Abstract
Objectives
To evaluate whether post-contrast cone-beam breast CT (CBBCT) alone is comparable to the current standard of combined pre- and post-contrast CBBCT regarding diagnostic accuracy and superior regarding radiation exposure.
Material and methods
This study included 49 women (61 breasts) with median age 57.9 years and BI-RADS 4/5 lesions diagnosed on mammography/ultrasound in density type c/d breasts. Two radiologists rated post-contrast CBBCT and pre- and post-contrast CBBCT with subtraction images on the BI-RADS scale separately for calculation of inter- and intra-observer agreement and in consensus for diagnostic accuracy assessment. Sensitivity, specificity, and area under the curve (AUC) were compared via McNemar test and DeLong method, respectively. Subtraction imaging misregistration were measured from 1 (no artifacts) to 4 (artifacts with width > 4 mm).
Results
A total of 100 lesion (51 malignant; 6 high risk; 43 benign) were included. AUC, sensitivity, and specificity showed no significant differences comparing post-contrast CBBCT alone versus pre- and post-contrast CBBCT (AUC 0.84 vs. 0.83, p = 0.643; sensitivity 0.89 vs. 0.85, p = 0.158; specificity 0.73 vs. 0.76, p = 0.655). Inter- and intra-observer agreement was excellent (intra-class correlation coefficient ICC = 0.76, ICC = 0.83, respectively). Radiation dose was significantly lower for post-contrast CBBCT alone versus pre- and post-contrast CBBCT (median average glandular radiation dose 5.9 mGy vs. 11.7 mGy, p < 0.001). High-degree misregistrations were evident in the majority of subtraction images (level 1/2/3/4 16.9%/27.1%/16.9%/39%), in particular for bilateral exams (3.2%/29.2%/8.3%/58.3%).
Conclusion
Diagnostic accuracy of post-contrast CBBCT alone is comparable to pre- and post-contrast CBBCT in type c/d breasts, while yielding a significant twofold radiation dose reduction.
Key Points
• The diagnostic accuracy of post-contrast CBBCT alone is comparable to dual acquisition of pre- and post-contrast CBBCT.
• Acquisition of the post-contrast CBBCT scan alone reduces radiation exposure compared to pre- and post-contrast CBBCT, thus countering one of the main limitations of CBBCT.
• High-degree misregistration artifacts limit the interpretation of subtraction images from pre- and post-contrast CBBCT studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cone-beam breast CT (CBBCT) is a novel dedicated breast-imaging technique that allows for acquisition of high-resolution and 3D datasets [1,2,3]. Compared to two-view mammography (MG), CBBCT reduces breast tissue overlap and improves conspicuity of breast lesions [4,5,6]. The current acquisition standard for contrast-enhanced CBBCT consists of two separate CBBCT scans: one pre-contrast CBBCT scan, followed by intravenous administration of iodinated contrast media, and one post-contrast CBBCT scan.
Contrast-enhanced CBBCT has been shown to further increase diagnostic accuracy of CBBCT, potentially by visualization of hypervascular breast lesions [6,7,8,9,10]. Several studies have compared contrast-enhanced CBBCT with MG and magnetic resonance imaging (MRI) of the breast: they demonstrated an increased accuracy for contrast-enhanced CBBCT over MG [6, 7, 9, 11] and comparable results with MRI [10].
Nevertheless, the radiation exposure of contrast-enhanced CBBCT is high, with an average glandular dose (AGD) of up to 33 mGy reported in the literature [7, 9]. In part, this high radiation dose results from acquisition of two separate CBBCT scans as detailed above [6,7,8,9,10, 12]. This technique allows for evaluation of the absolute enhancement of breast lesions compared to baseline values, as well as for acquisition of subtraction images. Still, to date, there are no studies evaluating the true diagnostic benefit of the pre-contrast scan for contrast-enhanced CBBCT, and to what degree misregistration limits CBBCT subtraction imaging.
