Abstract
Objectives
To explore if there is a correlation between T2WI histogram features of the primary tumor and the existence of regional lymph node (LN) metastasis in rectal cancer.
Methods
Eighty-eight patients with pathologically proven rectal adenocarcinoma, who received direct surgical resection and underwent preoperative rectal MRIs, were enrolled retrospectively. Based on pathological analysis of surgical specimen, patients were classified into negative LN (LN−) and positive LN (LN+) groups. The degree of differentiation and pathological T stage were recorded. Clinical T stage, tumor location, and maximum diameter of tumor were evaluated of each patient. Whole-tumor texture analysis was independently performed by two radiologists on axial T2WI, including skewness, kurtosis, energy, and entropy.
Results
The interobserver agreement was overall good for texture analysis between two radiologists, with intraclass correlation coefficients (ICCs) ranging from 0.626 to 0.826. The LN− group had a significantly higher skewness (p < 0.001), kurtosis (p < 0.001), and energy (p = 0.004) than the LN+ group, and a lower entropy (p = 0.028). These four parameters showed moderate to good diagnostic power in predicting LN metastasis with respective AUC of 0.750, 0.733, 0.669, and 0.648. In addition, they were both correlated with LN metastasis (rs = − 0.413, − 0.385, − 0.28, and 0.245, respectively). The multivariate analysis showed that lower skewness was an independent risk factor of LN metastasis (odds ratio, OR = 9.832; 95%CI, 1.171–56.295; p = 0.01).
Conclusions
Signal intensity histogram parameters of primary tumor on T2WI were associated with regional LN status in rectal cancer, which may help improve the prediction of nodal stage.
Key Points
• Histogram parameters of tumor on T2WI may help to reduce uncertainty when assessing LN status in rectal cancer.
• Histogram parameters of tumor on T2WI showed a significant difference between different regional LN status groups in rectal cancer.
• Skewness was an independent risk factor of regional LN metastasis in rectal cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
An accurate evaluation of lymph node (LN) status in patients with rectal cancer is essential for treatment planning [1, 2] and of an important prognostic factor for local recurrence and overall survival [3]. Magnetic resonance imaging (MRI) is the standard method for preoperative staging in rectal cancer, but it has limited accuracy in assessing LN metastasis with morphological criteria like short-axis diameter, shape, border smoothness, and signal heterogeneity [4, 5].
Previous studies reported the promising role of functional sequences to differentiate benign from malignant nodes, such as diffusion kurtosis imaging [6], intravoxel incoherent motion imaging [7], dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) [8], and chemical shift effect imaging [9]. Most of these studies were performed on a node-by-node basis with different methods to locate and match the nodes on MRI with pathological specimen correctly, which still remains the largest challenge in nodal research nowadays. On the other hand, studies have shown correlations between the depth of tumor invasion, the degree of tumor differentiation, and DCE-MRI parameters of the primary tumor with LN metastasis, indicating a potential association between tumor characteristics and LN status [10,11,12,13].
Texture analysis is a tool for extracting quantitative features from medical images and is an indicator of tumor heterogeneity [14]. Whole-tumor first-order statistical-based texture parameters, also known as histogram parameters, which reflect only the distribution of voxel gray-level intensity without spatial information or relationship of voxels, have a good repeatability [15]. Previous studies also showed its reliability in predicting tumor response to neoadjuvant chemoradiotherapy and survival in rectal cancer [16,17,18]. However, the correlation between texture parameters of tumor and regional LN metastasis is still unknown. Therefore, the purpose of this study was to explore there was a correlation between rectal tumor histogram parameters on T2WI and the existence of regional LN metastasis.
Methods and materials
This retrospective study was approved the Institutional Review Board of West China Hospital and informed consent was waived.
Patients
Between July 2017 and October 2018, 96 consecutive patients with histopathologically proven rectal adenocarcinoma, who underwent preoperative MRI examinations and received direct surgical treatment, were initially retrieved. In general, patients with lower cT3bN1 or with combined maximum one solitary liver metastasis were considered for direct surgical resection in our hospital. In addition, some cT4 patients may also receive surgical treatment after evaluating the extent of tumor invasion. Locally advanced rectal cancer usually received neoadjuvant chemoradiotherapy, except for some specific palliative situations. Eight patients were excluded for the following reasons: (1) insufficient T2WI quality to obtain measurements (n = 3), such as obvious motion artifact caused by respiration or intestinal peristalsis and (2) lack of pathological reports of surgical specimens (n = 5). Finally, 88 patients were enrolled retrospectively.
