Abstract
Objectives
To develop and validate a prognostic model of integrating whole-tumour apparent diffusion coefficient (ADC) from pretreatment diffusion-weighted (DW) magnetic resonance (MR) imaging with human papillomavirus (HPV) genotyping in predicting the overall survival (OS) and disease-free survival (DFS) for women with stage IB–IV cervical cancer following concurrent chemoradiotherapy (CCRT).
Methods
We retrospectively analysed three prospectively collected cohorts comprising 300 patients with stage IB–IV cervical cancer treated with CCRT in 2007–2014 and filtered 134 female patients who underwent MR imaging at 3.0 T for final analysis (age, 24–92 years; median, 54 years). Univariate and multivariate Cox regression analyses were used to evaluate the whole-tumour ADC histogram parameters, HPV genotyping and relevant clinical variables in predicting OS and DFS. The dataset was randomly split into training (n = 88) and testing (n = 46) datasets for construction and independent bootstrap validation of the models.
Results
The median follow-up time for surviving patients was 69 months (range, 9–126 months). Non-squamous cell type, ADC10 <0.77 × 10-3 mm2/s, T3-4, M1 stage and high-risk HPV status were selected to generate a model, in which the OS and DFS for the low, intermediate and high-risk groups were significantly stratified (p < 0.0001). The prognostic model improved the prediction significantly compared with the International Federation of Gynaecology and Obstetrics (FIGO) stage for both the training and independent testing datasets (p < 0.0001).
Conclusions
The prognostic model based on integrated clinical and imaging data could be a useful clinical biomarker to predict OS and DFS in patients with stage IB–IV cervical cancer treated with CCRT.
Key points
• ADC 10 is the best prognostic factor among ADC parameters in cervical cancer treated with CCRT
• A novel prognostic model was built based on histology, ADC 10 , T and M stage and HPV status
• The prognostic model outperforms FIGO stage in the survival prediction
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cervical cancer is the fourth most common cancer in women worldwide, with estimated new cases and deaths of 13,240 and 4,170 respectively in the United States in 2018 [1]. The standard treatment for locally advanced cervical cancer [International Federation of Gynaecology and Obstetrics (FIGO) stages IB2 to IVA] is concurrent chemoradiotherapy (CCRT) [2]. The 5-year survival rate is about 58-80% for stage IB-II cancer, but is only 15-35% for the stage III-IV cervical cancer [1]. Biomarkers identifying patients at higher risk of recurrence is urgently needed, to provide a better personalisation of cancer care in treatment decisions or follow-up strategies. Still, they may not be satisfactory for identifying before treatment. Clinicopathological factors for cervical cancer including FIGO stage have shown predictive values in prognosis [3]. The most important causative factor for cervical cancer, human papillomavirus (HPV), also provides valuable prognostic information [4], with the absence of HPV infections or presence of alpha-7 species resulting in a poorer prognosis, compared with alpha-9 species or multiple HPV infections [4].
Magnetic resonance (MR) imaging is useful in the staging of cervical carcinomas of FIGO stage IB1 or greater, supported by its accuracy for evaluating tumour size, detecting parametrial invasion, and high negative predictive values for excluding bladder and rectal invasion [3]. Diffusion-weighted (DW) MR imaging provides a quantitative readout—apparent diffusion coefficient (ADC) values, representing tumour cellularity [3]. Preliminary reports have shown the prognostic values of ADC derived from a single section [5,6,7,8,9,10,11,12], few of them applying the three-dimensional whole-tumour ADC values [13]. With the development of high-throughput extraction of quantitative features that result in the conversion of images into mineable data, [14], DW MR imaging demonstrated potentials in integrating ADC values with the established predictive markers such as HPV genotyping [15] and clinical FIGO stage [3], to improve the predictive accuracy.
The purpose of this study was to develop and evaluate the values of a prognostic model based on an integration of pretreatment MR imaging, whole-tumour ADC values and HPV genotyping, in the prediction of survival and recurrence for women with stage IB-IV cervical cancer following CCRT.
