Abstract
Purpose
The goal of this study was to develop and evaluate a volumetric three-dimensional (3D) approach to improve the accuracy of ablation margin assessment following thermal ablation of hepatic tumors.
Methods
The 3D margin assessment technique was developed to generate the new 3D assessment metrics: volumes of insufficient coverage (VICs) measuring volume of tissue at risk post-ablation. VICs were computed for the tumor and tumor plus theoretical 5- and 10-mm margins. The diagnostic accuracy of the 3D assessment to predict 2-year local tumor progression (LTP) was compared to that of manual 2D assessment using retrospective analysis of a patient cohort that has previously been reported as a part of an outcome-centered study. Eighty-six consecutive patients with 108 colorectal cancer liver metastases treated with radiofrequency ablation (2002–2012) were used for evaluation. The 2-year LTP discrimination power was assessed using receiver operating characteristic area under the curve (AUC) analysis.
Results
A 3D assessment of margins was successfully completed for 93 out of 108 tumors. The minimum margin size measured using the 3D method had higher discrimination power compared with the 2D method, with an AUC value of 0.893 vs. 0.790 (p = 0.01). The new 5-mm VIC metric had the highest 2-year LTP discrimination power with an AUC value of 0.923 (p = 0.004).
Conclusions
Volumetric semi-automated 3D assessment of the ablation zone in the liver is feasible and can improve accuracy of 2-year LTP prediction following thermal ablation of hepatic tumors.
Key Points
• More accurate prediction of local tumor progression risk using volumetric 3D ablation zone assessment can help improve the efficacy of image-guided percutaneous thermal ablation of hepatic tumors.
• The accuracy of evaluation of ablation zone margins after thermal ablation of colorectal liver metastases can be improved using a volumetric 3D semi-automated assessment approach and the volume of insufficient coverage assessment metric.
• The new 5-mm volume-of-insufficient-coverage metric, indicating the volume of tumor plus 5-mm margin that remained untreated, had the highest 2-year local tumor progression discrimination power.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Image-guided thermal ablation is an increasingly common non-surgical treatment for colorectal cancer liver metastases (CRLM) [1,2,3]. When compared with surgery, percutaneous radiofrequency and microwave ablation of CRLM offer lower complication rates and serve as viable alternatives to surgery [4,5,6]. Despite potential advantages, widespread adoption of ablation for CRLM has been impeded by local tumor progression (LTP) rates of up to 48% [7,8,9,10]. The results of prior studies have shown that a minimum ablation margin is an independent predictor of LTP following ablation of CRLM [9, 11,12,13]. With the majority of the intrahepatic micrometastases found within 10 mm away from the boundary of the gross CRLM [14], it has been considered desirable to create an ablation zone that extends beyond the borders of the tumor with a 5–10-mm margin [15]. As a critical parameter of local ablation efficacy, accurate intraprocedural margin assessment would provide valuable feedback to the operator at the time of treatment [16].
Historically, assessment of the ablative margin mirrors the principles of surgical margin assessment where the reported margin size is the distance between the edge of the neoplasm and the edge of the transected tissue, as seen on microscopic evaluation. Following thermal ablation, margin assessment is performed by estimating the distance between the radiographic boundaries of the tumor and the ablation zone using manual measurements and visual comparison of pre- and post-ablation two-dimensional (2D) images [8, 9, 11, 13, 17, 18]. However, two issues compromise this conventional approach. First, performing any quantitative comparison between the pre- and post-ablation images is hindered by frequent misalignment of the liver due to breathing motion, positional differences, and heating-induced tissue changes. Second, the size of the minimal margin does not indicate the extent of the remaining tissue at risk of LTP, i.e., tissue that needs to be ablated to ensure adequate margins. Finally, ablation zones with 1–5-mm margins result in LTP rate of 43–60% [9, 13]; therefore, methodologies to improve predictive accuracy in this category may be particularly clinically impactful.
The goal of this study was to develop and evaluate a quantitative three-dimensional (3D) approach that overcomes the limitations of the current approach and improves the accuracy of margin assessment following thermal ablation of liver metastases.
Methods
A 3D quantitative ablation assessment technique was designed to accurately evaluate the size of the minimum ablation margin and to measure and display the volume of the tissue at risk for LTP due to insufficient ablation coverage. The latter 3D metric is referred to here as the volume of insufficient coverage (VIC). The 3D quantitative ablation assessment technique was evaluated in a retrospective study that was approved by our institutional review board with a waiver of informed consent.
