Abstract
Objectives
The present study aimed to compare the diagnostic performance of a machine learning (ML)–based FFRCT algorithm, quantified subtended myocardial volume, and high-risk plaque features for predicting if a coronary stenosis is hemodynamically significant, with reference to FFRICA.
Methods
Patients who underwent both CCTA and FFRICA measurement within 2 weeks were retrospectively included. ML-based FFRCT, volume of subtended myocardium (Vsub), percentage of subtended myocardium volume versus total myocardium volume (Vratio), high-risk plaque features, minimal lumen diameter (MLD), and minimal lumen area (MLA) along with other parameters were recorded. Lesions with FFRICA ≤ 0.8 were considered to be functionally significant.
Results
One hundred eighty patients with 208 lesions were included. The lesion length (LL), diameter stenosis, area stenosis, plaque burden, Vsub, Vratio, Vratio/MLD, Vratio/MLA, and LL/MLD4 were all significantly longer or larger in the group of FFRICA ≤ 0.8 while smaller minimal lumen area, MLD, and FFRCT value were noted. The AUC of FFRCT + Vratio/MLD was significantly better than that of FFRCT alone (0.935 versus 0.873, p < 0.001). High-risk plaque features failed to show difference between functionally significant and insignificant groups. Vratio/MLD-complemented ML-based FFRCT for “gray zone” lesions with FFRCT value ranged from 0.7 to 0.8 and the combined use of these two parameters yielded the best diagnostic performance (86.5%, 180/208).
Conclusions
ML-based FFRCT simulation and Vratio/MLD both provide incremental value over CCTA-derived diameter stenosis and high-risk plaque features for predicting hemodynamically significant lesions. Vratio/MLD is more accurate than ML-based FFRCT for lesions with simulated FFRCT value from 0.7 to 0.8.
Key Points
• Machine learning–based FFR CT and subtended myocardium volume both performed well for predicting hemodynamically significant coronary stenosis.
• Subtended myocardium volume was more accurate than machine learning–based FFR CT for “gray zone” lesions with simulated FFR value from 0.7 to 0.8.
• CT-derived high-risk plaque features failed to correctly identify hemodynamically significant stenosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coronary computed tomography angiography (CCTA) is an accurate imaging modality for ruling out obstructive coronary artery disease (CAD) when compared with invasive coronary angiography (ICA) [1,2,3]. However, these anatomy-based imaging methods lack functional information to determine the hemodynamic significance of coronary stenosis, which is more important for clinical decision-making. In contrast to the above imaging modalities, fractional flow reserve (FFR) is currently the gold standard for the evaluation of functional status of coronary lesions. According to previous large clinical trials, FFR is more favored than ICA for guiding revascularization strategy and leads to better clinical outcomes [4, 5].
Through computational fluid dynamics (CFD), it is possible to calculate FFRCT from standard CCTA [6,7,8]. However, this method is time-consuming. Recently, machine learning (ML)–based FFRCT has been introduced for differentiating flow-limiting and non-flow-limiting coronary stenosis with very short processing time and with promising preliminary results [9]. CT-derived high-risk plaque features might also be independent predictors of hemodynamic significance regardless of lesion’s geometrical features [10, 11]. In addition, Duke Jeopardy Score (DJS) is an angiography-based index to roughly estimate the amount of myocardium subtended by a coronary stenosis [12]. A previous study has shown that the extent of subtended myocardium as evaluated by DJS was a predictor of flow-limiting lesions [13]. With the development of computational technique, it is now technically feasible to use CCTA data to further absolutely quantify the subtended myocardium volume [14]. Consequently, we hypothesized that CT-derived plaque characteristics and absolute myocardial volume quantification might provide incremental values to the ML-based FFRCT method. Therefore, we aimed to investigate the diagnostic performance of ML-based FFRCT method combined with quantified myocardium volume as well as high-risk plaque features for the prediction of hemodynamically significant coronary stenosis.
