Abstract
Purpose
The purpose of this study is to investigate the detectability of pregnancy-associated breast cancer (PABC) in lactating glandular tissue on magnetic resonance imaging (MRI) by using pre- and post-contrast acquisitions and their derived postprocessed images and compare these results to ultrasound (US) and mammography (MG).
Materials and methods
We reviewed the electronic database for women with PABC and existing breast MRI. MR images (T2-weighted short inversion-recovery sequence [STIR], dynamic contrast-enhanced T1-weighted gradient echo sequence and postprocessed subtraction images [early post-contrast minus pre-contrast]) were retrospectively evaluated (image quality, parenchymal/tumour enhancement kintetics, tumour size and additional lesions). Supplemental subtraction images (latest post-contrast minus early post-contrast) to reduce plateau enhancement were additionally calculated and tumour conspicuity and size were measured. Findings were compared to US and MG reports.
Results
Nineteen patients (range 27–42 years) were included. Background parenchymal enhancement (BPE) was minimal (n=1), mild (n=3), moderate (n=7) and marked (n=8) with kinetics measured plateau (n=8), continuous (n=10) and not quantifiable (n=1). Tumour kinetics presented wash-out (n=17) and plateau (n=2). Eighteen of nineteen tumours were identified on the supplemental subtraction images. All tumours were visible on US; 12/19 were visible on MG (63.2%). MRI detected additional malignant lesions in two patients.
Conclusion
Despite high BPE of the lactating breast, MRI securely detects carcinomas and identifies satellite lesions. By using supplemental subtraction images, background enhancement can be eliminated to facilitate diagnosis. US remains a reliable diagnostic tool, but additional MRI is recommended to rule out satellite/contralateral lesions. MG interpretations can be difficult due to high parenchymal density.
Key Points
• Despite high background enhancement, MRI of the breast confidently detects carcinomas and identifies further lesions in the lactating breast.
• By using supplemental subtraction images, background enhancement in the lactating breast can be eliminated to facilitate diagnosis.
• US remains a reliable diagnostic tool. Mammography can be limited due to extremely dense breast tissue related to lactation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pregnancy leads to a number of anatomical and physiological changes in the breast. Higher hormone levels cause ductal and lobular growth leading to an increase in breast size and higher density of glandular tissue [1, 2]. Clinical examination in these cases can be extremely challenging, yet pregnant or lactating women presenting with a palpable mass in the breast are not an uncommon situation in clinical practice [3]. Although about 70−80% of all findings detected during lactation are benign [3], it is of utmost importance to rule out suspicious or malignant masses in the breast as it affects both mother and child.
By definition, pregnancy-associated breast cancer (PABC) is a disease with onset during gestation or within the first year postpartum [4] and accounts for about 3% of all breast cancers [5] or 0.3/1000 pregnancies [3]. While it is still very rare [5], the numbers have been increasing in the past few years, which can mainly be attributed to the increasing age of childbearing women who already present a higher risk for breast malignancies [3].
A safe and quick way of breast imaging for these patients is ultrasound (US), which is considered the gold standard for the detection and characterisation of breast masses in pregnant women [2]. As most of these women are of young age, screening mammography (MG) is usually not performed and information gained through mammograms is often minimal due to high tissue density. Yet, it can deliver information on microcalcifications. So, when indicated, mammography in pregnant women can be performed with only little risk to the foetus by using abdominal shielding [3, 5]. Magnetic resonance imaging (MRI) of the breast with intravenous application of gadolinium-based contrast media is problematic during pregnancy due to an unknown risk of gadolinium to the foetus [5] and should only be performed in case of immediate risk to mother or child [6]. An MR examination postpartum, on the other hand, is unproblematic and breast-feeding is safe to be continued [7]. Nevertheless, radiologists are still confronted with the pregnancy-associated anatomical changes of the breast. Especially the assessment of lactating tissue in MRI has been discussed controversially in literature. In particular, the rapid enhancement of the lactating parenchyma – in contrast to non-lactating tissue – is often mentioned as a limiting factor in detecting neoplasms [3, 8, 9]. Previous studies on MRI in PABC reported that the background enhancement of the lactating gland did not interfere with lesion detection [1, 10,11,12]. Yet, similar to choosing the correct phase of the menstrual cycle [13], weaning can decrease the background enhancement in glandular parenchyma [14] to ensure lesion detection.
