Abstract
Introduction
This was a prospective, first-in-human trial to evaluate the feasibility and safety of insertion of biopsy introducer needles with our robot during CT fluoroscopy–guided biopsy in humans.
Materials and methods
Eligible patients were adults with a lesion ≥ 10 mm in an extremity or the trunk requiring pathological diagnosis with CT fluoroscopy–guided biopsy. Patients in whom at-risk structures were located within 10 mm of the scheduled needle tract were excluded. Ten patients (4 females and 6 males; mean [range] age, 72 [52–87] years) with lesions (mean [range] maximum diameter, 28 [14–52] mm) in the kidney (n = 4), lung (n = 3), mediastinum (n = 1), adrenal gland (n = 1), and muscle (n = 1) were enrolled. The biopsy procedure involved robotic insertion of a biopsy introducer needle followed by manual acquisition of specimens using a biopsy needle. The patients were followed up for 14 days. Feasibility was defined as the distance of ≤ 10 mm between needle tip after insertion and the nearest lesion edge on the CT fluoroscopic images. The safety of robotic insertion was evaluated on the basis of machine-related troubles and adverse events according to the Clavien-Dindo classification.
Results
Robotic insertion of the introducer needle was feasible in all patients, enabling pathological diagnosis. There was no machine-related trouble. A total of 11 adverse events occurred in 8 patients, including 10 grade I events and 1 grade IIIa event.
Conclusion
Insertion of biopsy introducer needles with our robot was feasible at several locations in the human body.
Key Points
• Insertion of biopsy introducer needles with our robot during CT fluoroscopy–guided biopsy was feasible at several locations in the human body.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Computed tomography (CT)–guided needle biopsy is an established method for pathologic diagnosis of lesions at various anatomic locations. Conventional CT systems are typically used as an imaging guide for needle insertion. With these systems, however, the physicians must rely on a technician for CT scanning, and visualization of the CT images requires time. CT fluoroscopy may allow for CT scanning by the physicians and provide almost real-time imaging display [1], thereby significantly reducing the procedure duration [2,3,4,5,6]. However, a major disadvantage of CT fluoroscopy is the possibility of radiation exposure to the physicians who are close to the CT gantry [7,8,9,10,11]. To address this issue, we have been developing a remote-controlled robot that enables needle insertion under CT guidance since January 2012. Although some robots for CT-guided intervention including Maxio (Perfint healthcare) [12], Innomotion (Innomedic) [13], and iSYS (iSYS Medizintechnik) [14] have already been commercialized, their task is confined to needle-guiding. That is, the robot can position and orient a needle holder on the basis of preprocedural CT images, but needle insertion must still be done manually through the holder by a physician. Thus, a disadvantage of those robots may be the difficulty of adjusting needle orientation to compensate for needle deviation and target movement that may occur during insertion.
A phantom study [15] showed that insertion of biopsy introducer needles with our robot under CT fluoroscopy guidance prevented radiation exposure to the physician, and the accuracy of robotic needle insertion was equivalent to that performed manually. Subsequent animal experiments showed that robotic insertion of biopsy introducer needles was safe and accurate in several in vivo anatomic locations [15]. In addition to prevention of radiation exposure to the physician, the ability to define exact angle and depth of the needle using our robot may be an advantage over manual needle insertion. Those results of the previous study encouraged us to conduct a first clinical trial. The purpose of the present study was to evaluate the feasibility and safety of robotic insertion of biopsy introducer needles during CT fluoroscopy–guided needle biopsy in humans.
Materials and methods
This was a single-center, single-arm, open-label, prospective, first-in-human, feasibility trial that was approved by the institutional review board. All patients provided written informed consent. The trial was registered on the University Hospital Medical Information Network (UMIN000030018) and Japan Registry of Clinical Trials (jRCTs062180001).
