Abstract
Objective
To evaluate simplified intravoxel incoherent motion (IVIM) diffusion-weighted imaging (DWI) for liver lesion characterisation at 3.0 T and to compare it with 1.5 T.
Methods
3.0-T DWI data from a respiratory-gated MRI sequence with b = 0, 50, 250, and 800 s/mm2 were analysed in 116 lesions (78 patients) and 27 healthy livers. Apparent diffusion coefficient ADC = ADC(0,800) and IVIM-based parameters D1′ = ADC(50,800), D2′ = ADC(250,800), f1′ = f(0,50,800), f2′ = f(0,250,800), D*′ = D*(0,50,250,800), ADClow = ADC(0,50), and ADCdiff = ADClow-D2′ were calculated voxel-wise and analysed on per-patient basis. Results were compared with those of 173 lesions (110 patients) and 40 healthy livers at 1.5 T.
Results
Focal nodular hyperplasias were best discriminated from all other lesions by f1′ and haemangiomas by D1′ with an area under the curve (AUC) of 0.993 and 1.000, respectively. For discrimination between malignant and benign lesions, ADC was best suited (AUC of 0.968). The combination of D1′ and f1′ correctly identified more lesions as malignant or benign than the ADC (99.1% vs 88.8%). Discriminatory power for differentiating malignant from benign lesions tended to be higher at 3.0 T than at 1.5 T.
Conclusion
Simplified IVIM is suitable for lesion characterisation at 3.0 T with a trend of superior diagnostic accuracy for discriminating malignant from benign lesions compared with 1.5 T.
Key Points
• Simplified IVIM is also suitable for liver lesion characterisation at 3.0 T.
• Excellent accuracy was reached for discriminating malignant from benign lesions.
• The acquisition of only three b-values (0, 50, 800 s/mm 2 ) is required.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In diffusion-weighted imaging (DWI), the intravoxel incoherent motion (IVIM) concept of Le Bihan [1] proposes a separate analysis of diffusion and perfusion effects by using a bi-exponential model. The acquisition of at least four b-values allows the determination of the true diffusion coefficient D, the perfusion fraction f, and the pseudodiffusion coefficient D* [2]. D represents the mobility of water molecules in tissue [3,4,5,6], f reflects the relative contribution of microvascular blood flow to the DWI signal, and D* depends on blood velocity and length of microvessel segments [1, 2, 7].
The role of IVIM imaging for lesion characterisation is still subject of investigation and remains controversially discussed [8,9,10,11,12,13,14,15,16,17,18,19]. A known problem is limited stability of IVIM analysis in case of low D* and/or f values as found in some malignant lesions and haemangiomas [8, 9, 16, 20, 21]. If D* is low (i.e. in the order of D), the signal decay is hardly bi-exponential. In cases with low f, the total IVIM effect can be very small. In both cases, the simultaneous determination of D, f, and D* by using unconstrained non-linear least squares fitting procedures leads to numerical instabilities, poor reproducibility of D* and f, and unreliable results [20, 21]. Improved stability can be achieved for IVIM approaches using a two-step constrained analysis methods like segmented fitting [9, 14, 16] and simplified IVIM [8, 15, 17, 22,23,24,25]. In simplified IVIM, explicit approximation formulas in combination with low number of acquired b-values are used. Thus, simplified IVIM is generally suitable for clinical routine applications. Recently, basic investigations were published for liver lesion characterisation at 1.5 T using a simplified IVIM approach with four b-values [8]. Data on simplified IVIM approaches at 3.0 T and comparisons on IVIM at 1.5 and 3.0 T are still missing.
Thus, the aim of this study was to perform basic investigations of simplified IVIM based on four b-values for liver lesion characterisation at 3.0 T and to compare results with 1.5 T in terms of optimal b-values, most suited IVIM parameters, threshold values, and accuracy.