In our institution, MRI has been playing a cardinal role in diagnostics of PABC for many years assessing the extent of pre-therapeutic disease in lactating patients and monitoring therapeutic response to neo-adjuvant chemotherapy. In our experience, as well as in recently published literature [1, 10,11,12], the background parenchymal enhancement (BPE) of lactating tissue in contrast-enhanced MRI does not or only to a minimal extent impair lesion conspicuity as malignant lesions and background parenchyma can usually be differentiated by their enhancement kinetics. Evaluation of enhancement kinetics is recommended on either fat-suppressed dynamic contrast-enhanced images or postprocessed subtraction images (by subtracting the non-contrast image from an early post-contrast image) [15]. Furthermore, we can use additional subtraction algorithms as previously reported [16, 17] to differentiate the enhancement characteristics of tumour and glandular parenchyma by subtracting the latest post-contrast dynamic series from an early post-contrast dynamic (i.e. supplemental subtraction images). By doing so, we can achieve an improved visualisation of an area presenting wash-out, a typical feature of malignant lesions, while at the same time reducing plateau enhancement, which is typically presented by glandular tissue and generally very prominent in lactating breasts [14]. This can be of further help when BPE is marked (which may increase the risk of false positive results) and diagnosis uncertain.
Thus, in this study we aim to investigate the detectability of breast cancer in lactating glandular tissue with focus on enhancement kinetics of background parenchyma and tumour lesions by using pre- and post-contrast acquisitions and their derived postprocessed subtraction images (conventional and supplemental). We will evaluate the detectability of lesions in the postprocessed images and assess the value of additionally calculated supplemental subtraction images. Furthermore, these results are set in comparison to US and MG findings to determine the impact of MRI in patients with PABC.
Materials and methods
This retrospective study was approved by the local institutional review board and written informed consent was waived. We reviewed the electronic database for women with PABC in the lactating breast that were examined in our institution between 12/2005 and 12/2017. PABC was defined as breast cancer with onset during gestation or within the first year post-partum [4], lactation was defined with the beginning of the secretory initiation 16 weeks after conception [18]. Only patients with a histologically confirmed primary breast malignancy were included, patients with recurrent disease or malignancy of skin appendages were excluded.
Corresponding mammography and ultrasound reports for comparison, as well as histopathology results and therapeutic concepts were drawn from electronic medical records and from reports of the senologic tumour board of our institution.
MR imaging protocol
MR images were acquired at 1.5 T (Achieva, Philips) using a dedicated breast surface coil with patients in prone position. The imaging protocol consisted of an axial T2-weighted short inversion-recovery (STIR) sequence (TR 2933 ms, TE 50 ms, TI 160 ms, Matrix 432x295, FOV 390/390 mm, slice-thickness 3.5 mm), as well as an axial dynamic T1-weighted gradient echo sequence without fat-saturation (TR 8.2 ms, TE 4.1 ms, Flip angle 20°, Matrix 488/468, FOV 420/420 mm, slice-thickness 1.5 mm) with intravenous application of 0.1−0.16 mmol/kg gadolinium-chelate (Magnevist® 0.5 mmol/mL or Gadovist® 1.0 mmol/mL, Bayer Health Care). One non-contrast and seven post-contrast series were generated with an acquisition time of 75 s. Postprocessing included: Axial subtraction images (generated for each contrast-enhanced image by subtracting the non-contrast image from the respective post-contrast sequence), supplemental subtraction images (generated by subtracting the last post-contrast dynamic series from the second post-contrast dynamic series for better visual differentiation of background enhancement and tumour enhancement; i.e. improved visualisation of wash-out while reducing plateau kinetics), and maximum intensity projections (MIP) in axial and sagittal orientation.