In the current study, we hypothesized that assessment of the post-contrast CBBCT scan alone would yield comparable diagnostic accuracy compared to the current standard of pre- and post-contrast CBBCT while yielding a lower radiation dose.
Materials and methods
Patients
This study evaluated CBBCT images of patients that were acquired as part of an earlier study comparing CBBCT to MG and prospective MRI [10]. This manuscript adds novel assessments of post-contrast CBBCT alone and compares these to pre- and post-contrast CBBCT. Two further studies focused on optimal contrast-enhanced CBBCT acquisition time and correlation of CBBCT contrast enhancement with immunohistochemical breast cancer subtypes [12, 13]. This paired study was conducted in accordance with the Declaration of Helsinki and received approval by the Institutional Review Board. All patients provided written informed consent prior to study enrollment.
From December 2015 to March 2017, women aged 40 years and older who were referred to our breast-imaging center were enrolled with ACR Breast Imaging Reporting and Data System (BI-RADS) category 4 or 5 lesions diagnosed on MG and/or ultrasound and dense or very dense breast tissue (type c or d) [14]. Each patient thus had at least one breast lesion (malignant or benign) and was either confirmed by histology for suspected malignant lesions or followed up after at least 12 months for probably benign lesions [15].
Women with any contraindications for iodinated contrast agents, pregnant and breastfeeding women were excluded.
Digital MG was performed with two standard views (cranio-caudal and mediolateral oblique) as part of the routine workup. Each patient underwent CBBCT examinations as a study procedure within at median 3 days after MG.
CBBCT protocol
A dedicated breast CT scanner (Koning Breast CT, CBCT 1000, Koning Corporation) was used for CBBCT examinations. The CBBCT was conducted with a constant tube voltage of 49 kVp and variable tube currents (between 50 and 200 mA) depending on breast size and density [1, 2]. Tube current was automatically selected after an initial scout scan and kept constant for pre- and post-contrast CBBCT imaging. The CBBCT scanner used in our study only allowed the imaging of one breast at a time and was done in standard manner [1]. A complete contrast-enhanced breast CT scan comprised an initial pre-contrast scan and a post-contrast scan 2 min after an automated single-shot intravenous injection of 90 mL iodinated contrast agents (iopromide, Ultravist 300; Bayer-Schering) with a flow rate of 3 mL/s using a power injector, followed by a 30-mL saline chaser. To minimize contrast media dose, bilateral CBBCT examinations were performed by rapid repositioning of the other breast, at a mean time of 3 min after contrast media application. The exact details were previously described [10, 13]. All CBBCT examinations were performed independently of the menstrual cycle.
Post-acquisition image processing and reconstruction were performed to achieve isotropic reconstructed volumes using a soft tissue filter and a voxel size of (0.273 mm)3 (standard mode). Dose reports from DICOM files were used to report CBBCT and MG radiation dose.
CBBCT image analysis
Anonymized CBBCT images were evaluated with a 3D visualization software and computer workstation (Visage CS Thin Client/Server, Visage Imaging). All images were interpreted separately for calculation of inter- and intra-observer variability, and in consensus for assessment of diagnostic accuracy by two breast radiologists (S.W., U.F.) with more than 8 years of breast-imaging experience and 2 years of experience in dedicated CBBCT. The readers were blinded to patients’ history and clinical examination.
To compare post-contrast CBBCT alone versus dual acquisition as pre- and post-contrast CBBCT, image interpretation was done in two sessions. First, the readers evaluated only the post-contrast CBBCT images. With an interval of 4 weeks, both pre-contrast CBBCT and post-contrast CBBCT studies were interpreted together, as well as subtraction images. To obtain measures of intra-observer variability, one reader (S.W.) additionally evaluated another 4 weeks later all post-contrast CBBCT and as well as pre- and post-contrast CBBCT studies a second time.