Materials and methods
Patients
This study complied with the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) Statement. We retrospectively analysed three consecutive prospectively collected cohorts from February 2007 to December 2014 (IRB 95-1243B, IRB 97-2366B and IRB 102-0620A3), enrolling all the available and evaluable cases during the study period. There was no sample size estimation at the initial stage. Our institutional review board approved this study and informed consents were obtained. The study setting was conducted in a tertiary referral centre with a dedicated interdisciplinary gynaecological oncology team. The inclusion criteria for this study were: biopsy proved newly diagnosed cervical cancer clinical stage FIGO IB-IVA and willing to undergo CCRT treatment. The exclusion criteria were: patients who are judged to be non-compliant to treatment or not accessible for follow-up, patients having contraindications to MR scanning, or patients unable to cooperate for MR study due to mental status. The patient cohort is detailed in Fig. 1. We recorded the relevant clinical information, including age, histology, grade, HPV status and serum levels of squamous cell carcinoma antigen (SCC-Ag) and carcinoembryonic antigen (CEA). Serum SCC-Ag level is elevated in 28-88% of patients with squamous cell cervical cancer, and has shown prognostic significance for patients undergoing surgical treatment or radiation therapy [16]. Data collection was planned before the MR imaging acquisition. A total of 134 evaluable subjects entered final analysis in this study; age ranged from 24 to 92 years (median, 54 years).
MR methods
MR studies were conducted with a 3-T MR imaging unit (Tim Trio; Siemens Healthineers, Erlangen, Germany) before treatment. Patients were placed in the supine position with bladder emptied before the examination. The lower nine elements of the integrated spine coil and the lower six elements of the body-phased array coil were used to cover the entire pelvis. MR images of the pelvis were acquired in the sagittal and axial oblique planes perpendicular to the cervical canal (DW: single-shot echo-planar technique with fat suppression; TR/TE, 3,300/79; average, 4; b value = 0 and 1,000 s/mm2, section thickness, 4 mm; gap, 1 mm; matrix, 128 × 128; FOV, 20 cm; T2-weighted: 5,630/87; average, 3; matrix, 256 × 320; FOV, 20 cm). This study was performed during free-breathing at a minimal level. No premedication was administered [17].
Imaging analysis
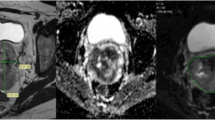
ADC maps were generated using a monoexponential decay model with b value of 0 and 1,000 s/mm2 (VB17a, Trio TIM; Siemens Healthineers). Anonymous data were exported offline to a personal computer. By using an in-house developed software based on Matlab (MathWorks, Natick, MA, USA), the first reader (Y.T.H, a gynaecological radiologist with 8 years of experience) drew regions of interest (ROIs) around the tumour on each slice on the ADC maps with reference to the high b value DW and T2-weighted images to delineate the whole tumour volume to minimise the slice selection bias. The second reader independently verified the ROIs (G.L., a gynaecological radiologist with 10 years of experience). Both readers were blinded to clinical outcome. Care was taken to avoid non-pathological structures in the ROIs, such as normal cervical stroma, vascular structures, fluid or Nabothian cysts in the cervix. The mean, minimum, 10th, 25th, 50th, 75th and 90th percentiles and maximal pixel ADC values (ADCmean, ADCmin, ADC10, ADC25, ADC50, ADC75, ADC90 and ADCmax, respectively) were generated, and the skewness and kurtosis of the histogram were recorded (Fig. 2). The tumour volume was calculated by multiplying the voxel size of DW image by the number of voxels in the ROI, where the voxel size = in-plane pixel size (1.5625 mm2) × (4 mm slice thickness + 1 mm gap) = 7.8125 mm3.