MRI scanning protocol
All patients underwent preoperative rectal MRIs on a 3.0-T scanner (Magnetom Skyra, Siemens Healthineers) using an 18-channel phased array coil in the supine position. Before the examination, two rectal suppository pills were used to empty the feces. Endorectal filling for distension was not applied. Routine high-resolution rectal MRI protocol consisted of turbo spin echo sagittal, oblique axial (angulated perpendicular to the rectal tumor axis), and oblique coronal (angulated parallel to the rectal tumor axis) T2WI and diffusion-weighted imaging (DWI) based on read-out segmented echo-planar imaging. The acquisition parameters of the oblique axial T2WI sequence were as follows: repetition time/echo time, 6890/100 ms; slices, 48; slice thickness, 3 mm; voxel size, 0.3 × 0.3 × 3 mm; averages, 3; field of view read, 200 mm; field of view phase, 90.6%; matrix, 384 × 346; parallel imaging technique mode, generalized auto-calibrating partial parallel acquisition with acceleration factor of 2; fat suppression, none; total scanning time, 5 min and 5 s.
Imaging analysis
One radiologist (Y.L., with 3 years of experience in rectal imaging) assessed the MR images and was blinded to the pathological results. Tumor location with distance from the transitional skin to the inferior edge of the tumor (lower rectum, < 5 cm; middle rectum, 5–10 cm; and upper rectum, > 10 cm) on sagittal T2WI was recorded, and the maximum diameter of tumor was measured on Syngo MMWP VE40C Station (Siemens Healthineers). Clinical T stage of each patient was also evaluated, and the extent (mm) of extramural growth in patients with T3 stage was recorded. If there was any ambiguity in the process of diagnosis, a senior radiologist was consulted (W.B., with 28 years of experience in rectal imaging).
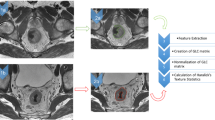
Texture analysis was performed independently on axial T2WI using Omni-Kinetics (Version V2.08, General Electric Medical Systems) by the same two radiologists (Y.L. and W.B., with respective 3 and 28 years of experience in rectal imaging) blinded to the pathological results. Regions of interest (ROIs) covering the whole tumor were manually delineated along the contour of the tumor on each section, excluding obvious necrosis and cystic areas (Fig. 1). The primary tumor was identified as abnormal wall thickening with intermediate signal intensity on T2WI, high-signal intensity on DWI (b = 1000 s/mm2), and corresponding low-signal intensity on apparent diffusion coefficient (ADC) map. Then, the histogram of signal intensity distribution on T2WI within whole-tumor ROIs was generated describing the frequency of occurrence of each voxel gray-level intensity with a bin size of 256 (Fig. 1). In the meantime, histogram statistics were automatically extracted without image filtration and no signal intensity normalization including skewness (asymmetry of intensity level distribution), kurtosis (peakedness of intensity level distribution), energy (homogeneity of the intensity level distribution), and entropy (randomness of the intensity level distribution).
Texture analysis of tumors on T2WI of a 61-year-old female patient (a) who was pathologically diagnosed pT2N0 after surgery and a 39-year-old male patient (b) who was diagnosed with pT3N1a after surgery. Whole-tumor regions of interest (ROIs) were manually drawing along the edge of the tumor. Then, whole-tumor histogram parameters were calculated and the histogram of gray-level distribution within ROIs (c and d) was generated, with skewness of 1.155 and 0.585, kurtosis of 4.63 and 0.788, energy of 0.019 and 0.011, and entropy of 6.196 and 6.809, respectively. The x-axis and y-axis in the histogram stand for voxel gray-level intensity and frequency of occurrence with imaging bin size of 256, respectively. No signal intensity normalization was used prior to texture analysis
Histopathologic analysis
We referred to the histopathological assessment of regional LN malignancy as the gold-standard. Pathological reports of surgically resected specimens contained the standard data set according to the eighth edition of the American Joint Committee on Cancer (AJCC 8th) TNM staging system. The degree of differentiation, depth of infiltration (pathological T stage, pT), and regional LN metastasis (pathological N stage, pN) of tumor were retrospectively collected, based on which the patients were grouped as LN-negative (LN−, pN0) and LN-positive (LN+, pN1-2).