To evaluate the proposed 3D assessment, it was compared with the manual 2D method in a cohort of patients who underwent radiofrequency ablation (RFA) of CRLMs and where the minimum margin was already measured [9]. Manual 2D margin assessment was carried out by comparing the diagnostic contrast-enhanced pre-RFA computed tomography (CT) images of the tumor and post-RFA CT images of the ablation zone, obtained 4–8 weeks after RFA, the first follow-up imaging exam after the treatment [13]. Although intended to improve intraprocedural margin assessment, evaluation of the proposed method using the diagnostic pre- and post-ablation images was the first step in investigation of its value.
Because the majority of LTPs are known to occur within the first 2 years following the RFA treatment [9], the diagnostic accuracy of the proposed 3D assessment metrics was evaluated to predict 2-year LTP. The LTP status information was available to both the readers performing the 2D manual margin assessment in [9] and to the reader performing the 3D margin assessment to whom the results of the 2D manual assessment were also available.
Study population
The data from the previously reported patient cohort for which the minimum margin size was already assessed using the 2D manual method described above was used for evaluation. The patients were reported in an investigation of the factors affecting the oncological outcomes of RFA of CRLMs [9], in which treatments of 233 CRLMs were analyzed, and the minimum margin was measured in 174 CRLMs. These 174 CRLMs from 130 consecutive patients who were treated between December 2002 and December 2012, with either CT or positron emission tomography/CT guidance, made up the potentially eligible patients cohort used in the current study. In our study, the data from patients were utilized solely to evaluate a new volumetric 3D margin assessment technique.
For each ablation, LTP was assessed using post-ablation imaging studies (contrast-enhanced CT, magnetic resonance imaging, or 18F-fluorodeoxyglucose positron emission tomography), performed 4–8 weeks after RFA, and then continued at 2–4-month intervals. The LTP status was established based on the radiology reports as has been described in detail in the previous study [8]. LTP was defined as either new peripheral or nodular enhancement within 1 cm, or an enlargement of the ablation zone, compared with that measured on the first follow-up imaging examination [15, 19]. LTP was differentiated by focal and multi-focal patterns of progression. A multi-focal pattern was defined as simultaneous appearance of multiple new nodules proximal, within 1 cm, and distal to the boundary of the ablation zone.
Additional exclusion criteria were utilized to determine the final cohort for this study, including (1) follow-up time is less than 2 years, and LTP did not develop; (2) multi-focal progression pattern; (3) data image format is incompatible with 3D processing; and (4) CT image slice thickness greater than 5 mm. When either pre- or post-RFA images were stored as image captures imported from the outside institutions, such data could not be processed and were defined as incompatible.
Volumetric 3D ablation margin assessment
A 3D volumetric margin assessment process was developed and executed by a medical physicist with radiographic imaging expertise. MIM MAESTRO® (MIM Software, Inc.) image-processing software was used to create a sequence of interactive semi- and fully automated steps programmed into a workflow that is described in detail in the Appendix. Key steps include (1) segmentation of the tumor; (2) generation of theoretical 5- and 10-mm 3D margins around the tumor, calculated by isotropically expanding the tumor boundary by 5 and 10 mm; (3) 3D rigid image registration of the pre- and post-ablation images; (4) segmentation of the ablation zone; and (5) generation of the 3D VIC assessment metrics. These VIC metrics were calculated and visualized for tumor and theoretical 5- and 10-mm margins (Fig. 1). Steps 2 and 5 were fully automated, and the other steps were semi-automated. The volumes generated in step 2, tumor plus 5 or 10 mm, were considered “target volumes” for ablation [20]. The term “Ideal necrosis edge” was used in another study to define the contour of particular theoretical margins around the tumor [21]. These volumes were adjusted when the tumor was proximal to the edge of the liver capsule, or a vessel. Prior to step 2, the edge of the liver and/or the edges of the vessels near the tumor were contoured manually. Whenever the edge of the liver, or vessel, was defined, the theoretical contours were automatically trimmed to not extend past the liver capsule, or vessel. The goal of the image registration was to achieve registration error, the distance between an arbitrary landmark on pre- and post-RFA images, of less than 3 mm [22]. If registration error was greater, the registration step was repeated to reduce the error. When the registration step could not achieve acceptable alignment, the margin assessment workflow was terminated without completing the 3D assessment.