Representative volumetric apparent diffusion coefficient (ADC) measurement. a Coloured scale bar, × 10-3 mm2/s. b Histogram analysis. A 47-year-old woman with moderately differentiated squamous cell carcinoma of uterine cervix, clinical stage T2b N0 M0, HPV genotype 18, maximal tumour diameter 7.3 cm, ADC10 0.68 × 10-3 mm2/s, ADC50 0.82 × 10-3 mm2/s (green), ADC90 1.00 × 10-3 mm2/s (red). This patient had persistent disease following concurrent chemoradiation therapy. Overall survival time, 8.1 months
Histopathology
Before treatment, histopathological types and tumour grades were evaluated in the consensus of a general pathologist and a specialised gynaecological pathologist (R.C.W.). If the primary site of a tumour was uncertain, an additional immunohistochemical study was performed to exclude the possibility of endometrial cancer and confirm the diagnosis of cervical cancer.
HPV
Poor prognostic HPV genotypes were defined as alpha-7 species (HPV18, 39, 45) and absence of HPV infection in this study, as opposed to favourable prognostic alpha-9 species (HPV16, 31, 33, 52, 58) and multiple HPV genotypes [4, 18]. The procedure for the HPV genotyping has been reported previously [19]. Briefly, DNA was extracted from paraffin-embedded specimens and amplified by polymerase chain reaction (PCR) for the L1 open reading frame with biotinylated GP6+ and SPF1 consensus primers, then hybridised with the Easychip® HPV Blot membranes (King Car, I-Lan, Taiwan), to determine the final HPV genotypes. Type-specific PCR reactions were performed to validate multiple types and HPV-negativity on HPV Blot. In cases of discordance between results, a repeat of HPV Blot, SPF1/GP6+ PCR, and direct sequencing was performed to resolve the discrepancy.
Treatment and outcome
All patients underwent standard external beam radiation therapy (daily fraction of 1.8–2 Gy in five fractions weekly; total dose, 40–45 Gy) and cisplatin-based CCRT, six cycles of weekly intravenous cisplatin infusions (40-50 mg/m2 body surface) during the radiation therapy course [4]. Post-therapy surveillance was the same as that in our previous report [4]. The primary outcome was overall survival (OS), defined as the time from the first fraction of radiation therapy to the date of death. Disease-free survival (DFS) was determined as the time from the first fraction of radiation therapy to the development of any recurrence (local, regional or distant). Evidence of recurrence was based on the pathology documentations. When the histological/cytological tissues were unable to be obtained due to anatomical limitations or patient conditions, we used clinical proof of recurrence confirmed by at least two imaging modalities—18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT), MR or CT. Cases lost to follow-up, non-cancer-related death and those alive at the end of the follow-up period were considered censored observations. Patients with persistent diseases were regarded as having relapsed on the first day when completing radiation therapy.
Statistical analysis
The data were analysed using the Statistical Package for Social Sciences, version 11 (SPSS; IBM, Armonk, New York, USA), MedCalc for Windows, Version 9.2.0.0 (MedCalc Software, Ostend, Belgium), or R Package for Statistical Computing (www.r-project.org). Survival curves were generated using the Kaplan-Meier method, and comparisons were made using the log-rank test. Pearson's chi-squared test was used to evaluate the association between the variables. Continuous variables were analysed with Student’s t-test (two-group comparisons). The dataset was randomly split into training (n = 88) and testing (n = 46) datasets. Based on the training set, univariate Cox proportional hazard regressions were used to assess associations of ADC parameters, tumour volume and relevant clinical variables (i.e. age, histological type, differentiation, TNM stage, HPV risk, serum levels of SCC-Ag and CEA) with OS and DFS. Variables significant in the univariate analysis or by prognostic interest were subsequently entered into a multivariate analysis using the Cox proportional hazards ratio model. Their hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using the Wald test. Continuous variables were determined by the recursive partitioning method for optimal cut-point analysis. By using the selected independent prognostic variables based on the training dataset, outcome-predicting models were formulated. The testing data, uniformly resampled from the testing dataset under the bootstrapping procedure to duplicate the testing sample size, was applied for independent validation of the models. We used likelihood ratio tests to compare the goodness-of-fit between models. Missing data was excluded from the analysis case-wise. All tests were two-sided, and p < 0.05 was considered statistically significant.