Materials and methods
Patients’ population
The Institutional Review Board of the hospital approved this retrospective study, and the informed consent was waived as well. We retrospectively searched the hospital database from January 2012 to December 2017 to include patients with clinically suspected CAD who underwent both CCTA and invasive coronary angiography (ICA)/FFR measurement. The FFR measurement was clinically indicated to assess the hemodynamic significance of coronary stenosis in order to optimize the treatment strategy (revascularization or medical treatment). The inclusion criterion was the interval between the CCTA examination and FFRICA measurement within 2 weeks.
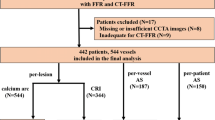
The exclusion criteria were as follows: (I) previous history of coronary revascularization; (II) previous history of myocardial infarction; (III) insufficient image quality of CCTA examination; (IV) patients with coronary anomalies or concomitant cardiomyopathy; and (V) the interval between CCTA and FFR measurement was longer than 2 weeks (Fig. 1).
CCTA protocol
A 128-slice multidetector CT (Definition AS+, Siemens Healthineers) was used for data acquisition. In all patients with an initial heart rate of > 65 bpm, 25–75 mg β-blocker (Betaloc ZOK, AstraZeneca) was administrated orally 1 h prior to the examination. Nitroglycerin was administered sublingually in all patients. Retrospective ECG-gated CTA was employed in patients with a final heart rate of ≥ 70 bpm whereas prospective ECG-triggered sequential acquisition was performed in patients with a final heart rate of < 70 bpm. The details of CCTA acquisition were given in online appendix.
Reconstruction and CT-derived plaque analysis
Data were transferred to an offline workstation (Syngo.via, Siemens Healthineers) for reconstruction and post-processing. The image quality was evaluated by using a 4-point Likert scale: 4 = excellent (in the absence of artifact), 3 = good (in the presence of mild artifact), 2 = sufficient (in the presence of moderate artifact, but still diagnostic), and 1 = poor (in the presence of severe artifact, non-diagnostic). Only patients with the image quality of grades 3–4 were included for further analysis.
The plaque characterization was performed according to CCTA findings and a series of quantified plaque features were measured by using a dedicated plaque analysis software (Coronary Plaque Analysis, version 2.0, Siemens Healthineers). The recorded parameters were as follow: (1) a remodeling index; (2) low-attenuation plaque (LAP); (3) a spotty calcification; (4) napkin-ring sign (NRS) as defined by previous study [15]; (5) lesion length; (6) plaque volume; (7) plaque burden;( 8) the minimal lumen area (MLA) and the minimal lumen diameter (MLD); (9) the diameter stenosis and area stenosis; and (10) DJS. The detailed definitions of the above parameters were given in online appendix.
The amount of perfused myocardium subtended by the target stenosis was quantified according to the concept of the Voronoi algorithm by using a commercially available software (Ziostation, Ziosoft). In brief, the location of each target lesion was manually marked by observers. Then, the algorithm automatically calculated the subtended myocardial volume by aggregating all myocardial voxels connected to the voxels on the coronary arteries that were distal to the target lesion. The volume (Vsub) and percentage (Vratio) of the subtended myocardium were consequently generated by the software.
The results of a previous study revealed that the ratio of DJS versus MLD (DJS/MLD) outperformed other combinations of morphological parameters, such as DJS/MLA and the ratio of LL versus the fourth power of MLD (LL/MLD4), for the prediction of hemodynamically significant lesions [13]. In the present study, we replaced DJS with a more precise parameter, Vratio, to represent the extent of myocardium subtended by coronary stenosis and therefore tested the diagnostic performance of different combinations (Vratio/MLD, Vratio/MLA, and LL/MLD4).
Two cardiovascular radiologists (with 10 and 8 years of experience on cardiac imaging), who were blinded to ICA and FFRICA results, independently analyzed the lesions. The mean values of quantitative parameters measured by two observers were used for further analysis.