Various scenarios of the ablation zone’s coverage of the tumor and the tumor plus 5- and 10-mm theoretical margins were divided into five categories (top row), based on the size of the minimum ablation margin (magenta arrows). The bottom row illustrates the three-dimensional assessment metrics, volumes of insufficient coverage (VICs): tumor VIC (red), 5-mm VIC (purple), 10-mm VIC (cyan)
In practice, the main goal of margin assessment is to determine whether additional ablation is needed to adequately cover the tumor and margins and, if so, how much tissue needs to be treated. Assuming that the tissue near the tumor is a 3D layer of normal liver tissue likely containing micrometastases [14], it is rational to quantify and visualize not only the discrete minimum margin point but also the tissue at risk containing untreated viable tumor cells, or the micrometastases. Hence, the 3D VICs were automatically computed as volumes of the remaining at risk volume of tumor, or tumor plus the theoretical 5- or 10-mm margins (Fig. 1). These volumes were displayed as color overlays on post-ablation images. The minimum margin was recorded according to the definition described in Table A1 of the Appendix.
CT images had an in-plane resolution of 0.8 mm × 0.8 mm to 1.0 mm × 1.0 mm and a slice thickness of 5 mm. The total time required to complete 3D margin assessment was recorded. Sub-optimal alignment was defined as having at least one instance of the minimum distance between the corresponding landmarks in pre- and post-ablation images greater than 3 mm. For qualitative comparison of the location of VIC contours and LTP, the follow-up images on which LTP was first observed were aligned to the post-ablation images.
Statistical analysis
Receiver operating characteristic area under the curve (AUC) analysis was used to evaluate the 2-year LTP discrimination power of the minimal margin size metric, as measured manually and with the 3D method. Additionally, the discrimination power of the 3D metrics, VICs, and the volume of the ablation zone were examined. The difference in prediction power between the metrics was studied by comparison of the area under the receiver operating characteristic curve using Delong’s method. A p value < 0.05 was considered statistically significant. All analyses were performed using SAS 9.4 (2013–2018, SAS Institute Inc.), R 3.1.2 packages ROCR (2015), and pROC (2018).
Results
After applying the exclusion criteria described above, 108 out of 174 (62%) CRLMs were eligible for 3D assessment evaluation (Fig. 2). In 15 of these eligible cases (13.8%), rigid registration did not ensure optimal image alignment. Complete 3D assessment was performed in 93 CRLMs in a total of 72 patients (Table 1). An example of visualization of the results of 3D assessment is shown in Fig. 3. The average time to perform 3D volumetric margin analysis was 4.26 ± 1.5 min for each ablation site.
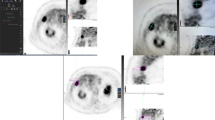
An example of 3D margin assessment. a Segmented tumor (yellow) and theoretical 5- (orange) and 10-mm (green) margin contours overlaid on the pre-ablation axial and reformatted sagittal and coronal computed tomography (CT) images. b Post-ablation images showing the segmented ablation zone. c 3D assessment metrics: tumor VIC (red), 5-mm volume of insufficient coverage (VIC) (purple), and 10-mm VIC (cyan). d Follow-up contrast-enhanced CT image showing the area of local tumor progression (LTP) (arrows) spatially corresponding to the location of the VICs in (C)
The median tumor diameter was 1.8 cm (range, 0.6–5.5 cm), and median volume was 1.77 cc (range, 0.13–35.9 cc). The median ablation zone volume was 28.9 cc (range, 1.7–103.4 cc). The ablation zone volume was greater than the volumes of the tumor plus 5- and 10-mm theoretical margins in 82 (88%) and 46 (49%) ablations, respectively. Fifty out of 93 CRLMs (54%) exhibited LTP within the first 2 years following ablation. In 39 (78%) out of all 50 ablations that had LTP, the VICs were spatially well co-localized with the area signifying LTP (Fig. 3c, d).
Table 2 shows the results of the 3D assessment. The greatest number of treatments where the margin was greater than 5 and 10 mm, without LTP, was found in the group of treatments where the ablation zone volume was also greater than the volume of the tumor plus the isotropic 10-mm margin. However, large ablation zone volume without the adequate minimum margin exhibited LTP. Due to the small number of cases in categories “margin > 10 mm” and “margin equals 0 mm,” the results were regrouped into three categories for statistical analysis and comparison with the manual 2D method. The treatments with 0-mm margin and incomplete ablation (based on the 3D analysis) were grouped together; and the treatments with margin > 10 mm were combined with those with 6–10-mm margins.
