaging and DCE-MR technique could be useful for the quantification of small bowel Crohn’s disease of adult patients. Therefore, it would be needed to assess the combined effect of perfusion and diffusion-weighted imaging techniques to evaluate the accurate histopathologic status.
In conclusion, our results suggest that quantitative parameters of DCE-MRI in pediatric Crohn’s disease patients can monitor disease activity and may indicate differences in inflammatory bowel loops between the active and inactive Crohn’s disease groups. The measurements of the Ktrans value might be possible in the assessment of Crohn’s disease activity in pediatric patients.
Abbreviations
- CDMI:
-
Crohn’s disease magnetic resonance imaging index
- CRP:
-
C-reactive protein
- DCE-MRI:
-
Dynamic contrast-enhanced magnetic resonance imaging
- K ep :
-
Rate constant
- K trans :
-
Volume transfer coefficient
- MRE:
-
Magnetic resonance enterography
- PCDAI:
-
Pediatric Crohn’s disease activity index
- TIC:
-
Time-intensity curve
- V e :
-
Extracellular extravascular volume fraction
References
Shikhare G, Kugathasan S (2010) Inflammatory bowel disease in children: current trends. J Gastroenterol 45:673–682
Zhu JG, Zhang FM, Zhou JF, Li HG (2017) Assessment of therapeutic response in Crohn’s disease using quantitative dynamic contrast enhanced MRI (DCE-MRI) parameters: a preliminary study. Medicine (Baltimore) 96:e7759
Yang L, Ge ZZ, Gao YJ et al (2013) Assessment of capsule endoscopy scoring index, clinical disease activity, and C-reactive protein in small bowel Crohn’s disease. J Gastroenterol Hepatol 28:829–833
Hyams J, Markowitz J, Otley A et al (2005) Evaluation of the pediatric Crohn disease activity index: a prospective multicenter experience. J Pediatr Gastroenterol Nutr 41:416–421
Levine A, Koletzko S, Turner D et al (2014) ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr 58:795–806
Mentzel HJ, Reinsch S, Kurzai M, Stenzel M (2014) Magnetic resonance imaging in children and adolescents with chronic inflammatory bowel disease. World J Gastroenterol 20:1180–1191
Ziech ML, Hummel TZ, Smets AM et al (2014) Accuracy of abdominal ultrasound and MRI for detection of Crohn disease and ulcerative colitis in children. Pediatr Radiol 44:1370–1378
Oto A, Kayhan A, Williams JT et al (2011) Active Crohn's disease in the small bowel: evaluation by diffusion weighted imaging and quantitative dynamic contrast enhanced MR imaging. J Magn Reson Imaging 33:615–624
Tielbeek JA, Ziech ML, Li Z et al (2014) Evaluation of conventional, dynamic contrast enhanced and diffusion weighted MRI for quantitative Crohn’s disease assessment with histopathology of surgical specimens. Eur Radiol 24:619–629
Zhu JG, Zhang FM, Luan Y et al (2016) Can dynamic contrast-enhanced MRI (DCE-MRI) and diffusion-weighted MRI (DW-MRI) evaluate inflammation disease a preliminary study of Crohn’s disease. Medicine (Baltimore) 95:e3239
Rimola J, Rodriguez S, Garcia-Bosch O et al (2009) Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut 58:1113–1120
Hyams JS, Ferry GD, Mandel FS et al (1991) Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr 12:439–447
Rimola J, Ordas I, Rodriguez S et al (2011) Magnetic resonance imaging for evaluation of Crohn’s disease: validation of parameters of severity and quantitative index of activity. Inflamm Bowel Dis 17:1759–1768
Gui X, Li J, Ueno A, Iacucci M, Qian J, Ghosh S (2018) Histopathological features of inflammatory bowel disease are associated with different CD4+ T cell subsets in colonic mucosal lamina propria. J Crohns Colitis 12:1448–1458
Li KL, Zhu XP, Waterton J, Jackson A (2000) Improved 3D quantitative mapping of blood volume and endothelial permeability in brain tumors. J Magn Reson Imaging 12:347–357
Braren R, Curcic J, Remmele S et al (2011) Free-breathing quantitative dynamic contrast-enhanced magnetic resonance imaging in a rat liver tumor model using dynamic radial T(1) mapping. Invest Radiol 46:624–631
Kim JH, Lee JM, Park JH et al (2013) Solid pancreatic lesions: characterization by using timing bolus dynamic contrast-enhanced MR imaging assessment-a preliminary study. Radiology 266:185–196