Misregistration of the subtraction imaging from movements between pre- and post-contrast CBBCT scans of one breast was classified by both readers in consensus and rated as level 1, no motions between both scans; level 2, intramammary motions with a maximum width of 2 mm; level 3, intramammary motions with a width between 2 and 4 mm; and level 4, intramammary motions with a maximum width of more than 4 mm (Fig. 1). Misregistration assessment followed the modified classification used in breast MRI [16].
Misregistration in post-contrast CBBCT subtraction imaging in axial view rated as level 1 (a no artifacts); level 2 (b arrow indicating artifacts with maximum width of 2 mm); level 3 (c arrow indicating artifacts with maximum width between 2 and 4 mm); and level 4 (d arrow indicating artifacts with maximum width of more than 4 mm)
To compare diagnostic accuracy of post-contrast CBBCT alone versus pre- and post-contrast CBBCT, the BI-RADS 5th edition classification was correlated with histopathological diagnoses, adapted for CBBCT imaging from the MG part [14]. Imaging studies were rated as BI-RADS 1, negative; BI-RADS 2, benign finding; BI-RADS 3, probably benign; BI-RADS 4, likely malignant; and BI-RADS 5, malignant. A priori, papillomas were categorized as “benign” for analysis purpose [17, 18].
Statistical analysis
Continuous variables were expressed as median with interquartile range (IQR) and categorical variables as absolute number with percent.
For assessment of inter-observer and intra-observer agreement of BI-RADS readings of the different CBBCT methods, the intra-class correlation coefficient (ICC) was used. An ICC less than 40% was considered as poor, 40–59% as fair, 60–74% as good, and 75–100% as excellent.
Diagnostic test accuracy was assessed lesion-based via test sensitivity, specificity, and area under the receiver-operating curve (AUC) separately for each CBBCT method. Confidence limits for sensitivity and specificity were based on exact binomial formulas, and for AUC based on 2.000 stratified bootstrap samples.
The BI-RADS score was dichotomized with labeling BI-RADS 1 and 2 as negative readings, and BI-RADS 3, 4, and 5 as positive readings. Sensitivity and specificity were calculated using standard 2 × 2 table methods. For calculation of ROC and corresponding AUC, a modified 4-point BI-RADS score was used as proposed by Jiang and Metz [19]: BI-RADS scores of 1 or 2 were summarized indicating no malignancy probability; all BI-RADS 0 lesions were discarded. Dependent AUCs were compared using the De Long method [20]. McNemar’s test was utilized to compare sensitivity and specificity.
Radiation doses were compared via the Wilcoxon rank sum test between post-contrast CBBCT alone and combined pre- and post-contrast CBBCT.
Statistical analyses were conducted using R version 3.3.2 and RStudio version 1.1.383. All p values reported are two-sided. An alpha level of 0.05 was considered statistically significant.
Results
Patient cohort
A total of 49 patients with median age of 57.9 years (IQR 49–66 years) were included. Seventeen patients (34.7%) were pre-menopausal and 32 patients (65.3%) were post-menopausal. Breast density was rated as type c in 34 patients (69.4%) and as type d in 15 patients (30.6%). A total of 59 breasts were included: the right breast was affected in 15 patients (31.2%), and the left breast in 22 patients (45.8%). Another 12 patients (25%) had bilateral breast involvement.
Misregistrations
Misregistration was absent in 10 (16.9%) of the 59 post-contrast CBBCT examinations of separate breasts (artifact level 1). Artifact levels 2, 3, and 4 were observed in 16 (27.1%), 10 (16.9%), and 23 (39%) breasts, respectively. Bilateral CBBCT scans with repositioning yielded in a higher prevalence of high-degree artifacts (level 1 n = 1, 4.2%; level 2 n = 7, 29.2%; level 3 n = 2, 8.3%; level 4 n = 14, 58.3%). Figure 1 depicts all four levels of CBBCT misregistration.