Statistical analysis
All statistical analyses were performed using SPSS 23.0 (IBM Corp). Interobserver agreement of texture analysis between two radiologists was evaluated by calculating intraclass correlation coefficients (ICCs, 0–0.4 poor agreement; 0.41–0.6 moderate agreement; 0.61–0.8 good agreement; and 0.81–1 excellent agreement). Averaged histogram results of two radiologists were used for further analysis. Categorical variables including gender, tumor location, cT stage, degree of differentiation, and pT stage were compared between the N− and LN+ groups using the chi-square test. Quantitative data including age, maximum diameter of tumor, and histogram parameters were compared by using independent t test or the Mann-Whitney U test, according to the normality of data distribution tested by the Kolmogorov-Smirnov method. Spearman correlation analysis was done to assess the correlation between texture parameters and LN metastasis. Receiver operating characteristic (ROC) curves were generated to assess the diagnostic performance of the histogram parameters in predicting LN status by calculating the area under the ROC curve (AUC). The optimal threshold was determined by the maximum Youden index, and corresponding sensitivity and specificity were calculated. Subsequently, multivariate binary logistic regression analysis was performed with entry of variables to identify independent predictors for LN metastasis after collinearity diagnosis (variance inflation factor, VIF larger than 10 represented the existence of collinearity). P value less than 0.05 was considered significant.
Results
Patient demographics
All included patients received radical surgical excision including low anterior resection (n = 52), ultra-low anterior resection (n = 15), intersphincteric resection (n = 5), abdominoperineal resection (n = 10), and extralevator abdominoperineal resection (n = 6). The median interval between preoperative MRI and surgery was 6 days (range, 0–62).
Eighty-eight patients were enrolled in this study, i.e., 58 male (39–91 years; mean, 61 years) and 30 female (42–85 years; mean, 62 years). Twenty-one tumors were located at the upper rectum, 48 at the middle rectum, and 19 at the low rectum. Maximum diameter of tumors measured on T2WI ranged from 1.7 to 8.2 cm, with a mean value of 3.8 cm. Five patients were clinically staged as cT1; 14 were cT2; 61 were cT3 (cT3a, 3; cT3b, 42; cT3c, 13; and cT3d, 3); and 8 were cT4 (cT4a, 4; and cT4b, 4).
Pathological reports showed that 78 tumors were well/moderately differentiated, and 10 were poorly differentiated. Six patients were pathologically staged as pT1; 32 were pT2; 41 were pT3; and 6 were pT4 (pT4a, 4; and pT4b, 2). Fifty-seven patients were pathologically assess as pN0, 25 were pN1 (pN1a, 10; pN1b, 11; and pN1c, 4), and 6 were pN2 (pN2a, 3; pN2b, 3). Then, 57 patients were grouped as LN− (pN0), and 31 were LN+ (pN1-N2).
Univariate analysis
Clinical and pathological characteristics
The clinical and pathological data between the LN− and LN+ groups were summarized in Table 1. On univariate analysis, patients were categorized as low-cT stage (cT1-T3b, tumor extends ≤ 5 mm beyond the muscularis propria, n = 64) and high-cT stage (cT3c-T4b, tumor extends > 5 mm beyond muscularis propria, n = 24) and low-pT stage (pT1-T3b, extramural infiltration ≤ 5 mm, n = 73) and high-pT stage (pT3c-T4b, extramural infiltration > 5 mm, n = 15). No significantly different distribution of age (p = 0.589), gender (p = 0.46), tumor location (p = 0.202), maximum diameter of tumor (p = 0.986), cT stage (p = 0.202), pT stage (p = 0.107), and degree of differentiation (p = 0.164) was observed between the two groups.
Histogram parameters
The histogram parameters between the two groups were shown in Table 2. Interobserver agreement was good for skewness (ICC = 0.745; 95%CI, 0.61–0.833), kurtosis (ICC = 0.626; 95%CI, 0.43–0.755), and entropy (ICC = 0.796; 95%CI, 0.688–0.866) and was excellent for energy (ICC = 0.826; 95%CI, 0.735–0.886). A significantly higher skewness (0.998 ± 0.376 vs. 0.692 ± 0.219, p < 0.001), kurtosis (3.018 vs. 1.876, p < 0.001), and energy (0.015 ± 0.003 vs. 0.013 ± 0.002, p = 0.004) were found in the LN− than in the LN+ group. And the LN− group also had a significantly lower entropy (6.435 ± 0.251 vs. 6.554 ± 0.212, p = 0.028). Skewness, kurtosis, and energy all revealed significantly negative correlations with LN metastasis (rs = − 0.413, − 0.385, and − 0.28, all p < 0.001), and the entropy had a positive correlation (rs = 0.245, p = 0.021) with LN metastasis.