Materials and methods
Subjects
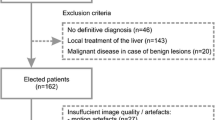
DWI data of consecutive clinical routine liver examinations from August 2012 to July 2016 acquired with four b-values at 3.0 T were reviewed. The study was approved by the local institutional review board of the University Hospital Bonn, which waived the need for informed patient consent because it was a retrospective analysis study of clinical routine examinations. One hundred forty-eight patients fulfilled the inclusion criterion of at least one focal hepatic lesion ≥ 1 cm detected in the examination using all available sequences. Seventy patients were excluded as outlined in Fig. 1 and data of 78 patients were finally analysed (Table 1). Diagnosis of liver lesions was undertaken within clinical routine. Cholangiocellular carcinomas (CCCs) were histologically proven. Hepatocellular carcinomas (HCCs) were either histologically proven or diagnosed according to the American Association for the Study for Liver Disease MRI criteria [26]. Diagnosis of metastasis was based on typical imaging features in combination with histologically proven primary cancer. Diagnosis of focal nodular hyperplasia (FNH) or haemangioma was established on the basis of typical radiological findings on contrast-enhanced MRI and was confirmed by at least one follow-up examination. In addition, healthy liver parenchyma, defined as normal appearing liver in MRI in combination with clinically absent liver disease, was investigated in 27 patients (Table 1), which served as reference (REFs). Hereby, 5 patients of the benign lesion groups were included who had no other liver disease based on clinical and radiological data. In addition, 22 randomly selected patients with non-specific abdominal symptoms or non-conclusive sonographic examinations and normal hepatic MRI examinations without liver disease and without malignant disease were included.
In addition to the 3.0-T data, the data of a different patient group (110 patients with liver lesions and 40 patients with healthy liver based on clinical and radiological criteria), which were examined with simplified IVIM at 1.5 T in a previous study [8], were included (see Fig. 1 and Table 1). In the previous study, basic investigations concerning simplified IVIM at 1.5 T had been performed, whereas in the present study, the data were used to analyse the diagnostic yield of simplified IVIM at 3.0 T in comparison with 1.5 T.
Magnetic resonance imaging
All examinations were performed on a clinical 3.0-T MRI system (Ingenia, 3.0 T, Philips Healthcare, gradient system: 80 mT/m maximum amplitude, 200 T/m/s maximum slew rate; equipped with dual-source RF transmission technology) using commercially available phased array surface coils for signal detection. As in the previous study [8], the DWI sequence (Table 2) was a respiratory-triggered single-shot spin-echo echo-planar imaging variant with four b-values (0, 50, 250, and 800 s/mm2). It was part of the standardised imaging protocol and always acquired before contrast agent injection. Isotropic (directionally independent) diffusion-weighted images were reconstructed from the images with diffusion-sensitised gradients in three orthogonal directions on the MRI system.
Postprocessing of the 3.0-T data
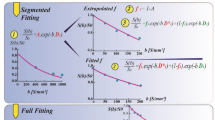
As in reference [8], two different approximations of D and f were calculated from the acquired b-values, one from b0 = 0, b1 = 50, b3 = 800 and one from b0 = 0, b2 = 250, b3 = 800 s/mm2:
From the four b-values, D* was approximated by using D2′ and f2′ and the reading for b1:
Moreover, the perfusion-sensitive parameters ADClow and ADCdiff and the conventional ADC were calculated:
Parameter maps were calculated offline in MATLAB (MathWorks).
Analysis of IVIM parameters at 3.0 T
Image analyses were performed in consensus by a board-certified radiologist with more than 14 years of experience in abdominal imaging and a physicist with more than 19 years of experience in DWI. For each lesion included, one region of interest (ROI) was analysed in a single slice that was centrally in the lesion and largely unaffected by motion artefacts, pixel misalignments, and susceptibility artefacts. The hand-drawn ROI was carefully placed on the DWI image with b = 800 s/mm2 by adapting the ROI to the most hyperintense structures excluding areas close to the lesion rim to avoid partial-volume effects. Areas with intratumoural necrosis, calcification, haemorrhage, or scar in a FNH were also excluded. After the anatomical position of each ROI was visually cross-checked for pixel misalignments between images with different b-values, the ROI was copied into the parameter maps. In case of healthy liver tissue, one large ROI per patient was placed in a central slice into the right lobe, excluding large vessels and benign lesions.