Breast lesions
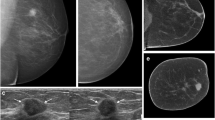
In the study cohort, a total of 100 breast lesions were identified. The median number of lesions per breast was 2 (range 1–8 lesions/breast). Histopathological assessment via core-needle biopsy was performed for 63 lesions (63%), and imaging follow-up over at least 1 year for 37 lesions (37%). Malignant breast lesions were diagnosed in 55 cases (55%), and benign disease in 45 cases (45%). Histopathological diagnoses are summarized in Table 1. Figures 2, 3, and 4 show exemplary pre-contrast CBBCT and post-contrast CBBCT cases with subtraction images.
Case of a 45-year-old female presenting with 3 intramammary lesions of the left breast in axial view. Both retromamillary lesions (a, c; indicated by arrowheads) showed no contrast enhancement on post-contrast CBBCT and were histopathologically approved as a benign duct ectasia. The central lesion (b, d, e; indicated by arrow) demonstrated contrast enhancement also seen on subtraction imaging and was histopathologically approved as an invasive lobular carcinoma (ILC), grade 2 with 11-mm diameter
Case of a 51-year-old female presenting with suspicious microcalcifications on MG (not shown). CBBCT in axial view shows linear distributed microcalcifications in the central part of the right breast on pre- and post-contrast CBBCT and contrast enhancement on post-contrast CBBCT that was histopathologically approved as an intermediate-grade DCIS (a, b; arrowhead). Post-contrast CBBCT highlights malignant calcifications. A simultaneous invasive ductal carcinoma (IDC), grade 2 with 24-mm diameter, was identified on post-contrast CBBCT and subtraction imaging only via marked contrast enhancement (a–c; arrow)
Case of a 47-year-old female where pre-contrast CBBCT in sagittal view showed no suspicious finding. Post-contrast CBBCT and subtraction imaging revealed an irregular breast lesion (13-mm diameter) with indistinct margin and avid contrast enhancement (b, c; arrow). The lesion was histopathologically approved as an invasive ductal carcinoma (IDC) grade 2. The lesion was missed on MG (not shown)
On post-contrast CBBCT, lesion margin was indistinct in 52 cases (52%), circumscribed in 23 cases (23%), spiculated in 8 cases (8%), and other margin in 17 cases (17%). Lesion shape was irregular in 57 cases (57%), oval/round in 32 cases (32%), and other shape in 11 cases (11%).
Diagnostic accuracy
In two reader consensus reading, both approaches showed comparable AUC, sensitivity, and specificity for assessment of breast lesions malignancy. No significant differences in diagnostic accuracy were detected comparing post-contrast CBBCT alone to pre- and post-contrast CBBCT (AUC 0.84 vs. 0.83, p = 0.643; sensitivity 0.89 vs. 0.85, p = 0.158; specificity 0.73 vs. 0.76, p = 0.655). Table 2 summarizes the diagnostic accuracy of pre- and post-contrast CBBCT versus post-contrast CBBCT alone with according 95% confidence intervals.
On separate reading, inter-observer and intra-observer agreement was excellent with ICC = 0.76 (95% CI 0.66–0.83) and ICC = 0.83 (95% CI 0.76–0.88). All lesions identified on combined pre- and post-contrast CBBCT were seen on post-contrast CBBCT alone as well.
Radiation dose
The average glandular radiation dose (AGD) per breast was lower for post-contrast CBBCT alone (median AGD 5.9 mGy, IQR 5.9–8 mGy) compared to combination of pre- and post-contrast CBBCT (median AGD 11.7 mGy, IQR 11.7–15.9 mGy; p < 0.001). In those patients with breast lesions identified on MG (n = 50), median AGD for MG was lower than post-contrast CBBCT alone (3.2 mGy, IQR 2.7–4.3 mGy vs. 5.9 mGy, IQR 5.9–8 mGy, p < 0.001).