With a respective cutoff value of < 1.002, < 2.108, < 0.013, and > 6.477, skewness, kurtosis, energy, and entropy showed moderate to good diagnostic power in predicting LN metastasis with an AUC of 0.750 (95%CI, 0.651–0.849), 0.733 (95%CI, 0.625–0.841), 0.669 (95%CI, 0.555–0.783), and 0.648 (95%CI, 0.530–0.766), yielding sensitivities of 93.5%, 74.2%, 54.8%, and 71% and specificities of 52.6%, 71.9%, 73.7%, and 56.1%, respectively (Fig. 2).
Multivariate analysis
Multivariate analysis results were shown in Table 3. The degree of differentiation, pT stage, skewness, kurtosis, energy, and entropy were preliminarily selected as covariates for multivariate logistic regression analysis, with a p value less than 0.2 for the univariate analysis. Entropy and energy demonstrated a strong collinearity (VIF = 27.229), and entropy was subsequently excluded because of its less sensitivity compared with energy (p = 0.028 vs. 0.004 in the univariate analysis and AUC = 0.648 vs 0.669 in the ROC curves analysis). In addition, histogram parameters were included in the logistic model after being transformed into categorical variables using the cutoff value determined in ROC curve analysis. So, five covariates entered in the final logistic model including the degree of differentiation, pT stage, skewness category (< 1.002 or > 1.002), kurtosis category (< 2.108 or > 2.108), and energy category (< 0.013 or > 0.013). The logistic regression analysis demonstrated that lower skewness (< 1.002) was an independent risk factor of LN metastasis (odds ratio, OR = 9.832; 95%CI, 1.717–56.295; p = 0.01) in the model. The degree of differentiation (OR = 4.137; 95%CI, 0.816–20.965; p = 0.086), pT stage (OR = 3.201; 95%CI, 0.778–13.163; p = 0.107), kurtosis (OR = 3.21; 95%CI, 0.849–12.142; p = 0.086), and energy (OR = 1.159; 95%CI, 0.339–3.962; p = 0.814) were not independent risk factors. In addition, the model showed good accuracy of predicting regional LN status with an overall correct percentage of 76.1% and goodness of fit (p value of 0.686) in the Hosmer and Lemeshow test.
Discussion
This study performed a texture analysis of primary tumor on preoperative T2WI and explored the correlation between signal intensity histogram parameters and the existence of regional LNs. The results are that the histogram parameters of tumor have a potential role in predicting pathological regional LN metastasis.
Currently, nodal staging in rectal cancer remains a challenging issue [4]. A meta-analysis showed that rectal MRI had a poor accuracy in assessing LN with a sensitivity of 77% and a specificity of 71% based on the size and morphological features [19]. Grovik [13] et al identified that Ktrans value of tumor was associated with pathological nodal status, suggesting that the nodal metastasis may be closely related to the microscopic characteristics of the primary tumor. De Cecco [16], Horvat [17], and Jalil [18] et al demonstrated that texture parameters derived from T2WI of the rectal cancer were indicators of tumor response to neoadjuvant chemoradiotherapy and long-term survival. However, the value of texture analysis in predicting LN metastasis is still unclear, and no previous research on the correlation between T2WI signal intensity histogram parameters and LN status was found. Our study found that patients in the LN− group had significant higher skewness, kurtosis, and energy and had lower entropy of tumors on T2WI than in LN+ group. Furthermore, all histogram features were significantly correlated with LN status. A higher value of skewness reflects that the distribution is more asymmetric. Higher kurtosis indicates that the distribution tends to have heavy tails or outliers, and the mass of the distribution is concentrated. Higher energy reflects that the distribution of intensity levels is very narrow. Entropy is the opposite index to energy. Altogether, our results suggested that tumors with LN metastasis might manifest more heterogeneous on T2WI and tended to have a more symmetric and light-tailed histogram of voxel value distribution within tumors.