Results
Patient characteristics and clinical outcome
Table 1 lists the clinical and demographic features of the study population. The median follow-up was 69 months (range, 9–126 months) for surviving patients. Of the 134 women analysed, 87 (65%) were free of disease at last follow-up. Twenty-two patients had persistent cervical cancer and were never free of disease. Of the 25 patients with recurrence, 12 had distant failure alone (one bone, two lungs, lymph nodes at the supraclavicular fossa, mediastinum, inguina, three para-aortic lymph nodes, one having both para-aortic and cardiophrenic lymph nodes, and two having multiple sites including lung and multiple lymph nodes at the supraclavicular fossa and mediastinum), 1 had regional lymph node recurrence alone and 5 were local failure alone (four cervix and one vaginal cuff). The remaining seven patients had more than one site of failure: one local-regional-distant (vaginal stump, pelvic lymph node and lung), two local-distant (one cervix and lung, another supra-vesicle mass and para-aortic lymph node), four regional-distant (one pelvic and para-aortic lymph nodes, one pelvis, para-aortic and mediastinal lymph nodes, two pelvis, para-aortic and supraclavicular fossa lymph node, respectively). Recurrences were confirmed by at least two imaging modalities in all cases, and in 16 patients also proven by biopsy. The time to recurrence ranged from 7 to 61 months after primary treatment. The overall 2-year OS rate was 81.2% (95% CI, 73.5–86.9%), and the 2-year DFS rate was 83.5% (95% CI, 76.0–88.8%). Forty-one patients died of persistent or recurrent disease.
Whole-tumour ADC10 is an independent prognostic factor for survival
We developed the prognostic factors based on the training dataset. Our two readers agreed in 747 of the total 825 image slices, with an agreement rate of 0.905. All the ADC parameters (ADCmean, ADCmin, ADC10, ADC25, ADC50, ADC75, ADC90 and ADCmax) correlated one another significantly (p < 0.0001). Univariate Cox regression analyses for OS and DFS are shown in Tables 2 and 3. Based on stepwise selection procedure, it showed that adenocarcinoma or adenosquamous carcinoma cell type, ADC10, ADCmax, T stage 3-4, distant metastasis (M1) and high-risk HPV status were independent predictors for both poor OS and DFS (Table 2 and 3). In subgroup analysis, adenocarcinoma or adenosquamous carcinoma cell type, ADC10, T stage 3-4 and high-risk HPV status were also independent predictors for both poor OS and DFS in the patients without distant metastasis (M0). ADC10 values showed no significant correlations with the tumour volume, serum CEA or SCC-Ag levels (Supplementary Table 1), and showed no significant differences between tumour cell type (adenocarcinoma or adenosquamous carcinoma vs squamous cell carcinoma), T (T1-2 vs T3-4), N and M stages, as well as HPV risk (Supplementary Table 2).
Outcome-predicting models
Outcome-predicting models were constructed using the five covariates, including non-squamous cell type, ADC10 <0.77 × 10-3 mm2/s, T3-4, M1 stage and high-risk HPV status. The prognostic score was low, intermediate and high in presence of 0-1, 2-3 and 4-5 risk covariates respectively. The probability of dying was lowest in the low-risk group (HR 0.01), followed by the intermediate-risk group (HR 0.02) when compared with the high-risk group (HR 1, p = 0.001; Table 4). Two-year OS rates of the low-, intermediate- and high-risk groups were 89.6%, 74.4% and 0%, respectively. The probability of cancer recurrence was lowest in the low-risk group (HR 0.03), followed by the intermediate-risk group (HR 0.12) when compared with the high-risk group (HR 1, p < 0.0001; Table 4). We also found significant differences between the low and intermediate prognostic groups in predicting the OS (p = 0.002) and DFS (p = 0.002). For both OS and DFS, the prognostic model improved the prediction compared with outcome classification using FIGO stage and was consistent and stable on the testing dataset (Figs. 3 and 4). The prognostic model has significant improvement compared with the FIGO staging system, based on the likelihood ratio tests to compare the goodness-of-fit (p < 0.0001). Adding the ADC10 to the model significantly improve the predictive value in OS (p = 0.035) and DFS (p = 0.038) in the testing dataset.