MR image analysis
MR images of patients with histologically confirmed breast cancer were retrospectively evaluated independently and in a blinded fashion by two radiologists (SB and JT with 10 and 3 years of experience in breast imaging) on a dedicated workstation (ViewForum, Philips) according to the American College of Radiology (ACR) BI-RADS®catalogue of 2013 [19]. Images were assessed in following order: T2-weighted STIR, T1-weighted sequence, postprocessed subtraction images, supplemental subtraction images and MIP images.
Conspicuity and size of the histologically confirmed tumour lesions were determined in the subtraction images. In a second step, tumour detectability and size were assessed in the supplemental subtraction images. MIPs were used to measure tumour expansion in all three dimensions.
BPE of normal breast tissue was categorised according to the BI-RADS®lexicon [19] as 1: minimal, 2: mild, 3: moderate, and 4: marked. Enhancement kinetics of breast tissue and tumour lesions were evaluated in a region-of-interest (ROI)-based analysis using the dynamic contrast series. ROIs were positioned according to the recommendations of the Breast Imaging Working Group of the German Radiological Society in the area of peak enhancement including a minimum of nine pixels [20]. Kinetics were divided into continuous enhancement, plateau enhancement (initial upslope followed by a plateau in delayed contrast phase) and wash-out (initial upslope followed by a drop of >30% in delayed phase).
Overall image quality was rated on a 5-point Likert scale (5=excellent, 1=non-diagnostic).
Image evaluation
The MR-findings were compared to the corresponding MG and US. It was evaluated weather the tumour was detected in MG and US (yes/no) using the corresponding reports in the electronic medical records. If the lesion was displayed, the documented tumour size as measured in the corresponding examination was noted. Additionally, detected lesions in MR images were classified along the BI-RADS®-classification system. Lesions in the categories BI-RADS®4 and 5 were noted and compared to histopathology, if available.
Statistical analysis
Descriptive statistical data analysis was performed with dedicated software (SPSS Version 23.0; IBM Corporation).
Results
We identified 22 patients with pregnancy-associated breast cancer, out of which three patients were excluded due to Paget´s disease (n=1) and recurrent disease (n=2). Thus, 19 patients (mean age 34.4 years, range 27–42 years) were included in the final analysis. Each patient received MG, US and MRI of the breast, as well as an ultrasound-guided biopsy for histological confirmation (biopsy of the most prominent lesion was performed). Most patients did not have risk factors. A BRCA1-mutation was present in four patients.
All patients were symptomatic with a palpable lump in the breast. MR images were obtained: At the 19th week of pregnancy in n=1, during breast feeding in n=4 and after weaning (of 2 days – 4 weeks) in n=14. Time between giving birth and MRI was 4 days to 5 months.
MR image quality was excellent with a mean of 4.5 (range 3-5). Background enhancement was rated minimal in n=1, mild in n=3, moderate in n=7 and marked in n=8. The kinetics of the background enhancement were described as “plateau” in n=8, “continuous” in n=10 and “not quantifiable” in one patient with minimal enhancement. All lesions showed an enhancement which was categorised as “wash-out” in n=17 and “plateau” in n=2 patients. No tumour presented non-mass enhancement.
Mean tumour size as measured in the postprocessed MR subtraction images was 39.6 ±24.8 mm (range 11−100 mm). In the supplemental subtraction images the lesions were equally displayed in n=18 patients with no difference in measured tumour size, no additional lesions were detected. In one patient the supplemental subtraction images did not display the lesion, in this case background parenchymal enhancement and tumour presented a plateau-like kinetic. On ultrasound, all tumours were detected with a mean tumour size of 29.5 ±17.1 mm (range 11−90 mm). In mammography, a correlate to the tumour identified in MRI was found in n=12 (63.2 %), in n=7 patients the tumour was not detected; mean tumour size was 30.4 ±6.6 mm (range 22−43 mm). In one patient the lesion was associated with microcalcifications. Breast density in mammography was classified as following: ACR B in n=2, ACR C in n=4, ACRC/D in n=4, and ACR D in n=9. Representative images are displayed in Figs. 1, 2, and 3.