ROC curves showed that skewness had the best performance in predicting LN malignancy among histogram parameters with the cutoff value of 1.002, yielding AUC of 0.75, an excellent sensitivity of 93.5% and a low specificity of 52.6%. This indicates that skewness may reliably select patients without LN metastasis, who might benefit from local excision instead of radial total mesorectal excision in early rectal cancer, with less surgical complications and higher quality of life. Besides, kurtosis also had a similar good predictive power (AUC = 0.733), with a balanced sensitivity of 74.2% and a specificity of 71.9%. Therefore, kurtosis is likely to reduce false-positive cases when predicting LN status by skewness alone thus could also help to avoid excessive treatment in some patients. Nevertheless, energy and entropy showed lower predictive ability than skewness and kurtosis, indicating that they may be inferior parameters in predicting LN status.
Multivariate analysis was used to further demonstrate the correlation between texture parameters and LN status. As a result, lower skewness was found to be an independent risk factor of LN metastasis with an OR of 9.832. Previous studies on gastric cancer [20] and lung adenocarcinoma [21] also reported the effectiveness of skewness in predicting postoperative LN status. This reflected that skewness of the primary rectal cancer could be a valuable reference to radiologists when diagnosing nodal stage. Moreover, Liu et al [22] performed texture analysis on the apparent diffusion coefficient maps and identified that the entropy of tumor was an independent predictor of positive nodal status. Although entropy in our study also showed a difference between the LN− and LN+ groups, it was not an independent risk factor. This revealed that texture parameters generating from different MR sequences could have a different value in assessing LN malignancy. Furthermore, previous studies demonstrated that pT stage and degree of differentiation were independently associated with the presence of LN metastasis [10,11,12]. For this reason, they were included in our multivariate logistic regression model as confounding factors, with p value less than 0.2 for the univariate analysis. However, neither of them could significantly predict LN metastasis. This may be due to the relatively small population and imbalance among the degree of differentiation and pT stage of patients.
Interobserver variability of texture analysis of two radiologists was also assessed in our study, and an overall good interobserver agreement was found, with ICCs ranging from 0.626 to 0.826. This was in accordance with a previous study proving that statistical-based first-order texture parameters had good repeatability in clinical practice [15]. Many other researches explored different approaches to improve the diagnostic accuracy of MRI with various functional sequences and different quantitative parameters on a node-by-node basis [6, 7]. It is still rather difficult to repeat these experiments and verify the effectiveness of those indices, mainly because of the lack of valid methods to precisely match the pathological results with radiological imaging. Moreover, the limited spatial resolution of functional imaging, the difference between scanning protocols and MR vendors, and the small size of LN itself may all contribute to this poor reproducibility.
Our study has some limitations. First, this was a retrospective trial with a relatively small sample size, containing only 31 patients (35.2%) with pathological LN+. Second, there was a potential selection bias in the present study, as most LN+ cases identified by primary MRI with suspicious LN would receive neoadjuvant chemoradiotherapy and were therefore not included in this retrospective study. Third, we only included patients who went directly to surgical resection rather than those who firstly received neoadjuvant chemoradiotherapy. Therefore, our results may not apply to advanced rectal cancer. Fourth, in order to keep data consistency, clinical diagnosis in our study, including tumor location, the maximum diameter of tumor, and cT stage, was performed mostly by the less experienced radiologist. It might be better to evaluate all cases by both junior and senior radiologists with consensus to further improve the diagnostic accuracy. Fifth, our study only used histogram features on T2WI; other texture features such as gray-level co-occurrence matrix and other imaging sequences may also be investigated. Sixth, whole-tumor ROI drawing is a time-consuming task. The time required for texture analysis of each case was about 15 min for those radiologists who are familiar with the software. This could be largely shortened with the development of automatic or semi-automatic image segmentation technologies.
In conclusion, our study showed that the signal intensity histogram features of the primary rectal tumor on T2WI significantly correlated with regional LN status and that skewness was an independent risk factor of regional LN metastasis. If texture analysis was routinely available on workstation during the standard reporting workflow, radiologists could use easily tumor histogram features to reduce uncertainty when assessing LN status.