FFRCT analysis
A machine learning–based algorithm (cFFR, version 3.0, Siemens Healthineers) was used for FFRCT simulation [9]. This model was trained on a large database of synthesized coronary anatomies, where the reference values are computed using a CFD-based model. For on-site processing, few steps have to be taken manually to determine the vessel centerline, luminal contour, and coronary stenosis before the final computation could be finished [16]. More details regarding the mechanism and processing procedure of this approach are given in online appendix. Two cardiovascular radiologists (with 10 and 8 years of experience on cardiac imaging), who were blinded to ICA and FFR results, independently performed the FFRCT simulation and the mean values of lesions were used for further analysis.
ICA and FFR measurement
ICA was performed using a standard method and at least two views were obtained for each major coronary artery. The images were independently evaluated by two interventional cardiologists (with 26 and 20 years of experience on coronary intervention), who were blinded to the results of CCTA as well as FFRCT. The stenotic extent of each lesion was recorded according to visual assessment. FFRICA was clinically indicated to assess the necessity for revascularization. FFR was measured by using a 0.014-in. pressure guidewire (St Jude Medical) as previously described [17]. Hyperemia was induced by intravenous infusion of adenosine at the dose of 140 μg per kilogram of body weight per minute. Besides, FFR ≤ 0.8 was considered physiologically significant stenosis.
Statistical analysis
Statistical analysis was performed by using a commercial statistical software (MedCalc Statistical Software, version 15.2.2; MedCalc Software bvba). One-sample Kolmogorov-Smirnov test was used to check the assumption of normal distribution. Normally distributed continuous quantitative variables were expressed as mean ± standard deviation (SD), or median with first to third quartiles. Student’s t test was used for normally distributed data, and the Mann-Whitney U test was used for the data that were not normally distributed. Categorical variables were reported as count (%) and compared by the Fisher’s exact test or chi-square test, according to the data cell size. Intra-observer and inter-observer agreements of all parameters were examined for intra-class correlation coefficients (ICC). All lesions were then classified as functionally significant or functionally non-significant (according to FFR values) for evaluating the association between the respective variables and the hemodynamic relevance of the lesions. The correlations between FFR value and all parameters were assessed by Pearson’s correlation coefficient when data were normally distributed or according to Spearman’s rank correlation coefficient when data were not normally distributed. The Bland-Altman method was used to plot the difference between FFRCT and FFRICA versus the average of FFRCT and FFRICA measurements. Receiver operating characteristic (ROC) curve analyses were performed to calculate the area under the ROC curve (AUC). The optimal cut-off values for various parameters were determined by Youden’s index, and the maximum sum of sensitivity and specificity at ROC curve analysis was calculated based on a method developed by DeLong et al [18]. The combined performance of FFRCT with other parameters was investigated using binary logistic regression (details in Online Appendix). In addition, a stepwise approach based on FFRCT with restrictive use of Vratio/MLD was designed. Lesions with FFRCT values within previously reported “gray zone” range (FFRCT value ranging from 0.7 to 0.8) [19] were reclassified according to the results of Vratio/MLD. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were recorded as well. A two-tailed p value < 0.05 was statistically considered significant.
Results
Clinical characteristics
A total of 52,600 patients who underwent CCTA from January 2012 to December 2017 were initially reviewed. Besides, 50,412 patients without ICA and 2039 patients with ICA (but without FFRICA measurement) were excluded. Seven patients were subsequently excluded because the interval between CCTA and ICA was longer than 2 weeks whereas further exclusion of 12 patients was due to uninterpretable CCTA images. Ten patients with a history of target lesion revascularization were further excluded (Fig. 1).
Eventually, 180 patients (mean age, 63 ± 7.5 years), including 116 males (mean age, 62.1 ± 7.8 years) and 64 females (mean age, 64.2 ± 9.5 years; p = 0.54) with 208 lesions, were included in our study. The mean interval between ICA and CCTA was 7.3 ± 3.5 days. The mean dose length product of CCTA was 510.7 ± 102.6 mGy cm and mean effective dose was 7.6 ± 1.8 mSv. Detailed demographic data are given in Table 1. The average processing time for FFRCT calculation and CT-based myocardium quantification was 7.1 ± 2.8 and 10.2 ± 3.1 min, respectively.