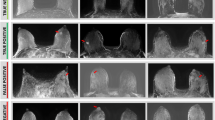
Images of a 34-year-old patient with invasive ductal carcinoma of no special type in the upper outer quadrant on the right. The tumour (white arrow) was displayed in the MR images (a T2 STIR, b contrast-enhanced subtraction image, c contrast-enhanced supplemental subtraction image), as well as on ultrasound (d). Due to the high density of the lactating breast tissue (ACR D) the mass was occult on mammography (e cranio-caudal view of right and left breast), but was easily detected on MR images with strong enhancement in the subtraction images (c). In the supplemental subtraction images, background parenchymal enhancement vanishes leaving only the slight enhancement of the tumour lesion. Note: Windowing of MR images was adapted accordingly
Images of a 40-year-old patient with invasive ductal carcinoma in the left breast at 9 o´clock. The tumour (white arrow) was displayed in the MR images (a T2 STIR, b contrast-enhanced subtraction image). Due to high density of the lactating breast tissue (ACR D) the mass was occult on mammography (c cranio-caudal and d mediolateral oblique view of both sides). Windowing of MR images was adapted accordingly
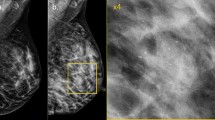
Images of a 37-year-old patient with invasive ductal carcinoma of no special type in the upper outer quadrant on the right breast. Due to high density of the lactating breast tissue (ACR D) the mass was occult on mammography (a mediolateral oblique view of both sides) but presented associated microcalcifications of 27 mm diameter (b enlarged mammographic view). The tumour (white arrow) was displayed in the MR images with 90 mm diameter (c contrast-enhanced subtraction image). Windowing of MR images was adapted accordingly
Histopathology revealed invasive ductal carcinoma of no special type (NST) in n=17 and medullary carcinoma in n=2 patients. In NST, seven patients presented a G3 tumour with a triple negative receptor status; in the medullary carcinoma group one patient presented this type. Receptor status concerning estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) were the following: ER+ PR+ HER2+ in n=5; ER+ PR- HER2- in n=1, and ER- PR- HER2- in n=9.
In six patients, additional lesions were detected through MRI, two of which were categorised as BI-RADS®4, n=4 were categorised as BI-RADS® 5. After MR-guided biopsy, both BI-RADS®4 lesions proved to be benign. On the BI-RADS®5 lesions, a second-look ultrasound was performed in which the lesions were detected and also characterised as BI-RADS®5. because of therapeutic relevance, two of these lesions were biopsied and proved malignant.
Five patients primarily received breast conserving therapy (BCT), 14 were treated with neoadjuvant chemotherapy. The mean tumour size in the final histopathological report was 17.3 ±8.9 mm (range 4−35 mm) in all patients; mean size in patients receiving BCT was 19.4 ±7.0 mm (range 10−26 mm) and 15.7 ±10.3 mm (range 4−35 mm) in patients receiving neoadjuvant chemotherapy. In patients having received BCT, tumour size as measured in MRI was 20.6 ±8.3 mm (range 11-32 mm) and in US 20 ±4.9 mm (range 15−26 mm). MG only detected three out of the five carcinomas with mean size of 25 ±3 mm (range 22−28 mm).