Statistical analysis
Statistical analysis was conducted using SPSS (version 24.0, IBM) and MedCalc (version 18.11, MedCalc Software). As for 1.5-T data [8], analysis of the 3.0-T data was performed on a per-patient basis, whereby mean parameter values were used in case of multiple lesions per patient. Statistical significance (p < 0.05) for differences between groups was tested with univariate analysis of variance after proving the Gaussian distribution with the Kolmogorov-Smirnov test. The Levene test showed non-equal variances in the different groups and thus the post hoc Games-Howell test was chosen. ROC analysis was performed for lesion discrimination and dependent ROC curves obtained at 3.0 T were compared with the method of DeLong et al. Furthermore, the independent ROC curves obtained at 3.0 T and 1.5 T were compared with the method of DeLong et al. For comparison of parameter values obtained at 3.0 T and 1.5 T, an independent samples Student’s t test (parametric test) was performed for each lesional subgroup separately after proving Gaussian distribution using Kolmogorov-Smirnov test and after variance analysis by the Levene test.
Results
Analysis of IVIM parameters at 3.0 T
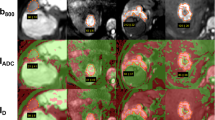
Mean parameter values were determined in 143 ROIs placed in 36 HCCs, 5 CCCs, 33 metastases of colorectal carcinomas (CRCs), 20 metastases of breast cancer (BCs), 14 haemangiomas, 8 FNHs, and 27 REFs. Hereby 1/2/3/4/5 lesions per patient were included in 26/2/2/−/− patients with HCCs, 5/−/−/−/− patients with CCCs, 3/4/3/2/1 patients with CRCs, 3/4/3/−/− patients with BCs, 10/2/−/−/− patients with haemangiomas, and 8/−/−/−/− patients with FNHs. In the REF group, 1 ROI per patient was included. Seventy lesions were located in the right and 46 in the left liver lobe. The 27 REFs were located in the right liver lobe. Mean lesion size diameter was 45 mm (10–160 mm). Mean ROI sizes were 540 mm2 (23–4985 mm2) in lesions and 1027 mm2 (413–2911 mm2) in REFs. Nineteen CRCs and 18 BCs were known to be treated by systemic chemotherapy. Obtained parameter values are given in Table 3, example images in Fig. 2.
Typical examples of intravoxel incoherent motion (IVIM)–based parameter maps for different liver lesions at 3.0 T. From left to right, images for focal nodular hyperplasia (FNH) and two haemangiomas (a), multifocal hepatocellular carcinoma (HCC) (b), and metastases of colorectal carcinoma (CRCs) (c) and breast cancer (BCs) (d) are shown. Original diffusion-weighted images with b = 0, 50, 250, 800 s/mm2 are presented together with conventional ADC, diffusion-sensitive D1′ and D2′ parameter maps, and perfusion-sensitive f1′, f2′, D*′, ADClow, ADCdiff parameter maps. The parameter maps are displayed as colour-coded overlays over DWI b = 0. If bad data quality due to voxel misalignment, motion influence, or limited SNR led to negative parameter values especially for f1′ or f2′ or to not defined values of the ln(x) in the equation for D*′, these voxels were not colorised. Regions of interest analysed are marked in white (haemangiomas, HCC, CRC, BC) and yellow (FNH). The FNH reveals medium diffusion and high perfusion parameter values, similar to healthy liver tissue not including large vessels. The haemangiomas show high values of diffusion parameters in combination with very low values of perfusion parameters. Malignant lesions (HCC, CRC, and BC) exhibit similar or slightly lower diffusion parameter than healthy tissue or FNH in combination with low perfusion parameters
According to univariate analysis of variance, differences between groups (6 different liver lesion types, healthy tissue) were statistically significant (p < 1 × 10−10) for all parameters. Results of the post hoc tests are given in Table 4. The main results were as follow: Haemangiomas had the largest values of diffusion sensitive parameters (ADC, D1′, and D2′) in comparison with all other groups, with the exception of non-significant differences in D2′ between haemangiomas and CCCs. FNHs (and REFs) had larger perfusion-sensitive parameters (f1′, f2′, D*′, ADClow, ADCdiff) than all lesion groups, with the exception of non-significant differences in f2′ between FNHs and haemangiomas and in f2′ and D*′ between FNHs and CCCs.