Kaplan-Meier curves for overall survival according to outcome prediction model (upper) and FIGO stage (lower) in cervical cancer patients undergoing CCRT, for training, testing and all datasets. The prognostic model has significant improvement in the training, test and overall data, compared with FIGO stage, based on the likelihood ratio tests to compare the goodness-of-fit (p < 0.0001 for all)
Kaplan-Meier curves for disease-free survival according to outcome prediction model (upper) and FIGO stage (lower) in cervical cancer patients undergoing CCRT, for training, testing and all datasets. The prognostic model has significant improvement in the training, test and overall data, compared with FIGO stage, based on the likelihood ratio tests to compare the goodness-of-fit (p < 0.0001 for all)
Discussion
In the present study, we integrated clinical and imaging data—non-squamous cell type, lower tumour ADC10 value, T3-4, M1 stage and high-risk HPV status—to generate a prognostic model which outperformed the current FIGO stage to predict OS and DFS for patients with cervical cancer following CCRT. We constructed independent validation bootstrap samples to estimate prediction error through randomly selecting datasets (training and testing), and extending the size of the testing set by bootstrap replication, in order to address the sample size limitation issue of independent validation [20]. This approach would lend stronger confidence in the data and a higher chance to have actionable models. Our principal findings based on ADC histogram analysis on the entire tumour volume showed that ADC10 is an independent prognostic indicator for OS and DFS. As lower ADC values imply an increase in cellularity [3], the lower ADC10 in this study might represent a more aggressive subpopulation in the cervical tumours. The low ADCmin of the primary tumour before CCRT has been reported as an independent prognostic factor for both OS and DFS based on single-slice ADC analysis [21]. Pretreatment ADCmean for patients with complete remission were significantly lower than those of partial remission following CCRT [11]. Furthermore, a lower ADCmean, along with FIGO stage, LN metastases and metabolic tumour volume (MTV) on 18F-FDG PET/CT were adverse predictors of both OS and DFS, by univariate Cox analysis [9]. In the only study analysing volumetric whole-tumour DW MR imaging for outcome prediction, Gladwish et al [13] showed a lower volumetric ADC90 or ADC95 might predict DFS in patients with locally advanced cervical cancer treated with CCRT, for both squamous cell carcinoma and adenocarcinoma. Our results indicate that the prognostic model could help oncologists accentuate the follow-up for patients with a high risk of local recurrence to assess for recurrence [22], or potentially tailor the prospective trial designs, as a reduced-dose intensity-modulated radiation therapy with concurrent chemotherapy might be an alternative in favourable-risk patients with HPV-associated oropharyngeal cancer [23].