Statistical analysis showed that the minimum margin size measured using the 3D method had higher discrimination power compared with the manual margin assessment, with an AUC of 0.893 vs. 0.790 (p = 0.01). The 3D approach, as compared with manual 2D assessment, categorized a larger proportion of ablations as incomplete, or 0 mm margin (37 vs. 26), and smaller proportion as margin greater than 5 mm (27 vs. 34).
All VIC metrics were found to be predictive of 2-year LTP with AUC values greater than 0.75. In comparison to the manual 2D approach, a 5-mm VIC (i.e., the volume of untreated tumor plus 5-mm margin) with an AUC of 0.923 had significantly higher discrimination power (p = 0.004). For the optimal threshold value of 0.72 cc, the 5-mm VIC metric had specificity of 0.86 and sensitivity of 0.94. Compared to the 3D minimal marginmethod, the 5-mm VIC had marginally greater discrimination power (p = 0.06). The AUC value of the volume of the tumor was 0.72, and it was significantly higher than the AUC of the tumor diameter (p = 0.0009). The volume of the ablation zone did not have discrimination power.
In the 29 cases for which minimum margin size was greater than 0 mm but less than 5 mm, as measured with the 3D method, the 2-year LTP rate was 52%. Analysis in this subset of cases showed that a 5-mm VIC metric had an AUC of 0.82 (p = 0.002) and could be used to discriminate between the cases traditionally categorized as minimum margin of 1–5 mm.
Discussion
This work describes the development and evaluation of a new quantitative 3D method for accurate volumetric evaluation of the margins following percutaneous image-guided hepatic ablation. The proposed approach is an interactive comprehensive workflow that encompasses semi-automated 3D registration and segmentation of the tumor and ablation zone as well as automated computation of 3D margin assessment metrics. Retrospective evaluation shows improved measurement accuracy for minimum margin size, as demonstrated by a higher 2-year LTP discrimination power when measured using the 3D method compared with a manual 2D approach. In addition, the methodology developed in this study enables a new 3D assessment metric that is designed to identify the extent of the tissue at risk for LTP, i.e., VICs. The VICs are automatically calculated and provide both quantitative and visible representations of the ablation coverage deficiencies. High discrimination power as well as increased sensitivity and specificity was found for the 5-mm VIC metric signifying that the volume of unablated tissue within 5 mm from the boundary of the tumor may be a helpful metric in predicting the risk of LTP.
Image registration of pre- and post-ablation images, which helps to establish an accurate 3D relationship between the tumor and ablation zone, has been explored the most in the ablation margin assessment studies of RFA of hepatocellular carcinoma [21, 23,24,25,26]. For example, Kim et al showed that it improved interobserver agreement during margin assessment [24], and Sakakibara et al and Shin et al showed that it enabled identification of more incomplete ablations compared with the conventional method [25, 26]. Similarly, this study showed that registration-based 3D margin assessment identified more cases with incomplete or 0-mm minimum margin compared with the conventional method.
Supplementing registration with 3D volumetric assessment metrics could further improve the accuracy of ablation zone evaluation. In several studies, feasibility and the potential value of 3D margin assessment were evaluated. In nine liver metastases, Silverman et al computed the tumor volume, the percentage of tumor covered, and the percentage of target volume coverage [20]. The target volume was defined as the volume of tumor plus the digitally created margin of 10 mm. Aiming to measure the exact size of the minimum margin and display the location of the minimum margin, Tani et al evaluated a new 3D distance map technique in 21 hepatic tumors [27] and demonstrated that the thinnest margin of 0 mm was associated with LTP. Hocquelet et al measured the area of the tumor surface exposed to the ablation margin of ≤ 5 mm and evaluated this metric in 16 patients undergoing RFA of hepatocellular carcinoma, showing the potentially greater value of this metric compared to the minimum margin [23]. Although valuable margin assessment concepts were introduced in these studies, the small sample size and the inhomogeneous data (various tumor, image guidance, and ablation modalities) hinder reliable evaluation and clinical translation of these concepts.
This work introduces a method that is interactive, semi-automated, and implemented using commercial image-processing software used clinically in radiology and radiation oncology. Although this implementation can be performed on several general image-processing software platforms, using a clinically relevant software platform may facilitate translation of this margin assessment method into clinical practice. Additionally, full automation or semi-automation of the steps in our method may shorten 3D margin assessment time and reduce the barrier for adoption of this method by imaging technologists.