Discussion
In this evaluation, MRI of the lactating breast was proven to be a reliable diagnostic tool in the assessment of suspected breast neoplasms. In early literature, a higher background enhancement of the lactating gland was thought to be associated with a reduced detection rate of malignancies in MRI [3, 8, 9] caused by an equally high uptake of contrast media of both tumour and glandular tissue [9]. Quite recently, smaller cohort studies disproved this hypothesis [1, 10, 11]. In a study with five patients, Espinosa and colleagues described a plateau-like enhancement of lactating tissue, which could easily be distinguished to the wash-out kinetic of the malignant lesions [10]. In a following evaluation, Taylor et al demonstrated similar results in six patients, and brought up the potential of unsuspected lesion detection through MRI as described in one patient of their cohort [11]. Another recent investigation lead by Oh et al includes nine patients with PABC, all of which received MRI of the breast. This study nicely demonstrates that lesion conspicuity was not impaired by higher background enhancement of the lactating tissue. In MRI, Oh et al identified additional lesions and found tumour size to be more accurately assessed when compared to ultrasound and mammography (with the limitation that only 4/9 patients received mammography in that study) [1]. The most recently published study by Myer et al investigated the MR-imaging features of PBAC and its impact on surgical management when performed preoperatively. Their results suggest a sensitivity of MRI of 98% in the detection of PABC and more accurate measurements of tumour size in comparison to US and MG [12]. The high sensitivity of MRI in tumour detection is in accordance with our study results. Our study includes 19 patients with PABC, all of which were detected in the MR images regardless of the amount of background enhancement. In the majority of patients, the lactating breast demonstrated a relevant background enhancement (moderate to marked) with continuous or plateau-like contrast kinetics. The histologically confirmed malignant lesions – on the other hand – almost exclusively demonstrated wash-out kinetics, which clearly delineated these masses from lactating glandular tissue. We can take advantage of these different enhancement kinetics by calculating additional subtraction images (called supplemental subtraction images). By subtracting the last post-contrast dynamic series from the second early post-contrast dynamic we can achieve an improved visualisation of the area presenting wash-out (a typical feature of malignant lesions) while at the same time reducing plateau enhancement (typically presented by glandular tissue and generally very prominent in lactating breasts) which is a further help in tumour detection. The utility of this method has been proven in a previously conducted study by Choi et al investigating different subtraction algorithms in breast MRI. Choi and colleagues demonstrated an improved detection of early enhancement/wash-out by using the so-called reverse subtraction images (in which the latest dynamic phase was subtracted from the respective dynamic images) [16]. In our study, we identified malignant lesions in 18/19 patients in the supplemental subtraction images (in one patient the lesion was not displayed because of the same plateau-enhancement of tumour and tissue) leading to a detection rate of 95% in these datasets. When comparing conventional vs. supplemental subtraction images, there was no difference in measured tumour size. No further lesion was detected in the additionally calculated supplemental subtraction images in our collective. Nevertheless, this method of reducing the background parenchymal enhancement to a minimum and displaying the area of wash-out kinetics can be of great help to securely detect a malignant lesion, rule out satellite lesions or identify tumour spread in the surrounding tissue, especially when BPE is marked (which is the case in lactating women). While we could not demonstrate a superiority of these supplemental subtraction images, it can be considered a supporting tool to assure diagnosis.
All carcinomas identified in MRI were also picked up by ultrasound. With a sensitivity of almost 100% ultrasound is still the method of choice and considered the most suitable modality for detection and characterisation of intramammary findings during gestation and lactation [21, 22]. Yet, we believe that the high sensitivity of US in our collective can be explained by the generally large tumour size (with a mean of 29.5 ±17.1 mm on US). In these cases, the additional pre-therapeutic MRI was mainly indicated to detect further satellite lesions and to rule out greater tumour extent or malignancies of the contralateral breast. Indeed, MRI identified additional lesions in six patients, two of which were reassessed due to therapeutic relevance by second-look ultrasound and characterised as malignant. Again, this demonstrates the diagnostic potential of an MR-examination and its relevance in assessing the pre-therapeutic disease extent.
