Abstract
Objectives
To evaluate the correlation between psoas muscle area (TPA) on CT images and pectoralis muscle area (PMA) on MRI in breast cancer patients.
Methods
This retrospective study was institutional review board approved and women involved gave written informed consent. Twenty six patients with both body CT and breast MRI available were evaluated. Two radiologists calculated TPA on 1.25-mm and 5-mm body CT images. Two radiologists measured PMA on axial T1-weighted images. Statistical analysis included inter- and intra-reader agreement and correlation between TPA on CT and PMA on MRI.
Results
The Pearson r correlation coefficient was 0.70 (95% CI 0.41–0.81) and the coefficient of determination was 0.49. The inter-reader agreement was k = 0.85 and k = 0.79 for axial 1.25-mm and 5-mm CT images, respectively. The intra-reader agreement of reader 1 was k = 0.98 and k = 0.94 for 1.25-mm and 5-mm CT images, respectively. The intra-reader agreement of reader 2 was k = 0.95 and k = 0.94 for 1.25-mm and 5-mm CT images, respectively. On axial T1-weighted images, the inter-reader agreement for radiologists evaluating the PMA was k = 0.61. Intra-observer agreement of reader 1 and reader 2 for PMA estimation was good (0.62 and 0.64), respectively.
Conclusion
The correlation between TPA on CT images and PMA on MRI was very good. Pectoralis muscle area on breast MRI could be useful to estimate muscle mass in women with breast cancer.
Key Points
• Pectoralis muscle area can be estimated on breast MRI
• Total psoas area on CT and pectoralis muscle area on MRI are strongly correlated
• Pectoralis muscle area on breast MRI could estimate the skeletal muscle mass
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The estimated first-time definition of “sarcopenia” was given by Baumgartner et al, who used this term to describe the age-related loss of skeletal muscle mass [1]. Sarcopenia is correlated with negative outcomes, such as psychophysical disability, poor quality of life and death [2].
Cancer is probably the most important pathological condition that leads to loss of muscle mass. With over 1.5 million new cases per year, breast cancer is the most common cancer and leading cause of cancer mortality in women worldwide [3, 4]. Sarcopenia has been described as a powerful independent predictor of poor survival for breast cancer patients [5,6,7,8,9]. Indeed, sarcopenia is associated with impaired functional status, decreased ability to tolerate chemotherapy, surgery and radiotherapy with many complications. Many studies demonstrated that breast cancer patients have an excess of toxicity from oncological therapies and a lower response to chemotherapy; for example, in breast cancer patients the response to capecitabine may be poor. In addition, Prado et al showed that sarcopenia is associated with a shorter time to tumour progression relative to survival in women with metastatic breast cancer [6]. Computer tomography (CT) is considered the gold standard to measure muscle area and density in Hounsfield units. Indeed, total psoas area (TPA) is strongly correlated with total body muscle mass and it can be easily measured on a single cross-sectional CT image [10, 11].
In women with breast cancer, CT scan for patients with clinically positive axillary nodes, large tumours, aggressive biology or clinical signs, symptoms or laboratory values suggesting the presence of metastases can be used [12]. Indeed, in early breast cancer, routine staging evaluations with different techniques (mammography, ultrasound, tomosynthesis and magnetic resonance imaging, MRI) are directed at locoregional disease.
MRI is widely adopted in breast cancer evaluation and potentially enhanced by recently introduced abbreviated MRI protocols [13].
Recent studies revealed a high correlation between the psoas muscle area and pectoralis muscle area (PMA) using CT [14, 15]. PMA could also be evaluated on routine MRI examinations. Therefore, the aim of our study is to evaluate if there is a correlation between PMA on MRI and psoas muscle area on CT scan in breast cancer patients to assess women’s muscular status directly on breast MRI.
Material and methods
Patients
This is a sub-study of different IRB (institutional review board)-approved studies for which women involved gave written informed consent. We retrospectively reviewed all consecutive breast cancer patients who were examined in our university hospital between January 2016 and January 2018.