ADC parameters were significantly lower than those of normal cervical tissues [24,25,26,27,28], which make it easy to contour the volumetric whole-tumour ADC histogram. Compared with adenocarcinoma, the tumour ADCmean [24,25,26,27,28,29] or ADC50 [26] is significantly lower for squamous cell carcinoma, possibly attributed to the glandular content of adenocarcinoma [30]. However, we found ADC10 values are independent of tumour volume, serum CEA or SCC-Ag levels, and show no significant differences in tumour cell types. In line with our study, there is no significant difference between the ADC histogram of cervical cancer when divided by FIGO stage [5], lymphovascular space invasion [25, 29], depth of cervical infiltration metastases [29] or lymph node metastases [25, 29], although a lower tumour ADCmean had been reported to be an independent predictor of pathological parametrial invasion [31]. Hence the ADC10 might represent tumour microenvironmental changes in cellularity, necrosis or interstitial fluid pressure [13]. Tumour subvolumes with greater cell density by DW MR imaging also have increased metabolic activity on FDG-PET [32]. An inverse correlation was reported between ADCmin and SUVmax [6, 33], with their combination being an independent prognostic factor for both DFS and OS [6]. The changes of ADCmean and SUVmax before and after 4 weeks of CCRT treatment were significantly correlated [34]. A recent small scaled study argued that the pre-treatment volume-based quantitative parameters of FDG PET may have better potential than ADC histogram for predicting treatment response and event-free survival in patients with locally advanced cervical cancer [35]. To this end, we postulated that the ADC10 might represent the more aggressive subpopulation in the target tumour, suggesting that the combination PET-MR might play a complementary role for the clinical assessment of tumour heterogeneity.
There were some limitations to our study. The primary technical limitation of this study is that, to reduce the adverse effects of medication and patient discomfort, we did not administrate anti-peristaltic agents in the prospective studies. Nevertheless, no remarkable bowel motion artefacts affected the quality of DW MR images in our study cohort. Second, although we excluded tumour volume less than 1 cm3 to prevent partial volume effects, we may still have included extreme ADCs resulting from DW imaging and ADC map misregistration artefacts. We can presume that the ADC10 or ADC90 obtained from histograms were less affected by these artefacts that ADCmin or ADCmax, and therefore, may be more reliable for histographical analysis. We gave equal weight to each predictor that had a different HR to facilitate clinical use. Despite a relatively short median follow-up of 2 years, the majority of treatment failures in cervical cancer occur within the first 2 years. Although our data are based on a single-institutional research, the ADC parameters have been proven to be robust across 1.5-T and 3-T scanners from different vendors using clinically available protocols, and might be suitable for use in multi-centre clinical studies and trials [36]. Nevertheless, our data are based on well-standardised prospective studies with internal validation and an independent testing dataset.
In conclusion, the prognostic model based on integrated clinical and imaging data—non-squamous cell type, lower tumour ADC10 value, T3-4, M1 stage and high-risk HPV status—may be a useful clinical biomarker to predict survival and recurrence in patients with stage IB-IV cervical cancer treated with CCRT. The prognostic models could be useful for stratifying study patients in future clinical trials.
Abbreviations
- CCRT:
-
Concurrent chemoradiotherapy
- CEA:
-
Carcinoembryonic antigen
- DFS:
-
Disease-free survival
- FIGO:
-
International Federation of Gynaecology and Obstetrics
- HPV:
-
Human papillomavirus
- HR:
-
Hazard ratio
- OS:
-
Overall survival
- SCC-Ag:
-
Squamous cell carcinoma antigen
References