Correlation of CCTA-derived morphological parameters and FFRCT with functionally significant stenosis (FFR ≤ 0.8)
Lesions were divided into two subgroups for further analysis, by using a FFR value of 0.8 as a cut-off. Stenosis morphology as evaluated by CCTA, diameter stenosis, area stenosis, plaque burden, total lesion length, Vsub, Vratio, Vratio/MLD, Vratio/MLA, and LL/MLD4 were all significantly longer or larger in the group of hemodynamic significant lesions (FFR ≤ 0.8, p < 0.05) compared with the group of insignificant lesions (FFR > 0.8) (for all p < 0.001), as shown in Table 2. In addition, smaller MLD, MLA, and FFRCT were associated with functionally significant lesions (1.19 ± 0.30 vs. 1.50 ± 0.42; 1.61 ± 0.44 vs. 2.31 ± 0.86; 0.71 ± 0.10 vs. 0.86 ± 0.08, respectively; for all p < 0.001) (Figs. 2 and 3). However, there were no significant differences between the hemodynamic significant subgroup and the insignificant subgroup with respect to the risky plaque features as evaluated at CCTA (low-attenuation plaque, spotty calcification, napkin-ring sign, positive remodeling) (for all p > 0.05).
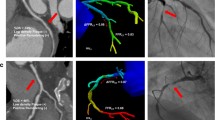
CCTA evaluation of severe coronary stenosis with an FFR of more than 0.8. a Three-dimensional MIP showed a severe coronary stenosis (white arrowhead) in the proximal LAD. b CPR showed the severe stenosis with non-calcified plaque at proximal LAD. The cross-section imaging revealed that the MLD was 1.9 mm. NRS and LAP were present as the color-coded plaque analysis showing low-density plaque component (< 30 HU, blue area) within the plaque. c ICA showed severe stenosis located at the proximal LAD with an FFR value of 0.85. d Left ventricle quantification demonstrated that the volume and percentage of subtended myocardium was 32 ml and 31.1%, respectively. The Vratio/MLD was 16.3, which was less than the best cut-off value and indicated hemodynamically insignificant of coronary stenosis. e FFRCT revealed that the LAD lesion had a simulated FFR value of 0.75, which was mismatched with FFRICA. CCTA, coronary computed tomography angiography; CPR, curved planar reformation; FFR, fractional flow reserve; ICA, invasive coronary angiography; LAD, left anterior descending; LAP, low-attenuation plaque; LL, lesion length; MIP, maximum intensity projection; MLD, minimal lumen diameter; NRS, napkin-ring sign
CCTA evaluation of moderate coronary stenosis with an FFR of greater than 0.8. a Three-dimensional MIP images showed moderate coronary stenosis (white arrowhead) in the middle RCA. b CPR revealed moderate stenosis with non-calcified plaque at the middle RCA. The cross-sectional imaging demonstrated that the MLD was 2.1 mm. High-risk plaque features were absent according to plaque analysis. c ICA showed moderate stenosis located at the middle RCA with an FFR value of 0.95. d Left ventricle quantification demonstrated that the volume and percentage of subtended myocardium were 22 ml and 23.5%, respectively. The Vratio/MLD was 11.2, which was less than the best cut-off value and indicated hemodynamically insignificant coronary stenosis. e FFRCT revealed that the LAD lesion had a simulated FFR value of 0.9, which was in accordance with FFRICA. CCTA, coronary computed tomography angiography; CPR, curved planar reformation; FFR, fractional flow reserve; ICA, invasive coronary angiography; LL, lesion length; MIP, maximum intensity projection; MLD, minimal lumen diameter; RCA, right coronary artery
Pearson correlation analysis demonstrated that the FFRCT, Vratio/MLD, and Vratio/MLA all correlated well with the FFRICA value (r = 0.72, − 0.62, and − 0.6, respectively; for all p < 0.001), whereas other parameters showed a poor correlation (Online Supplement Table E1). FFRCT showed a slight underestimation compared with FFRICA (Fig. 4). The intra-observer and inter-observer agreements of all parameters are shown in Online Supplement Tables E2–3.