Inclusion criteria were as follows:
-
For each patient, both total-body CT scans and breast MRI available.
-
The period between the two examinations not longer than 4 months. (This period was selected to have adequate follow-up time.)
-
High-quality imaging examinations.
Exclusion criteria were as follows: MRI or CT images not retrievable, time between the two examinations longer than 4 months, inaccurate images due to artefacts (e.g. motion artefacts; beam hardening CT artefacts, MRI artefacts for breast tissue expanders, surgical clips with considerable distortion). Women with breast sub-muscular implants or with sub-glandular prosthesis were also excluded.
A total of 26 women (mean age 51.1 ± 12.6 years, range 30–76 years) were included in the study. Clinical data and information on the primary tumour were also collected. Follow-up data were not considered relevant for the purpose of the study.
Images analysis: CT
All CT scans were acquired with different GE (GE Healthcare) CT scanners. Both 1.25-mm and 5-mm slice thickness with “standard body” kernel were available. All CT scans were performed with the patient in the supine position, head first on the scanner table and with the arms raised and placed behind the patient’s head, out of the scan plane. The whole body was scanned from the lung apex to the pubic symphysis.
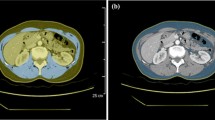
To assess measurement reproducibility, a strict method was used as follows. Reconstructed axial images with both a 1.25-mm and a 5-mm slice thickness were analysed using the software installed on the workstations of our radiology department (Suite-Estensa 1.9-Ebit-Esaote Group Company. 2015). The third lumbar vertebra (L3), at the level in which both transverse processes are clearly visible, was used as a bony landmark to properly identify the psoas muscle. Vertebrae were counted down from the cervical spine using scout images of the whole body or multiplanar reformatted (MPR) images from source thin-section axial images using the software on our workstations. In this study scout images were available for each patient. If there is no scout image of the whole spine or reformatted images, it is possible to identify the first lumbar vertebrae (L1) that is the first vertebra without a rib attachment. Once L1 has been found, the transverse processes can be used to count downward to L3. The psoas muscle was manually contoured bilaterally and the muscle mass was calculated as the cross-sectional area (square millimetres) (Fig. 1). The bilateral masses of the psoas were measured separately and the two values were averaged.
An example of measurement of psoas muscle total areas on axial CT image. The third lumbar vertebra (L3), at the level in which both transverse processes are clearly visible, was used as bony landmark to identify the psoas muscle area, contoured bilaterally (light blue area). Vertebrae were counted down from the cervical spine using scout images of the whole body or multiplanar reformatted (MPR) images from source thin-section axial images using the software on our workstations (not illustrated)
After patient selection was done, two board-certified radiologists (A.T., the main observer, with more than 10 years of experience specialised in oncological imaging and in breast imaging and E.B. with 5 years of experience in oncological imaging) independently performed psoas muscle measurement using reconstructed axial images with 1.25-mm and 5-mm slice thickness for all patients to assess inter-reader variability. To assess intra-reader agreement, 4 weeks after the first evaluation, all measurements were calculated again by the same two radiologists.
Images analysis: MRI
All patients were examined by using a 1.5-Tesla equipment (Magnetom Avanto, Siemens Healthcare). Dedicated double breast coil was used. Following the guidelines from the European Society of Breast Imaging, the standard MRI protocol was used for all patients: axial T2-weighted fast spin echo and axial T1-weighted 2D spoiled gradient echo dynamic contrast-enhanced acquisition with fat saturation, first performed before contrast administration and repeated multiple times after contrast administration. A bolus injection of gadolinium-based contrast agent (from 0.05 to 0.2 mmol/kg body weight) followed by a saline flush of 20 ml was given. The calculation of subtraction images from the pre- and post-contrast series was performed. All images were analysed using the software installed on the workstations of our radiology department as done for CT images. The pectoralis muscle was identified at the level of the sternal angle of Louis (manubriosternal joint that lies at the level of the second costal cartilage) used as bony landmark. Pectoralis muscle was manually contoured bilaterally and the cross-sectional area (square millimetres) on axial pre-contrast T1-weighted gradient echo fat-saturated images was calculated (Fig. 2). In all patients, PMA on the left and right side was determined separately and the two values were averaged.