Results of ROC analysis are given in Table 5. Haemangiomas were best discriminated from all other lesions by D1′ with area under the curve (AUC) of 1.000 and cut-off value of 1501.150 × 10−6 mm2/s followed by ADC and D2′ with slightly but not significantly lower AUC values. FNHs were best discriminated from all other lesions by f1′ with AUC of 0.993 and cut-off value of 105.650 × 10−3 followed by ADCdiff, ADClow, and D*′ with AUC values not being significantly lower. Discrimination by ADC was also possible but considerably inferior (AUC of 0.764, p < 0.001). Benign and malignant lesions were best discriminated by ADC with AUC of 0.968 and cut-off value of 1341.250 × 10−6 mm2/s followed by D1′ (AUC of 0.909, p = 0.0107). All other parameters had significantly lower AUC values. For the ADC cut-off value, 88.8% of the lesions (103 of 116) could be correctly identified as malignant or benign (Fig. 3a). We also used a combination of D1′ and f1′ parameters for the discrimination between benign and malignant lesions. Hereby the cut-off values obtained for discrimination of haemangiomas and FNHs were used (1501.150 × 10−6 mm2/s and 105.650 × 10−3, respectively). Lesions with D1′ and f1′ values lower than the cut-off values were assigned as malignant and lesions with D1′ or f1′ values higher than the cut-off values, as benign. For this parameter combination, 99.1% of the lesions (115 of 116) were correctly identified as malignant and benign (Fig. 3b, c).
Overview to ADC (a), D1′ (b), and f1′ (c) values measured in healthy liver tissue (REFs), focal nodular hyperplasias (FNHs), haemangiomas (HEMs), hepatocellular carcinomas (HCCs), cholangiocellular carcinomas (CCCs), and metastases of colorectal carcinomas (CRCs) and breast cancer (BCs) at 3.0 T. For an ADC cut-off value of 1341.250 × 10−6 mm2/s (black line in (a)), 88.8% of the lesions (103 of 116) were correctly identified as malignant and benign. For a combination of D1′ cut-off value of 1501.150 × 10−6 mm2/s (black line in (b)) and f1′ cut-off value of 105.650 × 10−3 (black line in (c)), 99.1% of the lesions (115 of 116) were correctly identified
When excluding CRCs and BCs to avoid the potential influence of treatment, similar results were obtained compared with the whole data set: For an ADC cut-off point of 1341.250 × 10−6 mm2/s, 92.1% of the lesions (58 of 63) were correctly identified as malignant and benign. For a combination of D1′ cut-off value of 1431.100 × 10−6 mm2/s and f1′ cut-off value of 105.650 × 10−3, 100.0% of the lesions (63 of 63) were correctly identified as malignant and benign.
Comparison between 3.0 T and 1.5 T
The comparison of ROC curves obtained at 3.0 T and 1.5 T revealed a slightly, but not significantly, higher AUC for the discrimination between haemangiomas and all other lesions by D1′ (AUC of 1.000 vs 0.994, p = 0.163) and between FNHs and all other lesions by f1′ (AUC of 0.993 vs 0.989, p = 0.737). For the discrimination between malignant and benign lesions, at 3.0 T, a significantly higher AUC was found for f2′ (AUC of 0.831 vs 0.630, p = 0.024) and a trend of higher AUC for ADC (AUC of 0.968 vs 0.915, p = 0.102) and D1′ (AUC of 0.909 vs 0.858, p = 0.364). All other parameters showed non-significant AUC differences. The comparison of the parameter values between 3.0 T and 1.5 T revealed no significant differences for most lesion groups (REFs, FNHs, haemangiomas, CCCs, BCs). For HCCs, a tendency of larger D(250,800) and lower f(250,800) was found at 3.0 T (p = 0.019 and p = 0.048, respectively). For CRCs, all perfusion-sensitive parameters were lower at 3.0 T (p value of 0.009 for ADC(0,800), < 0.00001 for f(50,800), 0.001 for f(250,800), 0.001 for D*, < 0.000001 for ADC(0,50), and 0.00001 for ADCdiff). Moreover, the standard deviations within the ROIs (RSDs) did not differ between 3.0 T and 1.5 T for most lesion groups (FNHs, haemangiomas, HCCs, CCCs, BCs), see Table 6. Lower RSD values at 3.0 T compared with 1.5 T were only found for REFs in case of ADC(0,800) (p = 0.035), D(250,800) (p = 0.019), f(50,800) (p = 0.021), f(250,800) (p = 0.008), ADC(0,50) (p = 0.024), and ADCdiff (p = 0.038) and for CRC in case of ADC(0,800) (p < 0.001), D(50,800) (p < 0.001), D(250,800) (p < 0.0001), f(50,800) (p = 0.001), D* (p = 0.046), ADC(0,50) (p = 0.002), and ADCdiff (p < 0.001). The inter-individual standard deviations of the parameters for the different lesion groups at 3.0 T were similar or lower than at 1.5 T.