There are limitations to this study. First, evaluation of the method using the diagnostic pre- and post-ablation images of 5-mm slice thickness may limit the accuracy of the assessment in slice direction. Ideally, future studies should aim to obtain thinner slices. Second, extrapolation of the potential value of the described method for intraprocedural use would require additional study in such a setting. In practice, post-ablation imaging will likely be acquired with the ablation applicator remaining in the tissue, which will introduce some degree of beam hardening artifact impacting segmentation performance. The third limitation is in the study design where the person performing evaluation of the new method was not blinded to the LTP-related outcomes and could be subject to bias. Future work will focus on evaluation and adaptation of this method for intraprocedural use.
In conclusion, this study demonstrated feasibility of 3D quantitative assessment of the ablation zone following RFA of liver metastases. The initial evaluation showed that 3D assessment of the minimum margin can improve accuracy of LTP prediction. Although evaluated in the CRLM patient population treated with RFA, the methodology proposed here may be applicable to assessment of other primary or metastatic liver tumors treated with other thermal ablation modalities.
Abbreviations
- LTP:
-
Local tumor progression
- 2D:
-
Two-dimensional
- 3D:
-
Three-dimensional
- AUC:
-
Area under the curve
- AZ:
-
Ablation zone
- CRLM:
-
Colorectal cancer liver metastases
- CT:
-
Computed tomography
- RFA:
-
Radiofrequency ablation
- VIC:
-
Volume of insufficient coverage
References
Gillams A, Lees W (2009) Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol 19(5):1206–1213
Stang A, Fischbach R, Teichmann W, Bokemeyer C, Braumann D (2009) A systematic review on the clinical benefit and role of radiofrequency ablation as treatment of colorectal liver metastases. Eur J Cancer 45(10):1748–1756
Gillams A, Goldberg N, Ahmed M et al (2015) Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontières meeting 2013. Eur Radiol 25(12):3438–3454
Loveman E, Jones J, Clegg AJ et al (2014) The clinical effectiveness and cost-effectiveness of ablative therapies in the management of liver metastases: systematic review and economic evaluation. Health Technol Assess 18(7):1–283
van Amerongen MJ, Jenniskens SF, van den Boezem PB, Fütterer JJ, de Wilt JH (2017) Radiofrequency ablation compared to surgical resection for curative treatment of patients with colorectal liver metastases–a meta-analysis. HPB(Oxford) 19(9):749–756
Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN (2012) Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology 265(3):958–968
Hamada A, Yamakado K, Nakatsuka A et al (2012) Radiofrequency ablation for colorectal liver metastases: prognostic factors in non-surgical candidates. Jpn J Radiol 30(7):567–574
Napoleone M, Kielar AZ, Hibbert R, Saif S, Kwan BY (2016) Local tumor progression patterns after radiofrequency ablation of colorectal cancer liver metastases. Diagn Interv Radiol 22(6):548
Shady W, Petre EN, Gonen M et al (2015) Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes—a 10-year experience at a single center. Radiology 278(2):601–611
Meijerink MR, Puijk RS, van Tilborg AAJM et al (2018) Radiofrequency and microwave ablation compared to systemic chemotherapy and to partial hepatectomy in the treatment of colorectal liver metastases: a systematic review and meta-analysis. Cardiovasc Intervent Radiol 41(8):1189–1204
Jeon SH, Cho YK, Choi SA, Kim MY, Lee HS (2017) Analysis of factors affecting local tumor progression of colorectal cancer liver metastasis after radiofrequency ablation. J Korean Soc Radiol 76(3):179–186
Shady W, Petre EN, Do KG et al (2017) Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol 29(2):268–275
Wang X, Sofocleous CT, Erinjeri JP et al (2013) Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol 36(1):166–175
Wakai T, Shirai Y, Sakata J et al (2008) Appraisal of 1 cm hepatectomy margins for intrahepatic micrometastases in patients with colorectal carcinoma liver metastasis. Ann Surg Oncol 15(9):2472–2481
Ahmed M, Solbiati L, Brace CL et al (2014) Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. J Vasc Interv Radiol 25(11):1691–1705
Puijk RS, Ruarus AH, Scheffer HJ et al (2018) Percutaneous liver tumour ablation: image guidance, endpoint assessment, and quality control. Can Assoc Radiol J 69(1):51–62
Nakazawa T, Kokubu S, Shibuya A et al (2007) Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol 188(2):480–488