An example of measurement of pectoralis muscle area on axial pre-contrast T1-weighted gradient echo MR images. On the basis of the scout view (not illustrated), the pectoralis muscle was identified at the level of the sternal angle of Louis (manubriosternal joint that lies at the level of the second costal cartilage) used as bony landmark. The pectoralis muscle area was countered bilaterally (red area)
Similarly, for psoas muscle area measurements, PMA was calculated by two observers (A.T., more than 10 years of experience in oncological imaging and in breast imaging and F.R., 5 years of experience in musculoskeletal imaging) on MRI images. To guarantee that all data were organised properly, a database was created by a professional data manager (F.V.), who did not perform the measurements. Intra- and inter-observer agreement for PMA was assessed as well.
Statistical analysis
Statistical analysis was performed using statistical software [SPSS, version 12.0.1 (SPSS, Inc.); Excel 2007 (Microsoft Corp.]; and MedCalc, version 11.4 (MedCalc Software)]. Descriptive statistics were used. The aim was to calculate inter- and intra-reader agreement in assessing the psoas cross-sectional area (square millimetres) on CT axial images with 1.25-mm and 5-mm slice thickness and then the PMA in square millimetres on MRI axial pre-contrast T1-weighted gradient echo fat-saturated images. Inter-reader agreement was evaluated using Cohen’s kappa test. Reliability coefficients were interpreted, respectively, as poor if less than 0.21, fair if between 0.21 and 0.4, moderate if between 0.41 and 0.6, good if between 0.61 and 0.8, and almost perfect agreement if 0.81–1 [16,17,18,19]. A p value of 0.05 was considered statistically significant. To evaluate the correlation between the PMA on MRI and the psoas muscle area on CT, the two-tailed Pearson’s test was performed using both 1.25-mm slice thickness and 5-mm slice thickness. The Pearson r correlation coefficient with 95% confidence intervals (CI) and the coefficient of determination (R2) were calculated. A p value of 0.05 was considered statistically significant. To have a significant correlation of an r = 0.06 with a type I error of 0.05 a total sample size of 19 was calculated as sufficient.
Results
According to the study protocol, 2/28 (7.1%) women were excluded for missing data. Indeed, MRI/CT exams were not retrievable (one patient) or image quality was not deemed acceptable (one patient with orthopaedic spinal devices). Therefore, a total of 26 breast cancer patients were evaluated bilaterally (mean age 51.1 ± 12.6 years, range 30–76 years; mean cancer size ± standard deviation 21 ± 11 mm). Standard clinical data and cancer characteristics (cancer size and type, histological grade, lymph node status, oestrogen and progesterone receptors, human epidermal growth factor receptor 2) were retrievable for 24/26 (92.31%). Mean values and standard deviation for measurement data were also reported (Table 1). No T4 cancers were included in this study and there were no cancers infiltrating the pectoralis muscle.
Inter- and intra-reader agreement
On axial 1.25-mm-slice-thickness CT images, measurements of psoas muscle area (square millimetres) were performed by two radiologists and according to Cohen’s kappa test, the inter-reader agreement was considered almost perfect (0.81–1). The inter-reader agreement was k = 0.85, p < 0.05. On axial 5-mm-slice-thickness CT images, measurements of psoas cross-sectional area (square millimetres) the inter-reader agreement was considered good (0.61–0.8). The inter-reader agreement was k = 0.79, p < 0.05. Intra-reader agreement of reader 1 was 0.98 and 0.94 for 1.25-mm and 5-mm, respectively. Intra-reader agreement of reader 2 was 0.95 and 0.94 for 1.25-mm and 5-mm, respectively.