Discussion
The main result of the present basic study is that simplified IVIM for liver lesion characterisation at 3.0 T achieved excellent accuracy in differentiating malignant from benign lesions by using the combination of parameters D and f approximated from b = 0, 50, 800 s/mm2 (D1′, f1′). Compared with 1.5 T, the achieved accuracy tended to be higher at 3.0 T [8]. All other parameters, including the conventional ADC calculated from b = 0 and 800 s/mm2, the approximations of D and f from b = 0, 250, 800 s/mm2 (D2′, f2′), and D*′ derived from four b-values turned out to be clearly inferior to the combined D1′ and f1′.
Up to now, there are only a few studies using a stable IVIM parameter analysis method like segmented fitting or explicitly calculating formulas in order to differentiate between subgroups of malignant and benign lesions, and this is the first study on simplified IVIM at 3.0 T [8, 9, 13,14,15,16,17, 19]. For lesion differentiation, the same parameters turned out to be optimal and the same combination of b-values was found as for simplified IVIM at 1.5 T. Furthermore, the cut-off values at 3.0 T for the discrimination between malignant and benign lesions were very similar to those at 1.5 T [8]. Diffusion-sensitive parameters were highest for haemangiomas with D1′ and ADC being the best single parameters to differentiate them from all other lesions. Perfusion-sensitive parameters were higher for FNHs than for HCCs, CCCs, metastases, and haemangiomas with f1′ being most suitable to differentiate FNHs from all other lesions. The lower D*′ and lower f1′ and f2′ values in malignant lesions may be caused by slow or stagnant blood flow through damaged tumour vessels and low density and/or diameter of microvascular vessels containing flowing blood as discussed in detail earlier [8]. For haemangiomas, this finding can possibly be explained by the presence of dilated vessels and pools of stagnant blood leading to low D*′ values [8]. If D*′ is in the order of D2′, the perfusion influence is not negligible at high b-values and the behaviour of ln(S(b)) is also non-linear for high b-values [15]. Compared with healthy liver tissue, FNHs had similar D*′ values, but slightly lower values of f1′, f2′, ADClow, and ADCdiff, because perfusion fraction is lower as a result from the longer relaxation times T1 and T2 as previously reported [8]. FNHs may reveal similar microcirculation properties as normal liver tissue because of its hyperplastic rather than neoplastic nature [8]. In order to discriminate malignant from benign lesions, the highest accuracy was obtained by ADC followed by D1′ with significantly higher values in benign lesions. By combining D1′ and f1′, discriminatory power for differentiation between benign and malignant lesions further improved.
Comparing field strengths with respect to diagnostic accuracy for discriminating between malignant and benign lesions, 3.0 T tended to be superior in comparison with 1.5 T. By using the combination of D1′ and f1′, 99.1% of the lesions could be correctly identified as malignant or benign at 3.0 T compared with 85.6% at 1.5 T [8]. For the single parameters ADC and D1′, AUC values of 0.968 vs 0.915 (p = 0.102) and 0.909 vs 0.858 (p = 0.364), respectively, were found at 3.0 T compared with 1.5 T [8]. Based on only the ADC, 88.8% of the lesions could be correctly identified as malignant and benign at 3.0 T in comparison with 82.1% at 1.5 T [8]. In concordance to this finding, in previous studies, discriminatory power between benign and malignant lesions was found to be high at 3.0 T (AUC of D, 0.98 at 3.0 T [9]) and low at 1.5 T (AUC of D, 0.723 [14]). In both studies, benign lesion group composition was comparable to our study (containing haemangiomas and focal nodular hyperplasias). However, if the benign lesion group contained also cysts or only haemangiomas (high ADC and D values), high AUC values were not only obtained at 3.0 T (0.933–0.98 for ADC and 0.96–0.971 for D [12]) but also at 1.5 T (0.967 for ADC and 0.837–0.98 for D [10, 16]). For the discrimination between malignant and benign lesions using perfusion parameters, for f2′, a significantly higher AUC was found in the present 3.0-T study as compared with 1.5 T (0.831 compared with 0.630, p = 0.024) [8]. The discriminatory power of perfusion parameters was significantly lower than for diffusion parameters, at both field strengths 3.0 and 1.5 T. This can be explained due to the fact that some benign lesions as cysts and haemangiomas have low values of perfusion parameters, which are within the range of the malignant lesions. The