Vogl TJ, Basten LM, Nour-Eldin N-EA et al (2017) Evaluation of microwave ablation of liver malignancy with enabled constant spatial energy control to achieve a predictable spherical ablation zone. Int J Hyperth 34(4):492–500
Goldberg SN, Grassi CJ, Cardella JF et al (2009) Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol 20(7):S377–SS90
Silverman SG, Sun MR, Tuncali K et al (2004) Three-dimensional assessment of MRI-guided percutaneous cryotherapy of liver metastases. AJR Am J Roentgenol 183(3):707–712
Passera K, Selvaggi S, Scaramuzza D, Garbagnati F, Vergnaghi D, Mainardi L (2013) Radiofrequency ablation of liver tumors: quantitative assessment of tumor coverage through CT image processing. BMC Med Imaging 13(1):3
Makino Y, Imai Y, Igura T et al (2015) Comparative evaluation of three-dimensional Gd-EOB-DTPA-enhanced MR fusion imaging with CT fusion imaging in the assessment of treatment effect of radiofrequency ablation of hepatocellular carcinoma. Abdom Imaging 40(1):102–111
Hocquelet A, Trillaud H, Frulio N et al (2016) Three-dimensional measurement of hepatocellular carcinoma ablation zones and margins for predicting local tumor progression. J Vasc Interv Radiol 27(7):1038–1045
Kim KW, Lee JM, Klotz E et al (2011) Safety margin assessment after radiofrequency ablation of the liver using registration of preprocedure and postprocedure CT images. AJR Am J Roentgenol 196(5):W565–WW72
Sakakibara M, Ohkawa K, Katayama K et al (2014) Three-dimensional registration of images obtained before and after radiofrequency ablation of hepatocellular carcinoma to assess treatment adequacy. AJR Am J Roentgenol 202(5):W487–WW95
Shin S, Lee JM, Kim KW et al (2014) Postablation assessment using follow-up registration of CT images before and after radiofrequency ablation (RFA): prospective evaluation of midterm therapeutic results of RFA for hepatocellular carcinoma. AJR Am J Roentgenol 203(1):70–77
Tani S, Tatli S, Hata N et al (2016) Three-dimensional quantitative assessment of ablation margins based on registration of pre-and post-procedural MRI and distance map. Int J Comput Assist Radiol Surg 11(6):1133–1142
Acknowledgements
We thank James Keller for his help with editing and preparing this manuscript.
Preliminary results were presented as an abstract during the RSNA 2017.
Funding
This research project was supported by an internal Department of Radiology seed grant and in part by the NIH/NCI Cancer Center Support Grant P30 CA008748.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Elena Kaye.
Conflict of interest
The authors of this manuscript declare relationships with the following companies.
C. T. Sofocleous has received research support from BTG, Ethicon (Neuwave); HS Medical, Angiodynamics; Sota Medical; and is a consultant for Ethicon and GE.
S. B. Solomon is a shareholder of Johnson & Johnson, Adgero, Immunomedics, Aspire Bariatrics, and Progenics; has received personal fees from Medtronics, BTG, Astra Zeneca, and Johnson & Johnson; and has a research grant from GE Heathcare.
J. Durack is an investor in and is on scientific advisory board of Adient Medical.
E. Kaye received consulting fees from Galil Medical.
The remaining authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Weiji Shi, PhD and Zhigang Zhang, PhD kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in:
1. Shady W, Petre EN, Gonen M, et al Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes—a 10-year experience at a single center. Radiology. 2015;278:601–11.
2. Wang X, Sofocleous CT, Erinjeri JP, et al Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol 2013;36(1):166–175.
3. Sofocleous CT, Garg S, Petrovic LM, et al Ki-67 is a prognostic biomarker of survival after radiofrequency ablation of liver malignancies. Ann Surg Oncol 2012;19(13):4262–4269.
4. Sofocleous CT, Petre EN, Gonen M, et al CT-guided radiofrequency ablation as a salvage treatment of colorectal cancer hepatic metastases developing after hepatectomy. J Vasc Interv Radiol 2011;22(6):755–761.
Methodology
• Retrospective
• Diagnostic or prognostic study
• Performed at one institution
Electronic supplementary material
ESM 1
(DOCX 6556 kb)
Rights and permissions
About this article
Cite this article
Kaye, E.A., Cornelis, F.H., Petre, E.N. et al. Volumetric 3D assessment of ablation zones after thermal ablation of colorectal liver metastases to improve prediction of local tumor progression. Eur Radiol 29, 2698–2705 (2019). https://doi.org/10.1007/s00330-018-5809-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5809-0