Diagnostic performance of CCTA-derived parameters and FFRCT for the prediction of functionally significant coronary stenosis (FFR ≤ 0.8)
For single parameters, according to ROC curve analysis, the FFRCT showed the largest AUC (AUC = 0.873, 95%CI = 0.820–0.915) for diagnosing functionally significant stenosis (Table 3, Fig. 5). Vratio/MLD (AUC = 0.854, 95%CI = 0.799–0.899) and Vratio/MLA (AUC = 0.839, 95%CI = 0.781–0.886) had a similar diagnostic performance compared with FFRCT, whereas other parameters were less accurate (Table 3). For combined analysis, FFRCT + Vratio/MLD was revealed to have the significant larger AUC (AUC = 0.935, 95%CI = 0.892–0.964) than any other parameters (Table 3). More specifically, the AUC of FFRCT combined with Vratio/MLD was significantly better than that of FFRCT alone (0.935 vs. 0.873, p = 0.0068).
ROC curve analysis of FFRCT, Vratio/MLD, and combined FFRCT + Vratio/MLD for the identification of functionally significant coronary stenosis. FFRCT + Vratio/MLD showed significant improvement over CCTA, FFRCT, or Vratio/MLD merely for diagnosing flow-limiting coronary stenosis. *FFRCT + Vratio/MLD had the largest AUC compared with other parameters (all p < 0.05). FFRCT and Vratio/MLD had similar AUC (p = 0.6204). AUC, area under curve; CCTA, coronary computed tomography angiography; FFR, fractional flow reserve; MLD, minimal lumen diameter; ROC, receiver operating characteristic
The overall diagnostic accuracy of FFRCT analysis was 81.2% (Table 4). However, the diagnostic accuracy of FFRCT markedly varied for vessels with FFRCT values below 0.70, 0.70 to 0.79, 0.80, and 0.89, and above 0.89 (see Table 5). In our cohort, 55 lesions (26.4%) had FFRCT values between 0.70 and 0.79. Among them, only 34 lesions were truly within that range as determined by FFRICA. The diagnostic accuracy of those “gray zone” FFRCT lesions (61.8%, 34/55) could be significantly improved to 80% (44/55) (p = 0.0001), if these lesions were evaluated with Vratio/ MLD instead of FFRCT. For the total 208 lesions, this stepwise approach correctly classified 189 lesions and provided incremental diagnostic accuracy over FFRCT or Vratio/ MLD alone (90.9% [189/208] vs. 82.7% [172/208]; 90.9% [189/208] vs. 80.3% [167/208]).
Discussion
The major finding of the present study is that ML-based FFRCT simulation and Vratio/MLD both performed well for the prediction of hemodynamic status. Vratio/MLD was more accurate than ML-based FFRCT for lesions with simulated FFR value ranging from 0.7 to 0.8. However, the high-risk plaque features failed to show significant correlation with the hemodynamic significance.
FFR-guided revascularization strategy is associated with better clinical outcomes as well as less unnecessary percutaneous coronary intervention procedures [20,21,22]. Recently, a ML-based FFRCT approach was developed as a method for non-invasive evaluation of the hemodynamic status of coronary stenosis. It enabled simulation of FFR value from a standard CCTA scanning at a remarkably shorter processing time compared with a CFD-based approach [9]. Despite its promising role, previous studies identified “gray zone” lesions, corresponding to FFRCT values ranging between 0.7 and 0.8: according to a meta-analysis, the diagnostic accuracy was only 46.1% for CFD-based FFRCT in such cases [19]. Our study had similar findings for ML-based FFRCT, showing excellent diagnostic performance when the simulated value was below 0.7 or above 0.8, and only 61.8% between 0.7 and 0.8.