tendency toward higher diagnostic accuracy at 3.0 T for the differentiation between malignant and benign lesions might be caused by (a) changes of measured perfusion parameters due to different relaxation times and TE values, (b) slight differences in group compositions, or (c) improved signal-to-noise ratio (SNR) and/or image quality. For clarification of (a), parameter values obtained in this study were directly compared with those obtained in the previous 1.5-T study [8]. However, no significant differences could be found for REFs, FNHs, haemangiomas, CCCs, and BCs, neither for the diffusion nor for the perfusion parameters. In general, the perfusion fraction and parameters influenced by the perfusion fraction might have different values at 1.5 T and 3.0 T, depending on the relaxation times and chosen TE values [27]. Different values of the perfusion fraction might have caused differences in diagnostic accuracy. By using a smaller TE at 3.0 T, changes in relaxation times have apparently been compensated. Only for CRCs, lower perfusion parameters and lower RSDs were found at 3.0 T. This may be explained by a larger amount of partially necrotic CRCs due to systemic treatment in this study (58% at 3.0 T, 9% at 1.5 T). Necrotic changes might lead to lower D* values and reduced heterogeneity within the lesions [5, 28]. For BCs, a similar number of treated lesions were included at 3.0 T and 1.5 T (90% at 3.0 T and 87% at 1.5 T) not leading to any differences. Explanation (b) is also rather unlikely if one considers that two different compositions of the malignant lesion group (with and without metastases) lead to similar results. As an additional test, we re-investigated discriminability at 1.5 T for a smaller benign lesion group with the same number of FNHs and haemangiomas as at 3.0 T (data not shown); however, AUC values did not increase but decline. Thus, explanation (c), the improved SNR and/or image quality, might be most relevant. Diffusion parameters at 1.5 T are not significantly larger than those at 3.0 T which does not speak for SNR limitations at 1.5 T. However, RSD values for healthy tissue are larger at 1.5 T compared with 3.0 T which might indicate lower SNR at 1.5 T. Furthermore, the inter-individual standard deviations of the parameters for the different groups were larger at 1.5 T what could be caused by lower measurement repeatability/reproducibility. In general, the image quality of DWI at 3.0 T is rather lower due to more prominent dielectric shading (e.g. in patients with ascites), more pronounced susceptibility and motion artefacts, and less uniform fat suppression [29,30,31,32]. However, in the present study, two advanced technologies were used to improve DWI image quality at 3.0 T, dual-source parallel RF excitation and transmission technology for improving RF uniformity and a combination of SPIR with slice-selective gradient reversal (SSGR) for improved fat suppression [29, 30, 33].
The results of the present study and the 1.5-T study [8] confirm the usefulness of the three b-value approaches chosen in previous 1.5-T studies on lesion characterisation [15, 17] and assessment of therapy [22,23,24,25]. D1′ and f1′ serve as standardised empirical biomarkers indicating non-specific tissue alteration or therapy response. The numerically stable, voxel-wise determination enables a visual assessment of heterogeneous lesions and the targeted quantitative analysis of necrotic or viable areas. The high diagnostic accuracy of 99.1% of correctly identified malignant and benign lesions at 3.0 T and 85.5% at 1.5 T is very promising and motivates future studies, e.g. a field strength comparison for measured parameter reproducibility. Moreover, it would be interesting to further evaluate the clinical impact of simplified IVIM for lesion characterisation with respect to the following questions: Is simplified IVIM suitable to replace contrast-enhanced images in certain cases as a “fast-MRI” perspective? And is the method based on the determined threshold values suitable to also correctly classify rarer and more atypical lesions?
General concerns regarding the simplified IVIM approach as for example the b-value choice have already been addressed in the previous 1.5-T study [8]. A limitation of the study is that only patients with common lesion types and definitive diagnosis (typical MRI findings or histologically proven) have been included. However, this design was chosen in order to basically evaluate the simplified IVIM approach. Another limitation is the inter-individual comparison of diagnostic accuracy between 3.0 T and 1.5 T.