On axial pre-contrast T1-weighted gradient echo fat-saturated MR images, measurements of PMA (square millimetres) were performed by two radiologists and according to Cohen’s kappa test, the inter-reader agreement was considered good (0.61–0.8). The inter-reader agreement was k = 0.61, p < 0.05.
Intra-observer agreement of reader 1 and reader 2 for PMA estimation were good (0.62 and 0.64), respectively.
Correlation
Comparing axial 5-mm-slice-thickness body CT images and T1-weighted breast fat-saturated MR images, the Pearson r correlation coefficient (– 1 < r < + 1) was 0.52 (95% CI 0.20–0.78). Comparing axial 1.25-mm slice thickness body CT images and T1-weighted breast MR images, the Pearson r (– 1 < r < + 1) correlation coefficient was 0.70 (95% CI 0.41–0.81) and the coefficient of determination (R2, 0 < R2 < 1) was 0.49, p < 0.05 (Fig. 3).
Discussion
The aim of this study was to evaluate if there was a correlation between the psoas muscle area on CT images and the PMA on MRI in breast cancer patients. We thus hypothesise that PMA, like the psoas area, may serve as a surrogate marker of patient survival and outcome that can easily be measured on standard breast MRI in patients affected by breast cancer. To the best of our knowledge, no previous studies with this purpose were conducted. Kinsey et al [14] hypothesised that PMA measured on chest CT scan can be associated with overall survival in non-small cell lung cancer; Go et al [15] compared, with no significant differences, the pectoralis versus lumbar skeletal muscle for defining sarcopenia in diffuse large B cell lymphoma. However, these studies were performed using CT images. Furtner et al [20] evaluated the prognostic relevance of temporal muscle thickness on MRI in brain metastasis in patients with breast and lung cancer staging with CT scans.
The pectoral muscle was chosen as the muscle of interest for our study because it is one of the few easily measurable thoracic muscles, which is assessed on routinely obtained breast MR images. Pectoralis muscle area is readily available and does not require specific research protocols.
The results of our study suggest that the inter-reader agreement between radiologists evaluating the psoas muscle area on 1.25-mm-slice-thickness CT images was almost perfect, better than on 5-mm images. The psoas muscle area measurements are stable and not dependent on the radiologist. The technique for measurement of PMA has also been shown to be highly reproducible between different readers. Indeed, the inter-reader agreement was good. Interestingly, inter-observer agreement was lower for PMA compared to TPA. Indeed, PMA estimation on breast MRI is not a standard evaluation made by radiologists and precise Lewis angle identification could be difficult, especially for breast radiologists. We believe that a short period of training will guarantee better results even among less experienced radiologists. On the contrary, TPA estimation at L3 level is a simple evaluation with very god reproducibility data in our study and in medical literature [21].
Comparing axial 1.25-mm-slice-thickness body CT images and T1-weighted breast MR images, the Pearson r correlation coefficient was 0.70 (95% CI 0.41–0.81) and the coefficient of determination was 0.49. To the best of our knowledge, no previous studies correlated muscle cross-sectional area assessed with two different imaging techniques. The results suggest that PMA assessed on breast MR images was strongly correlated with psoas muscle area on body CT images and it could be used to assess women’s muscular status directly on breast MRI even without body CT. The strong correlation suggests that both imaging techniques could be used to estimate muscle mass in cancer patients. Body CT could be less available in breast cancer patients [12] compared to breast MRI which is strongly recommended in preoperative staging of newly diagnosed breast cancer [22]. Indeed, according to recommendations, CT scans are not systematically used to assess extension in breast cancer [7].
Moreover, the American Cancer Society (ACS) and National Comprehensive Cancer Network (NCCN) guidelines suggest annual breast MRI screening in women with a familial increased risk for breast cancer and in women BRCA1+ or BRCA2+ [23,24,25,26,27]. Women who underwent radiation therapy of the chest wall or mediastinum before age 30 also belong to the high-risk group. Their risk starts about 10 years after radiation therapy. Abbreviated MRI protocols were recently introduced with equivalent performance compared to standard MRI protocols, making this a breast imaging modality that could be considered as an excellent screening tool even in non-high-risk populations [13]. Deluche et al stated that sarcopenia is an independent prognostic factor in early breast cancer; therefore, assessing body composition could be a simple and useful approach to integrate into patient management [7].
