Abstract
Objectives
To intraindividually compare the diagnostic performance of CT and MRI in differentiating non-diffuse-type autoimmune pancreatitis (AIP) from pancreatic ductal adenocarcinoma (PDA).
Methods
Sixty-one patients with non-diffuse-type AIP and 122 patients with PDA, who underwent dynamic contrast-enhanced CT and MRI with MR pancreatography, were included. Two blinded radiologists independently rated their confidence in differentiating the two diseases on a 5-point scale, and the diagnostic performances of CT and MRI were compared. The presence of key imaging features to differentiate AIP and PDA were compared between CT and MRI.
Results
The area under the receiver operating characteristic curve was significantly greater on MRI (0.993–0.995) than on CT (0.953–0.976) for both raters (p≤0.035). The sensitivities of MRI were higher than those of CT for the diagnosis of AIP (88.5–90.2% vs. 77–80.3%, p≤0.07) and PDA (97.5–99.2% vs. 91.8–94.3%, p≤0.031) for both raters, although the difference for AIP was statistically marginal (p=0.07) for rater 1. In AIP, multiple pancreatic masses, delayed homogeneous enhancement of the pancreatic mass, and multiple main pancreatic duct (MPD) strictures were observed significantly more frequently using MRI than CT (p≤0.008). In PDA, discrete pancreatic mass and MPD stricture were observed significantly more frequently using MRI than CT (p≤0.012).
Conclusions
The diagnostic performance of MRI is better for differentiating non-diffuse-type AIP from PDA, which is due to the superiority of MRI over CT in demonstrating the key distinguishing features of both diseases.
Key Points
• Imaging differential diagnosis of non-diffuse-type AIP and PDA is challenging.
• MRI has better diagnostic performance than CT in differentiating non-diffuse-type AIP from PDA.
• MRI is superior to CT in demonstrating key distinguishing features of non-diffuse-type AIP and PDA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Autoimmune pancreatitis (AIP) is a rare but distinctive type of chronic pancreatitis, which responds markedly well to steroid therapy [1]. The radiological and clinical features of AIP are similar to those of pancreatic ductal adenocarcinoma (PDA), making differential diagnosis important for avoiding unnecessary surgery in patients with AIP [2,3,4]. Diffuse-type AIP can be easily distinguished from PDA based on its characteristic imaging findings, i.e. diffuse sausage-like pancreatic swelling with peripancreatic capsule-like rim or halo sign [2,3,4]. Non-diffuse-type AIP typically manifests as a focal pancreatic mass and main pancreatic duct (MPD) stricture on imaging studies, and thus differential diagnosis from PDA is challenging. Some investigators have attempted to find differential imaging features between non-diffuse-type AIP and PDA, using computed tomography (CT) and magnetic resonance imaging (MRI) or magnetic resonance pancreatography (MRP), in various studies [5,6,7,8,9,10,11,12]. Several key imaging features, with regard to morphology of the pancreatic mass and MPD stricture pattern, were identified to be important to differentiate the two diseases in previous studies.
Currently, CT is the most commonly used, non-invasive imaging modality to evaluate both pancreatic and extrapancreatic findings in patients with suspected AIP. However, in our experience, CT has limitations in demonstrating some specific findings of non-diffuse-type AIP, including multiple pancreatic masses and multiple MPD strictures, while these findings appear to be somewhat clearly visualized on MRI/MRP. The excellence of MRI/MRP in depicting the characteristic findings of non-diffuse-type AIP has been addressed in previous studies [5,6,7,8,9]. However, little is known about the diagnostic performance of CT or MRI/MRP in AIP. Moreover, to the best of our knowledge, there have been no studies comparing the diagnostic performances of CT and MRI/MRP in differentiating the two diseases. Therefore, we conducted this study to intra-individually compare the diagnostic performance of CT and MRI with MRP in differentiating non-diffuse-type AIP from PDA.
Materials and methods
Study population
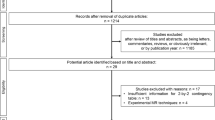
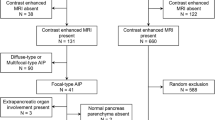
This retrospective study was approved by our institutional review board, and patient informed consent was waived. Through a search of our medical database, we identified 169 patients diagnosed with AIP, according to the Asian [13] or HISORt [14] criteria, or international consensus diagnostic criteria [15], in our institution between January 2007 and December 2016 (Fig. 1). Among these, 61 patients (44 men and 17 women; mean age ± standard deviation, 58.4 ± 11 years [range, 18–81 years]) were finally included in this study, based on the following inclusion criteria: (a) the presence of dynamic contrast-enhanced CT, including both arterial and portal phases; (b) dynamic contrast-enhanced MRI, including arterial, portal and delayed phases and MRP before steroid treatment; and (c) non-diffuse-type AIP. In criterion (c), the non-diffuse-type was defined as the presence of a focal mass involving less than half of the total pancreas or multifocal masses observed on CT and MRI. The cases with any mass involving more than half of the pancreas, regardless of lesion multiplicity, were considered diffuse type, and thus were excluded from the study. If diffuse or non-diffuse-type was equivocal, we referred to MRP and, if available, endoscopic retrograde pancreatography (ERP) findings. Among the 61 patients, histological diagnosis was performed for 38, and two had type 2 AIP [15].
From January 2013 to December 2015, 782 patients underwent curative-intent surgery for PDA in our institution (Fig. 1). To create a 1:2 matching with the AIP group, we randomly selected 122 (77 men and 45 women; mean age ± standard deviation, 62 ± 9.3 years [range, 40–83 years]) among the 564 patients with PDA who underwent both dynamic contrast-enhanced CT and MRI with MRP before surgery, using a commercially available random number generator (QuickCalcs, GraphPad Software).