Study endpoints, eligibility criteria, and study population
The primary and secondary endpoints were the feasibility and safety, respectively, of robotic insertion of the biopsy introducer needle. Eligibility criteria are shown in Table 1. The recruitment goal was 10 patients; this number was not based on statistical evidence because of the exploratory nature of the trial. Then, 10 patients (4 females and 6 males; mean age, 72 years; age range, 52–87 years) were enrolled between June and October 2018. The characteristics of the patients and lesions are summarized in Table 2. Target lesions were located in the kidney (n = 4), lung (n = 3), mediastinum (n = 1), adrenal gland (n = 1), and muscle (n = 1). The mean maximum diameter of the lesions was 28 mm (range, 14–52 mm). The schedules for subject enrollment, procedure, and assessments are summarized in Table E1. This study involved an interim evaluation, in-house monitoring, and audit.
Robotic system
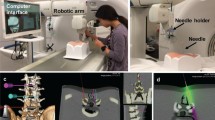
Details regarding our robotic system (Zerobot; Medicalnet Okayama) (Fig. 1) have been described elsewhere [15,16,17]. Briefly, the system aims to make a floor-mounted robot hold, position, orient, and insert a needle under CT guidance while a physician remotely operates the interface comprising a controller and a touch panel, thereby avoiding radiation exposure. The robot may be manipulated by either button operation of the controller or numerical inputs on software displayed on the touch panel. With the former technique, the robot moves while the buttons of the controller are manually pressed, whereas with the latter technique, the robot moves to a certain place semi-automatically after numerical inputs. However, in this study, the robot was manipulated only by button operation of the controller.
Biopsy procedure
The biopsy procedure was performed under conscious sedation in an inpatient setting. A commercially available coaxial biopsy needle system consisting of a biopsy introducer needle (TSK guide needle; TSK Laboratory) and a semiautomatic cutting biopsy needle (STAR CUT; TSK Laboratory) was used. A 19-gauge introducer needle was used for lung biopsy, while a 17-gauge one was used for biopsy in other locations. A sliding-gantry CT scanner (Aquilion 64; Cannon Medical Systems) was used as the imaging modality. The biopsy procedure involved robotic insertion of the biopsy introducer needle toward the lesion (i.e., robotic procedure), followed by manual acquisition of specimens using the biopsy needle (i.e., manual procedure).
The robot was physically placed on the CT table in an appropriate position. The patient was scanned by CT for planning needle insertion. A needle tract was determined on an axial CT image; the length and angle of the tract were measured at a CT console. Then, the skin entry point was marked on the patient. Local anesthesia was manually administered at the entry point.
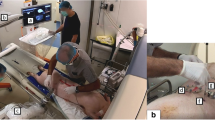
A photograph taken during the robotic procedure is shown in Fig. 2. The same physician (T.H., who had 23 years of experience in CT-guided intervention and has been involved in the development of the robot for 6 years) operated the robot in all cases. The physician wore an electronic dosimeter (Hitachi-Aloka Medical) on the upper chest outside the lead apron.
A photograph taken during the robotic procedure. The physician sits behind a lead shield a few meters away from the CT gantry. The physician operates the robot (black arrow) with the operation interface (black arrowheads), monitoring 3 continuous CT fluoroscopic images (white arrow) as well as the operative field (white arrowhead) displayed by a web camera (not shown). The small white arrow indicates the controller for operating the CT gantry
The physician operated the interface near the patient, in order to orient the needle at the predetermined angle and position the needle tip at the entry point. The physician moved behind a lead shield a few meters away from the CT gantry. Three continuous CT fluoroscopic images were acquired at the level of the entry point, followed by needle adjustment as needed. After a small skin incision was manually made by an assistant as necessary, robotic needle insertion started. CT fluoroscopy was used to check the needle intermittently or continuously during insertion. Needle insertion was completed when the physician judged the needle tip to be sufficiently near the lesion to acquire the specimens. Then, the needle was manually detached from the holder, leaving its tip in place. The robotic arm was removed from the operative field for the following manual procedure. Specimens were then obtained with the biopsy needle through the introducer needle. After removal of the needles, final CT images were obtained to evaluate adverse events.
Follow-up
The patients were kept in hospital for a night after the biopsy. The patients were asked to visit the hospital on day 14 for follow-up.