Discussion
CBBCT is a novel 3D breast-imaging technique showing superior diagnostic accuracy compared to MG and increased patient comfort [6, 7, 9, 11]. Initial trials also reported comparable diagnostic performance of contrast-enhanced CBBCT and breast MRI, while reducing examination time [10]. To date, one of the major limitations of contrast-enhanced CBBCT is the high radiation exposure with average glandular dose of up to 33 mGy due to dual acquisition of non-contrast and contrast-enhanced CBBCT [7, 9]. Radiation exposure might be reduced by implementation adapted CBBCT acquisition protocols, only including a single-scan post-contrast CBBCT.
Our study demonstrates that the diagnostic accuracy of post-contrast CBBCT alone is comparable to that of pre- and post-contrast CBBCT, measured by AUC, sensitivity, and specificity. At the same time, post-contrast CBBCT alone yields a significant twofold decrease in average glandular dose versus pre- and post-contrast CBBCT.
Considering AUC as the summary measure of diagnostic accuracy, post-contrast CBBCT alone versus pre- and post-contrast CBBCT did not show clinically relevant or statistically significant differences. Numerically, post-contrast CBBCT alone yielded a slightly lower sensitivity but higher specificity than pre- and post-contrast CBBCT that did not reach statistical significance. One might argue that by assessment of post-contrast CBBCT only, the false-positive rate could be reduced, thereby decreasing patient anxiety, unnecessary biopsies, and costs. Still, this finding has to be confirmed in larger and representative cohorts. Several earlier CBBCT studies demonstrated that intravenous contrast media administration improves detection and classification of breast lesions versus pre-contrast CBBCT, in particular for dense breast tissue [6,7,8, 10]. These studies showed that contrast-enhanced CBBCT had higher sensitivity (0.82–0.99) and specificity (0.85–0.86) compared to non-contrast CBBCT alone and to MG. These results are in line with the diagnostic accuracy observed for contrast-enhanced CBBCT alone in our study (sensitivity 0.85; specificity 0.73). Minor deviations in CBBCT specificity might result from discrepancies in patient cohorts, CBBCT scanner, and acquisition protocol. For example, both Aminololama-Shakeri et al and He et al included a relevant proportion of patients with type a/b breasts, and the study by Aminololama-Shakeri et al used the Boone breast CT at the University of California, San Diego [6, 8].
In our study, post-contrast CBBCT alone accounted for a twofold decreased average glandular dose compared to pre- and post-contrast CBBCT (5.9 mGy vs. 11.7 mGy, p < 0.001). A direct comparison of MG and post-contrast CBBCT radiation dose was only possible in a subset of patients with breast lesions identified on MG but showed a significantly lower AGD for mammography. Still, the radiation dose of post-contrast CBBCT alone in our study is comparable to that of non-contrast CBBCT alone shown in the literature: for example, He et al reported on an average glandular of 8 mGy for non-contrast CBBCT alone [6]. Prionas et al demonstrated a twofold lower radiation dose for pre-contrast CBBCT compared to pre- and post-contrast CBBCT (AGD 8–32 mGy) [9]. Considering these findings, decreases in radiation exposure for breast imaging might be achieved by avoidance of additional mammographic images such as spot compression and magnification views. This diagnostic algorithm could be further amended by implementation of CBBCT-guided biopsies that have been shown to be faster than standard stereotactic biopsies in prone position, while reducing radiation exposure [21]. Given the relevant radiation dose reduction observed in our study, post-contrast CBBCT alone might extend the diagnostic breast imaging spectrum in clinical practice, in particular if future studies can confirm a comparable diagnostic accuracy for CBBCT and breast MRI that was reported in initial trials [10]. CBBCT advantages include a fast imaging protocol and improved patient comfort as well as a high diagnostic accuracy for detection and assessment of microcalcifications, where breast MRI might face limitations [2, 8,9,10].