Even if evaluation of muscle mass could be achieved using sophisticated methods such as 3D sampling or muscle volume calculation, a strength of our study is to provide a quick and easy method to estimate muscle mass in breast cancer patients.
Some limitations of our study need to be acknowledged. A known limitation is the retrospective nature of our study with a suboptimal timing between CT and MRI. However, this data introduces a bias that could reduce the magnitude of the correlation; therefore, the positive correlation that we found could be even stronger in a prospective study with less time distance between CT and MRI. In addition, there were no T4 cancers or cancers invading the pectoralis muscle. It is possible that in patients with clear infiltration or disruption of the pectoralis muscle, the accuracy of PMA on breast MRI could be reduced.
Our data provide some evidence of potential clinical utility of PMA assessment. Further prospective studies are warranted to confirm these data on larger data sets regarding standard clinical endpoints.
A limitation of our study is that muscle quality was not evaluated. Indeed, muscle size, fibre type, architecture, aerobic capacity and intermuscular adipose tissue could contribute to muscle quality and to cross-sectional muscle area. Further studies could assess muscle quality with CT and MRI.
To increase measurement accuracy, precise evaluation and strict adherence to the predefined anatomical landmarks for PMA measurements are crucial. Therefore, a basic knowledge of musculoskeletal anatomy could be necessary even for breast radiologists. Another limitation is the relative small sample size that limits the possibility to detect differences in the subgroups of patients. We did not have the statistical power to further assess potential differences by histology in a stratified analysis. The assessment of muscle mass is an area of growing importance given its associations with adverse outcomes and the routine use of advanced imaging with many cancers.
Boutin et al showed that on body CT contrast enhancement of commonly measured muscles should be considered when using CT attenuation values as biomarkers of sarcopenia [28]. In addition, muscular CT enhancement may be significantly influenced by age, sex and unenhanced tissue attenuation [28]. Further studies are also warranted to evaluate the CT attenuation value of psoas muscle and pectoralis muscle in breast cancer patients. Patients with early stage breast cancer may not have CT imaging available and the possibility of using PMA instead when available could be useful.
In conclusion, our data show that PMA assessment could be used as a potential indicator of muscular status in women undergoing breast MRI. This data could be particularly useful in women with no standard body CT available (high-risk women or early breast cancer). PMA may help to better define muscular status in breast cancer patients and may optimise patient selection for therapeutic intervention or clinical trials.
Abbreviations
- ACS:
-
American Cancer Society
- CT:
-
Computer tomography
- EUSOBI:
-
European Society of Breast Imaging
- MRI:
-
Magnetic resonance imaging
- NCCN:
-
National Comprehensive Cancer Network
- PMA:
-
Pectoralis muscle area
- TPA:
-
Total psoas area
References
Baumgartner RN, Koehler KM, Gallagher D et al (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147:755–763
Muscaritoli M, Anker SD, Argilés J et al (2010) Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by special interest groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr 29:154–159
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108
Stewart B, Wild CP (2014) World cancer report 2014. World Health Organization, Geneva. http://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-2014. Accessed 26 Oct 2017
Del Fabbro E, Parsons H, Warneke CL et al (2012) The relationship between body composition and response to neoadjuvant chemotherapy in women with operable breast cancer. Oncologist 17:1240–1245
Prado CM, Baracos VE, McCargar LJ et al (2009) Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 15:2920–2926