Makanyanga JC, Pendse D, Dikaios N et al (2014) Evaluation of Crohn’s disease activity: initial validation of a magnetic resonance enterography global score (MEGS) against faecal calprotectin. Eur Radiol 24:277–287
Steward MJ, Punwani S, Proctor I et al (2012) Non-perforating small bowel Crohn’s disease assessed by MRI enterography: derivation and histopathological validation of an MR-based activity index. Eur J Radiol 81:2080–2088
Deepak P, Kolbe AB, Fidler JL, Fletcher JG, Knudsen JM, Bruining DH (2016) Update on magnetic resonance imaging and ultrasound evaluation of Crohn’s disease. Gastroenterol Hepatol (N Y) 12:226–236
Rozendorn N, Amitai MM, Eliakim RA, Kopylov U, Klang E (2018) A review of magnetic resonance enterography-based indices for quantification of Crohn’s disease inflammation. Therap Adv Gastroenterol 11:1756284818765956
Tofts PS, Kermode AG (1991) Measurement of the blood-brain-barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental-concepts. Magn Reson Med 17:357–367
Tofts PS, Brix G, Buckley DL et al (1999) Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 10:223–232
Orton MR, d’Arcy JA, Walker-Samuel S et al (2008) Computationally efficient vascular input function models for quantitative kinetic modelling using DCE-MRI. Phys Med Biol 53:1225–1239
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
O'Connor JP, Jackson A, Parker GJ, Jayson GC (2007) DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. Br J Cancer 96:189–195
Deban L, Correale C, Vetrano S, Malesci A, Danese S (2008) Multiple pathogenic roles of microvasculature in inflammatory bowel disease: a jack of all trades. Am J Pathol 172:1457–1466
Ziech ML, Lavini C, Caan MW et al (2012) Dynamic contrast-enhanced MRI in patients with luminal Crohn’s disease. Eur J Radiol 81:3019–3027
Taylor SA, Punwani S, Rodriguez-Justo M et al (2009) Mural Crohn disease: correlation of dynamic contrast-enhanced MR imaging findings with angiogenesis and inflammation at histologic examination--pilot study. Radiology 251:369–379
Otley A, Loonen H, Parekh N, Corey M, Sherman PM, Griffiths AM (1999) Assessing activity of pediatric Crohn’s disease: which index to use? Gastroenterology 116:527–531
Rimola J, Planell N, Rodriguez S et al (2015) Characterization of inflammation and fibrosis in Crohn’s disease lesions by magnetic resonance imaging. Am J Gastroenterol 110:432–440
Pomerri F, Al Bunni F, Zuliani M et al (2017) Assessing pediatric ileocolonic Crohn’s disease activity based on global MR enterography scores. Eur Radiol 27:1044–1051
Kim JS, Jang HY, Park SH et al (2017) MR Enterography assessment of bowel inflammation severity in Crohn disease using the MR index of activity score: modifying roles of DWI and effects of contrast phases. AJR Am J Roentgenol 208:1022–1029
Ordas I, Rimola J, Alfaro I et al (2019) Development and validation of a simplified magnetic resonance index of activity for Crohn’s disease. Gastroenterology 157:432–439
Evelhoch JL (1999) Key factors in the acquisition of contrast kinetic data for oncology. J Magn Reson Imaging 10:254–259
Ziech ML, Lavini C, Bipat S et al (2013) Dynamic contrast-enhanced MRI in determining disease activity in perianal fistulizing Crohn disease: a pilot study. AJR Am J Roentgenol 200:W170–W177
Li XH, Mao R, Huang SY et al (2018) Characterization of degree of intestinal fibrosis in patients with Crohn disease by using magnetization transfer MR imaging. Radiology 287:494–503
Hectors SJ, Gordic S, Semaan S et al (2019) Diffusion and perfusion MRI quantification in ileal Crohn’s disease. Eur Radiol 29:993–1002
Funding
This work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Young Hun Choi.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Seunghyun Lee and Young Hun Choi have significant statistical expertise.
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Lee, S., Choi, Y.H., Cho, Y.J. et al. Quantitative evaluation of Crohn’s disease using dynamic contrast-enhanced MRI in children and young adults. Eur Radiol 30, 3168–3177 (2020). https://doi.org/10.1007/s00330-020-06684-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06684-1