Misregistrations interfering with assessment of pre- and post-contrast CBBCT subtraction images were evident in the majority of our cases: only in 16.9% of all cases, no artifacts were observed, with higher prevalence of high-degree artifacts in bilateral exams. This distortion might pose a limitation when acquiring dual pre- and post-contrast CBBCT imaging, in particular in patients with bilateral breast involvement. Still, the diagnostic benefit, if any, of subtraction imaging in the context of CBBCT is not evaluated to this date.
Our study has several limitations. First, only Caucasian women with dense breast tissue were included, which may limit the generalizability of our results. In particular, radiation dose reduction with post-contrast CBBCT alone versus pre- and post-contrast CBBCT has to be confirmed in patients with density type a/b breasts as well. Inclusion of only BI-RADS 4/5 lesions identified on MG and/ or US might have biased lesion assessment by readers towards higher BI-RADS scores. This limits the generalizability of our results, in particular if the pre-contrast CBBCT scan has higher accuracy for assessment of benign lesions such as complicated cysts or those with dense content. Therefore, further studies assessing post-contrast CBBCT alone in larger cohorts with more BI-RADS 2/3 lesions might be warranted. Although most breast lesions underwent histopathological assessment, imaging follow-up was performed in several cases due to ethical constraints. Even at a follow-up time of 12 months, slow growing breast malignancies cannot be fully excluded. To avoid repetitive contrast administration for bilateral CBCCT exams, patients were rapidly repositioned which resulted in a delayed second post-contrast CBBCT scan (average 3 min versus 2 min for unilateral exam). This delay might distort contrast enhancement of otherwise similar lesions and is a technical CBBCT limitation as opposed to MRI allowing for simultaneous bilateral breast assessment. Finally, readers in our study were highly experienced in CBBCT imaging which might overestimate the diagnostic accuracy compared to less-experienced readers.
Conclusion
In conclusion, post-contrast CBBCT alone yields comparable diagnostic accuracy to the current standard of pre- and post-contrast CBBCT acquisition while demonstrating a twofold reduction of radiation dose in patients with type c/d breasts. Radiation dose reduction with post-contrast CBBCT alone counters one of the major limitations of CBBCT imaging and is expected to further decrease with technical advancements such as iterative reconstruction algorithms. High-degree misregistration was evident in the majority of pre- and post-contrast CBBCT imaging studies. These findings warrant future studies evaluating contrast-enhanced CBBCT in the diagnostic breast imaging algorithm and contrasting it to breast MRI.
Abbreviations
- ACR:
-
American College of Radiology
- AUC:
-
Area under the curve
- CBBCT:
-
Cone-beam breast CT
- IRB:
-
Institutional Review Board
- MG:
-
Mammography
- MRI:
-
Magnetic resonance imaging
- post-contrast CBBCT:
-
Contrast-enhanced cone-beam breast CT
- pre-contrast CBBCT:
-
Non-contrast cone-beam breast CT
- US:
-
Ultrasound
References
Wienbeck S, Lotz J, Fischer U (2016) Review of clinical studies and first clinical experiences with a commercially available cone-beam breast CT in Europe. Clin Imaging 42:50–59
O'Connell A, Conover DL, Zhang Y et al (2010) Cone-beam CT for breast imaging: radiation dose, breast coverage, and image quality. AJR Am J Roentgenol 195:496–509
Wienbeck S, Uhlig J, Luftner-Nagel S et al (2017) The role of cone-beam breast-CT for breast cancer detection relative to breast density. Eur Radiol 27:5185–5195
Lindfors KK, Boone JM, Nelson TR, Yang K, Kwan AL, Miller DF (2008) Dedicated breast CT: initial clinical experience. Radiology 246:725–733