The authors use now and would recommend to use b-values of 0, 50, and 800 s/mm2 for liver DWI.
In conclusion, simplified IVIM is suitable for lesion characterisation at 3.0 T with a tendency toward superior diagnostic accuracy for discriminating between malignant and benign lesions compared with 1.5 T. The combination of IVIM parameters D and f approximated from b-values 0, 50, and 800 s/mm2 provided more discriminatory power than the ADC determined from two b-values, D and f approximated from 0, 250, and 800 s/mm2, and D* approximated from four b-values.
Change history
21 May 2019
The original version of this article, published on 08 April 2019, unfortunately contained a mistake. The following correction has therefore been made in the original: The caption of Fig. 2 is wrong. The corrected version is given below.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Area under the curve
- CCC:
-
Cholangiocellular carcinoma
- DWI:
-
Diffusion-weighted imaging
- FNH:
-
Focal nodular hyperplasia
- HCC:
-
Hepatocellular carcinoma
- IVIM:
-
Intravoxel incoherent motion
- REF:
-
Reference tissue
- ROI:
-
Region of interest
References
Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M (1988) Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168:497–505
Koh DM, Collins DJ, Orton MR (2011) Intravoxel incoherent motion in body diffusion-weighted MRI: reality and challenges. AJR Am J Roentgenol 196:1351–1361
Padhani AR, Liu G, Koh DM et al (2009) Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 11:102–125
Takahara T, Kwee TC (2012) Low b-value diffusion-weighted imaging: emerging applications in the body. J Magn Reson Imaging 35:1266–1273
Taouli B, Koh DM (2010) Diffusion-weighted MR imaging of the liver. Radiology 254:47–66
Aoyagi T, Shuto K, Okazumi S et al (2012) Apparent diffusion coefficient correlation with oesophageal tumour stroma and angiogenesis. Eur Radiol 22:1172–1177
Cho GY, Kim S, Jensen JH et al (2012) A versatile flow phantom for intravoxel incoherent motion MRI. Magn Reson Med 67:1710–1720
Mürtz P, Sprinkart AM, Reick M et al (2018) Accurate IVIM model-based liver lesion characterisation can be achieved with only three b-value DWI. Eur Radiol 28:4418–4428
Wang M, Li X, Zou J et al (2016) Evaluation of hepatic tumors using intravoxel incoherent motion diffusion-weighted MRI. Med Sci Monit 22:702–709
Zhu L, Cheng Q, Luo W et al (2015) A comparative study of apparent diffusion coefficient and intravoxel incoherent motion-derived parameters for the characterization of common solid hepatic tumors. Acta Radiol 56:1411–1418
Yoon JH, Lee JM, Yu MH et al (2014) Evaluation of hepatic focal lesions using diffusion-weighted MR imaging: comparison of apparent diffusion coefficient and intravoxel incoherent motion-derived parameters. J Magn Reson Imaging 39:276–285
Watanabe H, Kanematsu M, Goshima S et al (2014) Characterizing focal hepatic lesions by free-breathing intravoxel incoherent motion MRI at 3.0 T. Acta Radiol 55:1166–1173
Colagrande S, Regini F, Pasquinelli F et al (2013) Focal liver lesion classification and characterization in noncirrhotic liver: a prospective comparison of diffusion-weighted magnetic resonance–related parameters. J Comput Assist Tomogr 37:560–567
Doblas S, Wagner M, Leitao HS et al (2013) Determination of malignancy and characterization of hepatic tumor type with diffusion-weighted magnetic resonance imaging: comparison of apparent diffusion coefficient and intravoxel incoherent motion–derived measurements. Invest Radiol 48:722–728
Penner A-H, Sprinkart AM, Kukuk GM et al (2013) Intravoxel incoherent motion model-based liver lesion characterisation from three b-value diffusion-weighted MRI. Eur Radiol 23:2773–2783
Ichikawa S, Motosugi U, Ichikawa T et al (2013) Intravoxel incoherent motion imaging of focal hepatic lesions. J Magn Reson Imaging 37:1371–1376
Coenegrachts K, Delanote J, Ter Beek L et al (2009) Evaluation of true diffusion, perfusion factor, and apparent diffusion coefficient in non-necrotic liver metastases and uncomplicated liver hemangiomas using black-blood echo planar imaging. Eur J Radiol 69:131–138