Interestingly, we found that the addition of Vratio/MLD to ML-based FFRCT improved the diagnostic accuracy from 61.8 to 80% for the “gray zone” lesions. Our previous study validated the diagnostic value of using DJS based on a CT morphological index for discrimination of flow-limiting and non-flow-limiting lesions [13]. However, DJS can only approximately evaluate the stenosis-subtended myocardial volume. In addition, the value of the index is limited when a coronary anomaly is present or major side branch vessels are absent. In the current study, we replaced DJS with absolute quantification of myocardial volume. A large myocardial volume was associated with significant inducible ischemia even with the same degree of stenosis [23]. Thus, the addition of absolute quantification of myocardial volume to anatomical stenosis may reduce the misdiagnosis of ischemic coronary stenosis with reference to FFRICA. Therefore, the potential clinical implication lies in the combined use of these parameters for more accurate functional assessment of coronary stenosis. In other words, for lesions with FFRCT values less than 0.7 or more than 0.8, ML-based FFRCT is an accurate approach with very high negative predictive value to safely rule out hemodynamically significant lesions and avoid unnecessary invasive procedures. For lesions with FFRCT values between 0.7 and 0.8, Vratio/MLD performed better than ML-based FFRCT and combined use of the above parameters would be recommended.
High-risk plaque features evaluated by CCTA were found to be irrelevant to the hemodynamic significance of coronary stenosis in our study. Discrepant results have been reported according to previous studies regarding the association between plaque histology and hemodynamic significance [24,25,26,27]. There were CCTA studies showing that the presence of a large necrotic core as well as the total LAP volume may contribute to the hemodynamic significance of coronary stenosis [24, 25]. In contrast, our results are more in line with other intravascular ultrasound studies that there was no association between plaque composition and FFR value [26, 27]. We found that the geometrical features, such as lesion length, entrance angle, exit angle, size of the reference vessel, and absolute blood flow relative to the territory supplied of coronary lesions, are more important factors than high-risk plaque features to affect the downstream myocardial perfusion. In contrast, high-risk plaque features are more likely to be linked to the risk of cardiac events [28,29,30]. Therefore, it seems reasonable to estimate that lesions with similar geometrical features and different plaque compositions tend to have comparable hemodynamic significance but heterogeneous prognosis.
The current study used a commercially available software to quantify the myocardial volume subtended by coronary stenosis. This technique is theoretically based on the concept of the Voronoi algorithm [31]: a voxel in the LV myocardium is linked to the nearest voxel on the coronary artery as its own territory. The accuracy of this approach regarding automatic myocardium segmentation has been validated by a recent animal study [14], with an excellent correlation of CT-derived myocardial volume to actual myocardial volume. Therefore, it is technically feasible to perform non-invasive myocardium quantification based on CT modality.
There are some limitations in our study. First, the retrospective design might lead to inclusion bias. Since FFRICA measurement was rarely used for the assessment of mild coronary stenosis (stenotic extent < 50%) in our hospital, the current analysis did not include mild lesions. Therefore, the diagnostic performance of ML-based FFRCT and subtended myocardial mas still needs to be validated in patients with mild stenosis. Second, CFD-based FFRCT was not used in the current investigation. Although previous studies showed a comparable performance of both two FFRCT approaches [32, 33], whether CFD-based FFRCT would result in similar results remains to be determined. Third, the present study only included a small fraction of patients who underwent CCTA in our institute. This is also a severe inclusion bias due to the retrospective nature of the study. Fourth, various other CT-based techniques or parameters have been recently reported to be able to accurately predict ischemic coronary stenosis [34,35,36,37]. Future head-to-head comparison studies are needed to determine the best method among current and those approaches. Finally, the relatively small sample size might also partially lead to the discrepant finding regarding the relationship between plaque characteristics and hemodynamic significance. For these reasons, future prospective studies with larger sample size are required to confirm the current finding.
In conclusion, ML-based FFRCT simulation and Vratio/MLD both performed well for predicting hemodynamic status. Vratio/MLD was more accurate than ML-based FFRCT for lesions with simulated FFR value ranging from 0.7 to 0.8. However, the high-risk plaque features failed to show a significant correlation with the hemodynamic significance.