Tumour detection in mammography was markedly reduced (63.2% detection rate), which is mainly caused by the high density of the lactating breast tissue reflected by ACR C-D in 89.5% of the patients. A decreased sensitivity of mammography for detection of PABC ranging from 78−90% has also been described in literature [22]. Although the detection rate on mammograms in our cohort was even less, MG is still recommended in all patients [23] as it may display suspicious microcalcifications which would otherwise go undetected. Suspicious microcalcifications were only detected in one patient (with NST G3 ER/PR+ Her2-), which might be explained by the histopathology of tumours in this collective. Breast cancer with triple negative receptor status has been described to be rarely associated with microcalcifications [24], yet this was the most frequent type in our study (present in eight patients).
The tumour size as measured in MRI was generally larger when compared to measurements in ultrasound or mammography. Correlation to histopathological tumour size could only be accurately performed in the five patients undergoing primary BCT, whereas 14 patients received neoadjuvant chemotherapy to downstage the initial tumour. In these five patients with histopathological correlation, ultrasound was most accurate regarding tumour size assessment, while MRI tended to overestimate the size. The non-significant overestimation of tumour size in MRI has been previously described: According to literature, a non-significant overestimation in tumour diameter in MR images can mainly be explained by MRI´s superior outline of soft tissue depicting larger DCIS-components or accompanying desmoplastic reactions of the surrounding tissue [25].
There are limitations to this study. Firstly, we only included a small number of patients, which can be explained by the extremely rare occurrence of this disease. Secondly, final correlation of tumour size measured in imaging (MRI, US, MG) to the size confirmed in histological specimen was only possible in the minority of patients. Most patients received neoadjuvant chemotherapy to reduce tumour size before final surgery, which is common in PABC as the diagnosis is usually made at advanced stage.
In conclusion, despite the marked background enhancement of the lactating tissue, MRI of the breast is able to confidently detect breast carcinomas and also identify additional lesions in the lactating breast, which is crucial for determining the appropriate therapeutic concept. By using additional postprocessing methods, i.e. supplemental subtraction images, the high background enhancement can be eliminated to facilitate diagnostics for better tumour visualisation. US remains a reliable and readily available diagnostic tool, while interpretations of MG can be difficult in these patients due to high parenchymal density.
Abbreviations
- ACR:
-
American College of Radiology
- BCT:
-
Breast conserving therapy
- BI-RADS®:
-
Breast Imaging Reporting and Data System
- ER:
-
Estrogen receptor
- FOV:
-
Field-of-View
- HER2:
-
Human epidermal growth factor receptor 2
- MG:
-
Mammography
- MIP:
-
Maximum intensity projection
- MRI:
-
Magnetic resonance imaging
- NST:
-
Invasive ductal carcinoma of no special type
- PBAC:
-
Pregnancy-associated breast cancer
- PR:
-
Progesterone receptor
- T:
-
Tesla
- TE:
-
Echo time
- TI:
-
Inversion time
- TR:
-
Repetition time
- US:
-
Ultrasound
References
Oh SW, Lim HS, Moon SM et al (2017) MR imaging characteristics of breast cancer diagnosed during lactation. Br J Radiol 90:20170203
Vashi R, Hooley R, Butler R, Geisel J, Philpotts L (2013) Breast imaging of the pregnant and lactating patient: imaging modalities and pregnancy-associated breast cancer. AJR Am J Roentgenol 200:321–328
Keleher AJ, Theriault RL, Gwyn KM et al (2002) Multidisciplinary management of breast cancer concurrent with pregnancy. J Am Coll Surg 194:54–64