Evaluation of outcomes
The feasibility of robotic insertion was evaluated by an independent Data Safety Monitoring Committee, based on the distance between the tip of the biopsy introducer needle after insertion and the nearest edge of the lesion measured on the CT fluoroscopic images. When it was ≤ 10 mm, the robotic insertion was judged as feasible. The safety of robotic insertion was evaluated on the basis of machine-related troubles and adverse events according to the Clavien-Dindo classification, version 2.0 [18].
Effective dose to the physician and time using CT fluoroscopy during the robotic procedure were recorded. The duration of the robotic procedure (interval between the first and last use of CT fluoroscopy) and of the entire biopsy procedure (interval between initiation of the first CT scan and completion of the final CT scan) was also recorded.
Statistical analyses
Characteristics of the patients and procedures were summarized by descriptive statistics (mean, standard deviation, median, etc.).
Results
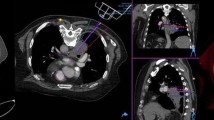
The results of the procedures are summarized in Table 3. In all cases, robotic insertion of the introducer needle was feasible (Fig. 3; Movies 1 and 2), following which a concordant pathological diagnosis was made. During the robotic procedure, the effective dose to the physician was 0 μSv in all cases and the mean time using CT fluoroscopy was 29 s (range, 4–124 s). Mean duration of the robotic procedure was 4 min (range, 0–23 min). The robotic procedure was completed within 4 min in 9 cases, whereas it took 23 min in 1 case (case 6). In case 6, we first planned a needle tract along the scapula and through the small intercostal space. We tried needle insertion 3 times, but the scapula and the rib always interfered with insertion, resulting in needle deviation. Therefore, a new tract through another intercostal space was planned. The robot was then repositioned, followed by a successful insertion. Mean duration of the entire biopsy procedure was 29 min (range, 17–53 min).
(MP4 23,001 kb)
(MP4 15,518 kb)
There was no machine-related trouble. A total of 11 adverse events (10 grade I and 1 grade IIIa) occurred in 8 patients, including 2 events unrelated to the procedure. The grade I events related to the procedure consisted of hemorrhage and pneumothorax, without the need for treatment, while the grade IIIa event was pneumothorax requiring chest tube placement. All patients were discharged on the following day.
Discussion
CT fluoroscopy–guided intervention may be associated with radiation exposure to the physician while using CT fluoroscopy [7,8,9,10,11]. Previous studies on CT fluoroscopy–guided biopsy showed that mean CT fluoroscopy time was 28–90 s [2, 10, 11]; the estimated effective dose to the physician was 0.054 mSv [10]; and the equivalent dose to the physicians was 4.7 μSv [11]. The results of this first-in-human trial indicated that robotic insertion of the biopsy introducer needles was feasible at several locations in the human body without radiation exposure to the physician. Robotic insertion was usually quick, requiring minimal use of CT fluoroscopy.
Robots to enable needle insertion under CT guidance have been also developed by several groups. Light Puncture Robot is a robot composed of a patient-mounted needle holder with 3 degrees of freedom (DOF) that is supported by a specific frame with 2 DOF [19]. Robopsy is a compact disposable patient-mounted robot with 4 DOF, one of which allows for gripping and releasing the needle [20]. Recently, Shahriari et al have proposed a robot containing the remote-center of a moving robotic arm with 2 DOF and a needle insertion device, which allows for real-time correction of needle orientation using fusion images of CT data and electromagnetic tracking data [21]. Won et al have developed a master-slave robot, which consisted of a floor-mounted manufacture robot and an end-effector that was attached to the robot arm [22]. More recently, XACT Robotics, Ltd. developed a patient-mounted robot with 5 DOF that enabled needle steering during stepwise correction of needle orientation based on the reconstructed CT images [23]. To our knowledge, however, those robots are still in the experimental stage.