Abbreviations
- AUC:
-
Area under the curve
- CCTA:
-
Coronary computed tomography angiography
- CFD:
-
Computational fluid dynamics
- DJS:
-
Duke Jeopardy Score
- FFR:
-
Fractional flow reserve
- ICA:
-
Invasive coronary angiography
- LL:
-
Lesion length
- ML:
-
Machine learning
- MLA:
-
Minimal lumen area
- MLD:
-
Minimal lumen diameter
- ROC:
-
Receiver operating characteristic
References
Miller JM, Rochitte CE, Dewey M et al (2008) Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 359(22):2324–2336
Westwood ME, Raatz HD, Misso K et al (2013) Systematic review of the accuracy of dual-source cardiac CT for detection of arterial stenosis in difficult to image patient groups. Radiology 267(2):387–395
Yang L, Zhou T, Zhang R et al (2014) Meta-analysis: diagnostic accuracy of coronary CT angiography with prospective ECG gating based on step-and-shoot, flash and volume modes for detection of coronary artery disease. Eur Radiol 24(10):2345–2352
Toth G, Hamilos M, Pyxaras S et al (2014) Evolving concepts of angiogram: fractional flow reserve discordances in 4000 coronary stenoses. Eur Heart J 35(40):2831–2838
Tonino PA, Fearon WF, De Bruyne B et al (2010) Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol 55(25):2816–2821
Taylor CA, Fonte TA, Min JK (2013) Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol 61(22):2233–2241
Nørgaard BL, Leipsic J, Gaur S et al (2014) Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (analysis of coronary blood flow using CT angiography: next steps). J Am Coll Cardiol 63(12):1145–1155
Min JK, Leipsic J, Pencina MJ et al (2012) Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA 308:1237–1245
Itu L, Rapaka S, Passerini T et al (2016) A machine-learning approach for computation of fractional flow reserve from coronary computed tomography. J Appl Physiol (1985) 121(1):42–52
Park HB, Heo R, ó Hartaigh B et al (2015) Atherosclerotic plaque characteristics by CT angiography identify coronary lesions that cause ischemia: a direct comparison to fractional flow reserve. JACC Cardiovasc Imaging 8(1):1–10
Driessen RS, Stuijfzand WJ, Raijmakers PG et al (2018) Effect of plaque burden and morphology on myocardial blood flow and fractional flow reserve. J Am Coll Cardiol 71(5):499–509
Califf RM, Phillips HR 3rd, Hindman MC et al (1985) Prognostic value of a coronary artery jeopardy score. J Am Coll Cardiol 5(5):1055–1063
Yu M, Zhao Y, Li W et al (2018) Relationship of the Duke jeopardy score combined with minimal lumen diameter as assessed by computed tomography angiography to the hemodynamic relevance of coronary artery stenosis. J Cardiovasc Comput Tomogr 12(3):247–254
Ide S, Sumitsuji S, Yamaguchi O, Sakata Y (2017) Cardiac computed tomography-derived myocardial mass at risk using the Voronoi-based segmentation algorithm: a histological validation study. J Cardiovasc Comput Tomogr 11(3):179–182
Min JK, Shaw LJ, Devereux RB et al (2007) Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol 50:1161–1170
Yu M, Lu Z, Li W, Wei M, Yan J, Zhang J (2018) CT morphological index provides incremental value to machine learning based CT-FFR for predicting hemodynamically significant coronary stenosis. Int J Cardiol 265:256–261
Pijls NH, De Bruyne B, Peels K et al (1996) Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med 334(26):1703–1708
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44(3):837–845
Cook CM, Petraco R, Shun-Shin MJ et al (2017) Diagnostic accuracy of computed tomography-derived fractional flow reserve: a systematic review. JAMA Cardiol 2(7):803–810
Tonino PA, De Bruyne B, Pijls NH et al (2009) Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 360(3):213–224
De Bruyne B, Pijls NH, Kalesan B et al (2012) Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 367(11):991–1001
De Bruyne B, Fearon WF, Pijls NH et al (2014) Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med 371(13):1208–1217
Leone AM, De Caterina AR, Basile E et al (2013) Influence of the amount of myocardium subtended by a stenosis on fractional flow reserve. Circ Cardiovasc Interv 6(1):29–36