American Cancer Society: cancer facts and figures (2018) American Cancer Society. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf. Accessed 27 June 2018
NCCN Clinical Practice Guidelines in Oncology: Cervical Cancer. National Comprehensive Cancer Network Web site. https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf. Accessed 27 June 2018
Sala E, Rockall AG, Freeman SJ, Mitchell DG, Reinhold C (2013) The added role of MR imaging in treatment stratification of patients with gynecologic malignancies: what the radiologist needs to know. Radiology 266:717–740
Wang CC, Lai CH, Huang HJ et al (2010) Clinical effect of human papillomavirus genotypes in patients with cervical cancer undergoing primary radiotherapy. Int J Radiat Oncol Biol Phys 78:1111-1120
Kuang F, Yan Z, Wang J, Rao Z (2014) The value of diffusion-weighted MRI to evaluate the response to radiochemotherapy for cervical cancer. Magn Reson Imaging 32:342–349
Nakamura K, Joja I, Kodama J, Hongo A, Hiramatsu Y (2012) Measurement of SUVmax plus ADCmin of the primary tumour is a predictor of prognosis in patients with cervical cancer. Eur J Nucl Med Mol Imaging 39:283–290
Heo SH, Shin SS, Kim JW et al (2013) Pre-treatment diffusion-weighted MR imaging for predicting tumor recurrence in uterine cervical cancer treated with concurrent chemoradiation: value of histogram analysis of apparent diffusion coefficients. Korean J Radiol 14:616-625
McVeigh PZ, Syed AM, Milosevic M, Fyles A, Haider MA (2008) Diffusion-weighted MRI in cervical cancer. Eur Radiol 18:1058–1064
Micco M, Vargas HA, Burger IA et al (2014) Combined pre-treatment MRI and 18F-FDG PET/CT parameters as prognostic biomarkers in patients with cervical cancer. Eur J Radiol 83:1169–1176
Himoto Y, Fujimoto K, Kido A et al (2015) Pretreatment mean apparent diffusion coefficient is significantly correlated with event-free survival in patients with International Federation of Gynecology and Obstetrics stage Ib to IIIb cervical cancer. Int J Gynecol Cancer 25:1079–1085
Liu Y, Bai R, Sun H, Liu H, Zhao X, Li Y (2009) Diffusion-weighted imaging in predicting and monitoring the response of uterine cervical cancer to combined chemoradiation. Clin Radiol 64:1067–1074
Kim HS, Kim CK, Park BK, Huh SJ, Kim B (2013) Evaluation of therapeutic response to concurrent chemoradiotherapy in patients with cervical cancer using diffusion-weighted MR imaging. J Magn Reson Imaging 37:187–193
Gladwish A, Milosevic M, Fyles A et al (2016) Association of apparent diffusion coefficient with disease recurrence in patients with locally advanced cervical cancer treated with radical chemotherapy and radiation therapy. Radiology 279:158–166
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures. they are data. Radiology 278:563–577
Lai CH, Chao A, Wang CC, Huang HJ (2014) Human papillomavirus and management of cervical cancer: does genotype matter. Curr Obstet Gynecol Rep 3:136–142
Kim BG (2013) Squamous cell carcinoma antigen in cervical cancer and beyond. J Gynecol Oncol 24:291–292
Lin G, Ng KK, Chang CJ et al (2009) Myometrial invasion in endometrial cancer: diagnostic accuracy of diffusion-weighted 3.0-T MR imaging—initial experience. Radiology 250:784–792
Cuschieri K, Brewster DH, Graham C et al (2014) Influence of HPV type on prognosis in patients diagnosed with invasive cervical cancer. Int J Cancer 135:2721–2726
Lin G, Lai CH, Tsai SY et al (2017) (1)H MR spectroscopy in cervical carcinoma using external phase array body coil at 3.0 tesla: prediction of poor prognostic human papillomavirus genotypes. J Magn Reson Imaging 45:899–907
Molinaro AM, Simon R, Pfeiffer RM (2005) Prediction error estimation: a comparison of resampling methods. Bioinformatics 21:3301–3307
Nakamura K, Joja I, Nagasaka T et al (2012) The mean apparent diffusion coefficient value (ADCmean) on primary cervical cancer is a predictive marker for disease recurrence. Gynecol Oncol 127:478–483
Jalaguier-Coudray A, Villard-Mahjoub R, Delouche A et al (2017) Value of dynamic contrast-enhanced and diffusion-weighted MR imaging in the detection of pathologic complete response in cervical cancer after neoadjuvant therapy: a retrospective observational study. Radiology. https://doi.org/10.1148/radiol.2017161299:161299