Acubot, developed at Johns Hopkins University, is a table-mounted robot with 6 DOF for needle insertion [24,25,26]. Acubot was first used in clinical cases of biopsy and radiofrequency ablation in several locations, neobladder access, and nephrostomy [24]. Robotic needle insertion was successful in all cases with no relevant complications. A randomized clinical trial in 20 cases of nerve and facet blocks under fluoroscopic guidance showed that robotic needle insertion had equivalent accuracy to manual insertion [25]. Another randomized study with 14 procedures of liver radiofrequency ablation revealed several advantages of robotic insertion over manual insertion, including fewer needle adjustments, shorter procedure time, and less radiation exposure for both physicians and patients [26]. Regrettably, however, Acubot has not been commercialized yet.
Although this study indicated the feasibility of insertion of the biopsy introducer needles with our robot in humans, a limitation to our robot was also revealed in a case. The needle deviated when the bone interfered with the insertion. The physician did not realize the interference before CT fluoroscopic images demonstrated the needle deviation. Haptic feedback could have informed the physician of the interference before needle deviation.
This was a preliminary study on the use of our robot in clinical procedures. Thus, the study suffered from limitations due to its exploratory nature, including a small study population in a single institution, a highly experienced single physician operating the robot, and study design without comparison of robotic insertion with manual insertion. Furthermore, we did not record time or the number of persons required for setup and removal of the robot. Roughly speaking, however, setup and removal took 20–30 min and 10–20 min, respectively, with at least two persons. Moreover, effective dose to the physician during the entire procedure was not measured.
In summary, the present first-in-human trial showed that insertion of the biopsy introducer needles with our robot was feasible at several locations in the human body. A randomized control trial comparing robotic to manual insertion with regard to needle insertion accuracy, radiation exposure, procedure time, adverse events, etc. using a larger population is now being planned.
Abbreviations
- CT:
-
Computed tomography
- DOF:
-
Degrees of freedom
- SD:
-
Standard deviation
References
Katada K, Kato R, Anno H et al (1996) Guidance with real-time CT fluoroscopy: early clinical experience. Radiology 200:851–856
Froelich JJ, Ishaque N, Regn J, Saar B, Walthers EM, Klose KJ (2002) Guidance of percutaneous pulmonary biopsies with real-time CT fluoroscopy. Eur J Radiol 42:74–79
Gianfelice D, Lepanto L, Perreault P, Chartrand-Lefebvre C, Milette PC (2000) Value of CT fluoroscopy for percutaneous biopsy procedures. J Vasc Interv Radiol 11:879–884
Kirchner J, Kickuth R, Laufer U, Schilling EM, Adams S, Liermann D (2002) CT fluoroscopy assisted puncture of thoracic and abdominal masses: a randomized trial. Clin Radiol 57:188–192
Carlson SK, Bender CE, Classic KL et al (2001) Benefits and safety of CT fluoroscopy in interventional radiologic procedures. Radiology 219:515–520
Silverman SG, Tuncali K, Adams DF, Nawfel RD, Zou KH, Judy PF (1999) CT fluoroscopy-guided abdominal interventions: techniques, results, and radiation exposure. Radiology 212:673–681
Paulson EK, Sheafor DH, Enterline DS, McAdams HP, Yoshizumi TT (2001) CT fluoroscopy-guided interventional procedures: techniques and radiation dose to radiologists. Radiology 220:161–167
Daly B, Krebs TL, Wong-You-Cheong JJ, Wang SS (1999) Percutaneous abdominal and pelvic interventional procedures using CT fluoroscopy guidance. AJR Am J Roentgenol 173:637–644
Matsui Y, Hiraki T, Gobara H et al (2016) Radiation exposure of interventional radiologists during computed tomography fluoroscopy-guided renal cryoablation and lung radiofrequency ablation: direct measurement in a clinical setting. Cardiovasc Intervent Radiol 39:894–901