Deluche E, Leobon S, Desport JC, Venat-Bouvet L, Usseglio J, Tubiana-Mathieu N (2018) Impact of body composition on outcome in patients with early breast cancer. Support Care Cancer 26:861–868
Klassen O, Schmidt ME, Ulrich CM et al (2017) Muscle strength in breast cancer patients receiving different treatment regimes. J Cachexia Sarcopenia Muscle 8:305–316
Villaseñor A, Ballard-Barbash R, Baumgartner K et al (2012) Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL study. J Cancer Surviv 6:398–406
Shen W, Punyanitya M, Wang Z et al (1985) Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol 1985:2333e8
Jones KI, Doleman B, Scott S, Lund JN, Williams JP (2015) Simple psoas cross-sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Colorectal Dis 17:20–26
Senkus E, Kyriakides S, Ohno S et al (2015) Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 5:8–30
Gilbert FJ, Selamoglu A (2018) Personalised screening: is this the way forward? Clin Radiol 73:327–333
Kinsey CM, San José Estépar R, van der Velden J, Cole BF, Christiani DC, Washko GR (2017) Lower pectoralis muscle area is associated with a worse overall survival in non-small cell lung cancer. Cancer Epidemiol Biomarkers Prev 26:38–43
Go SI, Park MJ, Song HN et al (2017) A comparison of pectoralis versus lumbar skeletal muscle indices for defining sarcopenia in diffuse large B-cell lymphoma - two are better than one. Oncotarget 8:47007–47019
Bland JM, Altman DG (2003) Applying the right statistics: analyses of measurement studies. Ultrasound Obstet Gynecol 22:85–93
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Bland JM, Altman DG (1996) Measurement error proportional to the mean. BMJ 313:106
Bland JM, Altman DG (1996) Measurement error. BMJ 313:744
Furtner J, Berghoff AS, Albtoush OM et al (2017) Survival prediction using temporal muscle thickness measurements on cranial magnetic resonance images in patients with newly diagnosed brain metastases. Eur Radiol 27:3167–3173
Rier HN, Jager A, Sleijfer S, van Rosmalen J, Kock MCJM, Levin MD (2017) Low muscle attenuation is a prognostic factor for survival in metastatic breast cancer patients treated with first line palliative chemotherapy. Breast 31:9–15
Mann RM, Balleyguier C, Baltzer PA et al (2015) Breast MRI: EUSOBI recommendations for women’s information. Eur Radiol 25:3669–3678
Strigel RM, Rollenhagen J, Burnside ES et al (2017) Screening breast MRI outcomes in routine clinical practice: comparison to BI-RADS benchmarks. Acad Radiol 24:411–417
Saslow D, Boetes C, Burke W et al (2007) American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 57:75–89
Warner E, Messersmith H, Causer P, Eisen A, Shumak R, Plewes D (2008) Systematic review: using magnetic resonance imaging to screen women at high risk for breast cancer. Ann Intern Med 148:671–679
Sardanelli F, Podo F, Santoro F et al (2011) High Breast Cancer Risk Italian 1 (HIBCRIT-1) Study (2011) Multicenter surveillance of women at high genetic breast cancer risk using mammography, ultrasonography, and contrast-enhanced magnetic resonance imaging (the high breast cancer risk Italian 1 study): final results. Invest Radiol 46:94–105
Lehman CD, Lee JM, DeMartini WB et al (2016) Screening MRI in women with a personal history of breast cancer. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djv349
Boutin RD, Kaptuch JM, Bateni CP, Chalfant JS, Yao L (2016) Influence of IV contrast administration on CT measures of muscle and bone attenuation: implications for sarcopenia and osteoporosis evaluation. AJR Am J Roentgenol 207:1046–1054
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Alberto Stefano Tagliafico.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional review board approval was obtained.
Methodology
• retrospective
• cross-sectional study
• performed at one institution
Rights and permissions
About this article
Cite this article
Rossi, F., Valdora, F., Barabino, E. et al. Muscle mass estimation on breast magnetic resonance imaging in breast cancer patients: comparison between psoas muscle area on computer tomography and pectoralis muscle area on MRI. Eur Radiol 29, 494–500 (2019). https://doi.org/10.1007/s00330-018-5663-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5663-0