Ahmadi A, Stone GW, Leipsic J et al (2016) Association of coronary stenosis and plaque morphology with fractional flow reserve and outcomes. JAMA Cardiol 1(3):350–357
Gaur S, Øvrehus KA, Dey D et al (2016) Coronary plaque quantification and fractional flow reserve by coronary computed tomography angiography identify ischaemia-causing lesions. Eur Heart J 13:1220–1227
Waksman R, Legutko J, Singh J et al (2013) FIRST: Fractional Flow Reserve and Intravascular Ultrasound Relationship Study. J Am Coll Cardiol 61:917–923
Brugaletta S, Garcia-Garcia HM, Shen ZJ et al (2012) Morphology of coronary artery lesions assessed by virtual histology intravascular ultrasound tissue characterization and fractional flow reserve. Int J Cardiovasc Imaging 28:221–228
Yu M, Lu Z, Li W et al (2018) Coronary plaque characteristics on baseline CT predict the need for late revascularization in symptomatic patients after percutaneous intervention. Eur Radiol 28(8):3441–3453
Yu M, Li W, Lu Z et al (2018) Quantitative baseline CT plaque characterization of unrevascularized non-culprit intermediate coronary stenosis predicts lesion volume progression and long-term prognosis: a serial CT follow-up study. Int J Cardiol 264:181–186
Ferencik M, Mayrhofer T, Bittner DO et al (2018) Use of high-risk coronary atherosclerotic plaque detection for risk stratification of patients with stable chest pain: a secondary analysis of the PROMISE randomized clinical trial. JAMA Cardiol 3(2):144–152
Guibas L, Stolfi J (1985) Primitives for the manipulation of general subdivisions and the computations of Voronoi diagrams. ACM Trans Graph 4:74–123
Tesche C, De Cecco CN, Baumann S et al (2018) Coronary CT angiography-derived fractional flow reserve: machine learning algorithm versus computational fluid dynamics modeling. Radiology 288(1):64–72
Coenen A, Kim YH, Kruk M et al (2018) Diagnostic accuracy of a machine-learning approach to coronary computed tomographic angiography-based fractional flow reserve: result from the MACHINE consortium. Circ Cardiovasc Imaging 11(6):e007217
Dey D, Gaur S, Ovrehus KA et al (2018) Integrated prediction of lesion-specific ischaemia from quantitative coronary CT angiography using machine learning: a multicentre study. Eur Radiol 28(6):2655–2664
von Knebel Doeberitz PL, De Cecco CN, Schoepf UJ et al (2018) Coronary CT angiography-derived plaque quantification with artificial intelligence CT fractional flow reserve for the identification of lesion-specific ischemia. Eur Radiol. https://doi.org/10.1007/s00330-018-5834-z
Siogkas PK, Anagnostopoulos CD, Liga R et al (2018) Noninvasive CT-based hemodynamic assessment of coronary lesions derived from fast computational analysis: a comparison against fractional flow reserve. Eur Radiol. https://doi.org/10.1007/s00330-018-5781-8
van Hamersvelt RW, Zreik M, Voskuil M, Viergever MA, Išgum I, Leiner T (2018) Deep learning analysis of left ventricular myocardium in CT angiographic intermediate-degree coronary stenosis improves the diagnostic accuracy for identification of functionally significant stenosis. Eur Radiol. https://doi.org/10.1007/s00330-018-5822-3
Funding
This study has received funding from the National Natural Science Foundation of China (Grant No.: 81671678), Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (Grant No.: 20161428), Shanghai Key Discipline of Medical Imaging (No.: 2017ZZ02005), and The National Key Research and Development Program of China (Grant Nos.: 2016YFC1300400 and 2016YFC1300402).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Jiayin Zhang.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by hospital IRB.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• comparative study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 33 kb)
Rights and permissions
About this article
Cite this article
Yu, M., Lu, Z., Shen, C. et al. The best predictor of ischemic coronary stenosis: subtended myocardial volume, machine learning–based FFRCT, or high-risk plaque features?. Eur Radiol 29, 3647–3657 (2019). https://doi.org/10.1007/s00330-019-06139-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06139-2