Marur S, Li S, Cmelak AJ et al (2017) E1308: Phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx—ECOG-ACRIN Cancer Research Group. J Clin Oncol 35:490–497
Liu Y, Ye Z, Sun H, Bai R (2015) Clinical application of diffusion-weighted magnetic resonance imaging in uterine cervical cancer. Int J Gynecol Cancer 25:1073–1078
Kuang F, Ren J, Zhong Q, Liyuan F, Huan Y, Chen Z (2013) The value of apparent diffusion coefficient in the assessment of cervical cancer. Eur Radiol 23:1050–1058
Lin Y, Li H, Chen Z et al (2015) Correlation of histogram analysis of apparent diffusion coefficient with uterine cervical pathologic finding. AJR Am J Roentgenol 204:1125–1131
Payne GS, Schmidt M, Morgan VA et al (2010) Evaluation of magnetic resonance diffusion and spectroscopy measurements as predictive biomarkers in stage 1 cervical cancer. Gynecol Oncol 116:246–252
Guan Y, Shi H, Chen Y et al (2016) Whole-lesion histogram analysis of apparent diffusion coefficient for the assessment of cervical cancer. J Comput Assist Tomogr 40:212–217
Xue H, Ren C, Yang J et al (2014) Histogram analysis of apparent diffusion coefficient for the assessment of local aggressiveness of cervical cancer. Arch Gynecol Obstet 290:341–348
Downey K, Riches SF, Morgan VA et al (2013) Relationship between imaging biomarkers of stage I cervical cancer and poor-prognosis histologic features: quantitative histogram analysis of diffusion-weighted MR images. AJR Am J Roentgenol 200:314–320
Park JJ, Kim CK, Park SY, Park BK, Kim B (2014) Value of diffusion-weighted imaging in predicting parametrial invasion in stage IA2-IIA cervical cancer. Eur Radiol 24:1081–1088
Olsen JR, Esthappan J, DeWees T et al (2013) Tumor volume and subvolume concordance between FDG-PET/CT and diffusion-weighted MRI for squamous cell carcinoma of the cervix. J Magn Reson Imaging 37:431–434
Ho KC, Lin G, Wang JJ, Lai CH, Chang CJ, Yen TC (2009) Correlation of apparent diffusion coefficients measured by 3T diffusion-weighted MRI and SUV from FDG PET/CT in primary cervical cancer. Eur J Nucl Med Mol Imaging 36:200–208
Park JJ, Kim CK, Park BK (2016) Prognostic value of diffusion-weighted magnetic resonance imaging and 18F-fluorodeoxyglucose-positron emission tomography/computed tomography after concurrent chemoradiotherapy in uterine cervical cancer. Radiother Oncol 120:507–511
Ueno Y, Lisbona R, Tamada T, Alaref A, Sugimura K, Reinhold C (2017) Comparison of FDG PET metabolic tumour volume versus ADC histogram: prognostic value of tumour treatment response and survival in patients with locally advanced uterine cervical cancer. Br J Radiol 90:20170035
Grech-Sollars M, Hales PW, Miyazaki K et al (2015) Multi-centre reproducibility of diffusion MRI parameters for clinical sequences in the brain. NMR Biomed 28:468–485
Funding
Supported by Chang Gung Medical Foundation grant CIRPG3E0022, CMRPG3F2241; National Science Council (Taiwan) MOST 104-2314-B-182A-095-MY3, NMRPD1E1051-3; Chang Gung IRB 95-1243B, 97-2366B, 102-0620A3 and 104-8300B. The authors acknowledge the assistance provided by the Cancer Center and the Clinical Trial Center, Chang Gung Memorial Hospital, Linkou, Taiwan, which was founded by the Ministry of Health and Welfare of Taiwan MOHW106-TDU-B-212-113005.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Chyong-Huey Lai.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Lan-Yan Yang, PhD. kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• diagnostic or prognostic study
• performed at one institution
Electronic supplementary material
ESM 1
(DOC 234 kb)
Rights and permissions
About this article
Cite this article
Lin, G., Yang, LY., Lin, YC. et al. Prognostic model based on magnetic resonance imaging, whole-tumour apparent diffusion coefficient values and HPV genotyping for stage IB-IV cervical cancer patients following chemoradiotherapy. Eur Radiol 29, 556–565 (2019). https://doi.org/10.1007/s00330-018-5651-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5651-4