Kim GR, Hur J, Lee SM et al (2011) CT fluoroscopy-guided lung biopsy versus conventional CT-guided lung biopsy: a prospective controlled study to assess radiation doses and diagnostic performance. Eur Radiol 21:232–239
Teeuwisse WM, Geleijns J, Broerse JJ, Obermann WR, van Persijn van Meerten EL (2001) Patient and staff dose during CT guided biopsy, drainage and coagulation. Br J Radiol 74:720–726
Smakic A, Rathmann N, Kostrzewa M, Schönberg SO, Weiß C, Diehl SJ (2018) Performance of a robotic assistance device in computed tomography-guided percutaneous diagnostic and therapeutic procedures. Cardiovasc Intervent Radiol 41:639–644
Wiewiorski M, Valderrabano V, Kretzschmar M et al (2009) CT-guided robotically-assisted infiltration of foot and ankle joints. Minim Invasive Ther Allied Technol 18:291–296
Groetz S, Wilhelm K, Willinek W, Pieper C, Schild H, Thomas D (2016) A new robotic assistance system for percutaneous CT-guided punctures: initial experience. Minim Invasive Ther Allied Technol 25:79–85
Hiraki T, Kamegawa T, Matsuno T et al (2017) Robotically driven CT-guided needle insertion: preliminary results in phantom and animal experiments. Radiology 285:454–461
Hiraki T, Matsuno T, Kamegawa T et al (2018) Robotic insertion of various ablation needles under computed tomography guidance: accuracy in animal experiments. Eur J Radiol 105:162–167
Hiraki T, Kamegawa T, Matsuno T, Komaki T, Sakurai J, Kanazawa S (2018) Zerobot®: a remote-controlled robot for needle insertion in CT-guided interventional radiology developed at Okayama University. Acta Med Okayama 72:539–546
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Bricault I, Zemiti N, Jouniaux E et al (2008) Light puncture robot for CT and MRI interventions: designing a new robotic architecture to perform abdominal and thoracic punctures. IEEE Eng Med Biol Mag 27:42–50
Walsh CJ, Hanumara NC, Slocum AH, Shepard JA, Gupta R (2008) A patient-mounted, telerobotic tool for CT-guided percutaneous interventions. J Med Devices 2:011007–011010
Shahriari N, Heerink W, van Katwijk T, Hekman E, Oudkerk M, Misra S (2017) Computed tomography (CT)-compatible remote center of motion needle steering robot: fusing CT images and electromagnetic sensor data. Med Eng Phys 45:71–77
Won HJ, Kim N, Kim GB, Seo JB, Kim H (2017) Validation of a CT-guided intervention robot for biopsy and radiofrequency ablation: experimental study with an abdominal phantom. Diagn Interv Radiol 23:233–237
Ben-David E, Shochat M, Roth I, Nissenbaum I, Sosna J, Goldberg SN (2018) Evaluation of a CT-guided robotic system for precise percutaneous needle insertion. J Vasc Interv Radiol 29:1440–1446
Solomon SB, Patriciu A, Bohlman ME, Kavoussi LR, Stoianovici D (2002) Robotically driven interventions: a method of using CT fluoroscopy without radiation exposure to the physician. Radiology 225:277–282
Cleary K, Watson V, Lindisch D et al (2005) Precision placement of instruments for minimally invasive procedures using a “needle driver” robot. Int J Med Robot 1:40–47
Patriciu A, Awad M, Solomon SB et al (2005) Robotic assisted radio-frequency ablation of liver tumors: randomized patient study. Med Image Comput Comput Assist Interv 8:526–533
Funding
This study has received funding from the Japan Society for the Promotion of Science (JSPS) (18K07677), Promotion of Science and Technology, Okayama Prefecture, Japan, Agency for Medical Research and Development (AMED) (15hk0102014h001, 15hk0102014h002, 15hk0102014h003), JSPS (25461882, 17K10439), Organization for Research Promotion & Collaboration, Okayama University; Japan Radiological Society, and Cannon Medical Systems Corporation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Takao Hiraki.
Conflict of interest
Drs. Hiraki and Kanazawa declare relationships with the following company: Cannon Medical Systems. Other authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Hiraki, T., Kamegawa, T., Matsuno, T. et al. Robotic needle insertion during computed tomography fluoroscopy–guided biopsy: prospective first-in-human feasibility trial. Eur Radiol 30, 927–933 (2020). https://doi.org/10.1007/s00330-019-06409-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06409-z