Petrek JA, Dukoff R, Rogatko A (1991) Prognosis of pregnancy-associated breast cancer. Cancer 67:869–872
Sabate JM, Clotet M, Torrubia S et al (2007) Radiologic evaluation of breast disorders related to pregnancy and lactation. Radiographics 27(Suppl 1):S101–S124
Committee on Obstetric Practice (2017) Committee Opinion No. 723: Guidelines for Diagnostic Imaging During Pregnancy and Lactation. Obstet Gynecol 130:e210–e216
American College of Radiology (2017) ACR Committee on Drugs and Contrast Media. https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf
Giess CS, Yeh ED, Raza S, Birdwell RL (2014) Background parenchymal enhancement at breast MR imaging: normal patterns, diagnostic challenges, and potential for false-positive and false-negative interpretation. Radiographics 34:234–247
Talele AC, Slanetz PJ, Edmister WB, Yeh ED, Kopans DB (2003) The lactating breast: MRI findings and literature review. Breast J 9:237–240
Espinosa LA, Daniel BL, Vidarsson L, Zakhour M, Ikeda DM, Herfkens RJ (2005) The lactating breast: contrast-enhanced MR imaging of normal tissue and cancer. Radiology 237:429–436
Taylor D, Lazberger J, Ives A, Wylie E, Saunders C (2011) Reducing delay in the diagnosis of pregnancy-associated breast cancer: how imaging can help us. J Med Imaging Radiat Oncol 55:33–42
Myers KS, Green LA, Lebron L, Morris EA (2017) Imaging Appearance and Clinical Impact of Preoperative Breast MRI in Pregnancy-Associated Breast Cancer. AJR Am J Roentgenol 209:W177–w183
Ellis RL (2009) Optimal Timing of Breast MRI Examinations for Premenopausal Women Who Do Not Have a Normal Menstrual Cycle. AJR Am J Roentgenol 193:1738–1740
Joshi S, Dialani V, Marotti J, Mehta TS, Slanetz PJ (2013) Breast disease in the pregnant and lactating patient: radiological-pathological correlation. Insights Imaging 4:527–538
Derakhshan JJ, McDonald ES, Siegelman ES, Schnall MD, Wehrli FW (2018) Characterizing and eliminating errors in enhancement and subtraction artifacts in dynamic contrast-enhanced breast MRI: Chemical shift artifact of the third kind. Magn Reson Med 79:2277–2289
Choi BG, Kim HH, Kim EN et al (2002) New subtraction algorithms for evaluation of lesions on dynamic contrast-enhanced MR mammography. Eur Radiol 12:3018–3022
Choi N, Han BK, Choe YH, Kim HS (2005) Three-phase dynamic breast magnetic resonance imaging with two-way subtraction. J Comput Assist Tomogr 29:834–841
Schanler RJ, Potak DC (2018) Physiology of lactation. https://www.uptodate.com/contents/physiology-of-lactation
D’Orsi CJSE, Mendelson EB, Morris EA et al (2013) ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. American College of Radiology, Reston
Breast Imaging Working Group of the German Radiological S (2014) Updated Recommendations for MRI of the Breast. Rofo 186:482–483
Hogge JP, De Paredes ES, Magnant CM, Lage J (1999) Imaging and Management of Breast Masses During Pregnancy and Lactation. Breast J 5:272–283
Liberman L, Giess CS, Dershaw DD, Deutch BM, Petrek JA (1994) Imaging of pregnancy-associated breast cancer. Radiology 191:245–248
Ayyappan AP, Kulkarni S, Crystal P (2010) Pregnancy-associated breast cancer: spectrum of imaging appearances. Br J Radiol 83:529–534
Gao B, Zhang H, Zhang SD et al (2014) Mammographic and clinicopathological features of triple-negative breast cancer. Br J Radiol 87:20130496
Gruber IV, Rueckert M, Kagan KO et al (2013) Measurement of tumour size with mammography, sonography and magnetic resonance imaging as compared to histological tumour size in primary breast cancer. BMC Cancer 13:328
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. med. Sonja Bahrs.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Rights and permissions
About this article
Cite this article
Taron, J., Fleischer, S., Preibsch, H. et al. Background parenchymal enhancement in pregnancy-associated breast cancer: a hindrance to diagnosis?. Eur Radiol 29, 1187–1193 (2019). https://doi.org/10.1007/s00330-018-5721-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5721-7