O'Connell AM, Kawakyu-O'Connor D (2012) Dedicated cone-beam breast computed tomography and diagnostic mammography: comparison of radiation dose, patient comfort, and qualitative review of imaging findings in BI-RADS 4 and 5 lesions. J Clin Imaging Sci 2:7
He N, Wu YP, Kong Y et al (2016) The utility of breast cone-beam computed tomography, ultrasound, and digital mammography for detecting malignant breast tumors: a prospective study with 212 patients. Eur J Radiol 85:392–403
Seifert P, Conover D, Zhang Y et al (2014) Evaluation of malignant breast lesions in the diagnostic setting with cone beam breast computed tomography (breast CT): feasibility study. Breast J 20:364–374
Aminololama-Shakeri S, Abbey CK, Gazi P et al (2016) Differentiation of ductal carcinoma in-situ from benign micro-calcifications by dedicated breast computed tomography. Eur J Radiol 85:297–303
Prionas ND, Lindfors KK, Ray S et al (2010) Contrast-enhanced dedicated breast CT: initial clinical experience. Radiology 256:714–723
Wienbeck S, Fischer U, Luftner-Nagel S, Lotz J, Uhlig J (2018) Contrast-enhanced cone-beam breast-CT (CBBCT): clinical performance compared to mammography and MRI. Eur Radiol. https://doi.org/10.1007/s00330-018-5376-4
Zhao B, Zhang X, Cai W, Conover D, Ning R (2015) Cone beam breast CT with multiplanar and three dimensional visualization in differentiating breast masses compared with mammography. Eur J Radiol 84:48–53
Uhlig J, Fischer U, von Fintel E et al (2017) Contrast enhancement on cone-beam breast-CT for discrimination of breast cancer immunohistochemical subtypes. Transl Oncol 10:904–910
Uhlig J, Fischer U, Surov A, Lotz J, Wienbeck S (2018) Contrast-enhanced cone-beam breast-CT: analysis of optimal acquisition time for discrimination of breast lesion malignancy. Eur J Radiol 99:9–16
Spak DA, Plaxco JS, Santiago L, Dryden MJ, Dogan BE (2017) BI-RADS(R) fifth edition: a summary of changes. Diagn Interv Imaging 98:179–190
Wallis M, Tardivon A, Helbich T, Schreer I; European Society of Breast Imaging (2007) Guidelines from the European Society of Breast Imaging for diagnostic interventional breast procedures. Eur Radiol 17:581–588
Fischer U (2012) Practical MR mammography: high-resolution MRI of the breast, 2nd edition edn. Georg Thieme Verlag, Stuttgart
Purushothaman HN, Lekanidi K, Shousha S, Wilson R (2016) Lesions of uncertain malignant potential in the breast (B3): what do we know? Clin Radiol 71:134–140
Hoffmann O, Stamatis GA, Bittner AK et al (2016) B3-lesions of the breast and cancer risk - an analysis of mammography screening patients. Mol Clin Oncol 4:705–708
Jiang Y, Metz CE (2010) BI-RADS data should not be used to estimate ROC curves. Radiology 256:29–31
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Wienbeck S, Lotz J, Fischer U (2017) Feasibility of vacuum-assisted breast cone-beam CT-guided biopsy and comparison with prone stereotactic biopsy. AJR Am J Roentgenol 208:1154–1162
Funding
The authors did not receive funding for work related to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Susanne Wienbeck.
Conflict of interest
The authors declare that they have no conflict of interest.
Statistics and biometry
One author has significant statistical expertise.
Informed consent
Written informed consent was obtained by all participants prior to study inclusion.
Ethical approval
This study was performed in accordance with the Declaration of Helsinki and received institutional review board approval (Goettingen University, No. 16/2/15).
Study subjects or cohorts overlap
Parts of the study population have been previously reported as detailed in the material and methods section as well as the references.
Methodology
• Observational
Rights and permissions
About this article
Cite this article
Uhlig, J., Fischer, U., Biggemann, L. et al. Pre- and post-contrast versus post-contrast cone-beam breast CT: can we reduce radiation exposure while maintaining diagnostic accuracy?. Eur Radiol 29, 3141–3148 (2019). https://doi.org/10.1007/s00330-018-5854-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5854-8