Yamada I, Aung W, Himeno Y et al (1999) Diffusion coefficients in abdominal organs and hepatic lesions: evaluation with intravoxel incoherent motion echo-planar MR imaging. Radiology 210:617–623
Luo M, Zhang L, Jiang X, Zhang W (2017) Intravoxel incoherent motion diffusion-weighted imaging: evaluation of the differentiation of solid hepatic lesions. Transl Oncol 10:831–838
Andreou A, Koh DM, Collins DJ et al (2013) Measurement reproducibility of perfusion fraction and pseudodiffusion coefficient derived by intravoxel incoherent motion diffusion-weighted MR imaging in normal liver and metastases. Eur Radiol 23:428–434
Kakite S, Dyvorne H, Besa C et al (2015) Hepatocellular carcinoma: short-term reproducibility of apparent diffusion coefficient and intravoxel incoherent motion parameters at 3.0T. J Magn Reson Imaging 41:149–156
Mürtz P, Penner A-H, Pfeiffer A-K et al (2016) Intravoxel incoherent motion model-based analysis of diffusion-weighted magnetic resonance imaging with 3 b-values for response assessment in locoregional therapy of hepatocellular carcinoma. Onco Targets Ther 9:6425–6433
Pieper CC, Sprinkart AM, Meyer C et al (2016) Evaluation of a simplified intravoxel incoherent motion (IVIM) analysis of diffusion-weighted imaging for prediction of tumor size changes and imaging response in breast cancer liver metastases undergoing radioembolization: a retrospective single center analysis. Medicine (Baltimore) 95:1–9
Pieper CC, Willinek WA, Meyer C et al (2016) Intravoxel incoherent motion diffusion-weighted MR imaging for prediction of early arterial blood flow stasis in radioembolization of breast cancer liver metastases. J Vasc Interv Radiol 27:1320–1328
Pieper C, Meyer C, Sprinkart AM et al (2016) The value of intravoxel incoherent motion model-based diffusion-weighted imaging for outcome prediction in resin-based radioembolization of breast cancer liver metastases. Onco Targets Ther 9:4089–4098
Bruix J, Sherman M, American Association for the Study of Liver Disease (2011) Management of hepatocellular carcinoma: an update. Hepatology 53:1020–1022
Lemke A, Laun FB, Simon D et al (2010) An in vivo verification of the intravoxel incoherent motion effect in diffusion-weighted imaging of the abdomen. Magn Reson Med 64:1580–1585
Wagner M, Doblas S, Daire JL et al (2012) Diffusion-weighted MR imaging for the regional characterization of liver tumors. Radiology 264:464–472
Mürtz P, Kaschner M, Träber F et al (2012) Diffusion-weighted whole-body MRI with background body signal suppression: technical improvements at 3.0 T. J Magn Reson Imaging 35:456–461
Mürtz P, Kaschner M, Träber F et al (2012) Evaluation of dual-source parallel RF excitation for diffusion-weighted whole-body MR imaging with background body signal suppression at 3.0 T. Eur J Radiol 81:3614–3623
Willinek WA, Gieseke J, Kukuk GM et al (2010) Dual-source parallel radiofrequency excitation body MR imaging compared with standard MR imaging at 3.0 T: initial clinical experience 1. Radiology 256:966–975
Chang JM, Lee JM, Lee MW et al (2006) Superparamagnetic iron oxide-enhanced liver magnetic resonance imaging: comparison of 1.5 T and 3.0 T imaging for detection of focal malignant liver lesions. Invest Radiol 41:168–174
Horie T, Ogino T, Muro I et al (2009) Optimization of fat suppression for 3.0T DWIBS. In: 17th Annual ISMRM Scientific Meeting and Exhibition 2009. pp 4614. Honolulu, Hawaii
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Petra Mürtz.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in Mürtz P, Sprinkart AM, Reick M, et al (2018) Accurate IVIM model-based liver lesion characterisation can be achieved with only three b-value DWI. Eur Radiol. doi: https://doi.org/10.1007/s00330-018-5401-7.
Methodology
• retrospective
• diagnostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mürtz, P., Pieper, C.C., Reick, M. et al. Is liver lesion characterisation by simplified IVIM DWI also feasible at 3.0 T?. Eur Radiol 29, 5889–5900 (2019). https://doi.org/10.1007/s00330-019-06192-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06192-x