Abbreviations
- ICCs:
-
Intraclass correlation coefficients
- LN−:
-
Lymph node negative
- LN:
-
Lymph node
- LN+:
-
Lymph node positive
- OR:
-
Odds ratio
References
Glynne-Jones R, Wyrwicz L, Tiret E et al (2017) Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 4:iv22–iv40
Beets GL, Figueiredo NF, Beets-Tan RGH (2017) Management of rectal cancer without radical resection. Annu Rev Med 1:169–182
Valentini V, van Stiphout RG, Lammering G et al (2011) Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol 23:3163–3172
Brouwer NPM, Stijns RCH, Lemmens VEPP et al (2018) Clinical lymph node staging in colorectal cancer; a flip of the coin? Eur J Surg Oncol 8:1241–1246
Beets-Tan RGH, Lambregts DMJ, Maas M et al (2018) Magnetic resonance imaging for clinical management of rectal cancer: updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol 4:1465–1475
Yu J, Dai X, Zou HH et al (2018) Diffusion kurtosis imaging in identifying the malignancy of lymph nodes during the primary staging of rectal cancer. Colorectal Dis 2:116–125
Qiu L, Liu XL, Liu SR et al (2016) Role of quantitative intravoxel incoherent motion parameters in the preoperative diagnosis of nodal metastasis in patients with rectal carcinoma. J Magn Reson Imaging 4:1031–1039
Armbruster M, D'Anastasi M, Holzner V et al (2018) Improved detection of a tumorous involvement of the mesorectal fascia and locoregional lymph nodes in locally advanced rectal cancer using DCE-MRI. Int J Colorectal Dis 7:901–909
Zhang H, Zhang C, Zheng Z et al (2017) Chemical shift effect predicting lymph node status in rectal cancer using high-resolution MR imaging with node-for-node matched histopathological validation. Eur Radiol 9:3845–3855
Chang HC, Huang SC, Chen JS et al (2012) Risk factors for lymph node metastasis in pT1 and pT2 rectal cancer: a single-institute experience in 943 patients and literature review. Ann Surg Oncol 8:2477–2484
Kajiwara Y, Ueno H, Hashiguchi Y, Mochizuki H, Hase K (2010) Risk factors of nodal involvement in T2 colorectal cancer. Dis Colon Rectum 10:1393–1399
Suh JH, Han KS, Kim BC et al (2012) Predictors for lymph node metastasis in T1 colorectal cancer. Endoscopy 6:590–595
Grovik E, Redalen KR, Storas TH et al (2017) Dynamic multi-echo DCE- and DSC-MRI in rectal cancer: low primary tumor K (trans) and DeltaR2* peak are significantly associated with lymph node metastasis. J Magn Reson Imaging 1:194–206
Lubner MG, Smith AD, Sandrasegaran K, Sahani DV, Pickhardt PJ (2017) CT texture analysis: definitions, applications, biologic correlates, and challenges. Radiographics 5:1483–1503
Gourtsoyianni S, Doumou G, Prezzi D et al (2017) Primary rectal cancer: repeatability of global and local-regional MR imaging texture features. Radiology 2:552–561
De Cecco CN, Ganeshan B, Ciolina M et al (2015) Texture analysis as imaging biomarker of tumoral response to neoadjuvant chemoradiotherapy in rectal cancer patients studied with 3-T magnetic resonance. Invest Radiol 4:239–245
Horvat N, Veeraraghavan H, Khan M et al (2018) MR imaging of rectal cancer: radiomics analysis to assess treatment response after neoadjuvant therapy. Radiology 3:833–843
Jalil O, Afaq A, Ganeshan B et al (2017) Magnetic resonance based texture parameters as potential imaging biomarkers for predicting long-term survival in locally advanced rectal cancer treated by chemoradiotherapy. Colorectal Dis 4:349–362
Al-Sukhni E, Milot L, Fruitman M et al (2012) Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: a systematic review and meta-analysis. Ann Surg Oncol 7:2212–2223
Liu S, Shi H, Ji C et al (2018) Preoperative CT texture analysis of gastric cancer: correlations with postoperative TNM staging. Clin Radiol 8:756.e751–756.e759
Gu Y, She Y, Xie D et al (2018) A texture analysis-based prediction model for lymph node metastasis in stage IA lung adenocarcinoma. Ann Thorac Surg 1:214–220
Liu L, Liu Y, Xu L et al (2017) Application of texture analysis based on apparent diffusion coefficient maps in discriminating different stages of rectal cancer. J Magn Reson Imaging 6:1798–1808
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Wu Bing.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, L., Liu, D., Fang, X. et al. Rectal cancer: can T2WI histogram of the primary tumor help predict the existence of lymph node metastasis?. Eur Radiol 29, 6469–6476 (2019). https://doi.org/10.1007/s00330-019-06328-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06328-z