Imaging examinations
CT techniques
The CT examinations were performed on 16-, 64- or 128-multidetector CT scanners (Somatom Sensation 16, Somatom Definition AS or AS+, Siemens Medical Systems; LightSpeed VCT, GE Healthcare), using dual-phase scanning, including the arterial and portal phases. The arterial and portal phase scans were obtained with a 15- to 25-s delay using a bolus-tracking technique and a fixed 70- to 80-s delay, respectively, after intravenous injection of 2–2.5 ml/kg of iopromide (Ultravist 370, Bayer Schering Pharma) at a rate of 3 ml/s, using an autoinjector. The parameters of the GE 64-multidetector scanner were a detector collimation of 0.625 mm, rotation time of 0.5 s, pitch of 0.98, tube voltage of 120 kV, automated tube current modulation with a noise index (200–400 mA) and reconstructed slice thickness of 2.5 mm. The parameters of the Siemens 16-, 64- and 128-multidetector scanner were detector collimations of 0.75, 0.6 and 0.6 mm, respectively; rotation time of 0.5 s; pitch of 1; tube voltage of 120 kV; automated dose modulation, using the maximum allowable tube current set at 200 mA; and reconstructed slice thickness of 3 mm.
MRI techniques
The MRI examinations were performed using a 1.5-T unit (Magnetom Avanto or Vision; Siemens Medical Solutions). The scan parameters were as follows: pre-contrast T1-weighted images with fat suppression using a fast low-angle shot (repetition time [TR]/echo time [TE], 224/2.5 ms; flip angle, 70°; section thickness, 6 mm; field of view [FOV], 240 × 350; matrix, 176 × 256); T2-weighted images with fat suppression using respiratory-triggered fast-spin echo (TR/TE, 3,600–5,100/96 ms; flip angle, 150°; echo train length, 13; section thickness, 6 mm; FOV, 240 × 350 mm; matrix, 264 × 384) or half-Fourier acquisition single-shot turbo spin-echo (TR/TE, infinite/154; flip angle, 150°; echo train length, 256; section thickness, 6 mm; FOV, 240 × 350 mm; matrix, 192 × 256); MRP with thick-slab (40 mm) images using single-shot rapid acquisition with relaxation enhancement sequence during one breath-hold (TR/TE, infinite/1,000 ms; flip angle, 180°; echo train length, 256; FOV, 300 × 300 mm; matrix, 320 × 320); and contrast-enhanced T1-weighted images with dynamic triple-phases (arterial, portal and delayed phase) using a fat-suppressed spoiled gradient-echo sequence (volumetric interpolated breath-hold examination; TR/TE, 4.1–4.2/1.5–1.7 ms; flip angle, 10°; section thickness, 4 mm; FOV, 280 × 350 mm; matrix, 256 × 320) at 10 s (arterial phase), 50 s (portal phase) and 3 min (delayed phase) after intravenous injection of gadobenate dimeglumine (MultiHance; Bracco SPA; 0.1 mmol/kg body weight) or gadoteric acid (Dotarem; Guerbet; 0.2 mmol/kg body weight) at a rate of 2 ml/s, using an autoinjector.
The time intervals between CT and MRI were 0–36 days (median, 5 days) in the AIP group and 0–34 days (median, 3 days) in the PDA group.
Image analysis
All images were reviewed using a local picture archiving and communication system monitor and digital imaging and communications in medicine imaging software. Two board-certified abdominal radiologists, who were highly experienced in diagnosing AIP and PDA, independently reviewed and rated the CT and MRI findings of all 183 patients (61 AIP and 122 PDA) in a random order. The radiologists were blinded to the clinical data, imaging results and final diagnosis, but were aware that the study population consisted of AIP and PDA. The raters used a 5-point scale to rate their confidence in differentiating between the two diseases for each CT and MRI finding: 1 – definite AIP; 2 – probable AIP; 3 – indeterminate; 4 – probable PDA; and 5 – definite PDA. A rating of 1 or 2 was considered to indicate a diagnosis of AIP; a rating of 3, indeterminate; and a rating of 4 or 5, PDA. They subjectively rated their confidence in the diagnosis without any given criteria. They considered both pancreatic and extrapancreatic (e.g. sclerosing cholangitis, renal involvement or retroperitoneal fibrosis in AIP) findings appropriately in the decision of diagnosis rating. The time interval between the reviews of CT and MRI was at least 1 month to avoid recall bias.
One month after completion of the diagnosis rating for all patients, the raters reviewed all the images again to determine, in consensus, the presence of the following key imaging features for differentiating between AIP and PDA for each CT and MRI finding: multiple pancreatic masses; discrete pancreatic mass (discernible focal hypoattenuating or hypointense mass, with visible border between the mass and the normal pancreas on unenhanced or contrast-enhanced images); delayed homogeneous enhancement of the pancreatic mass (hypoenhancement in the arterial phase and homogeneous iso- or hyperenhancement in the portal or delayed phase compared with the normal pancreas [16, 17]); halo sign (thin or thick, continuous, rim-like hypoenhancing soft tissue lesion outlining the pancreas); MPD stricture and its patterns (abrupt vs. tapered narrowing); multiple MPD strictures; marked upstream MPD dilatation (> 5 mm in maximum diameter); upstream pancreatic atrophy; and other organ involvement, including sclerosing cholangitis (intrahepatic or suprapancreatic extrahepatic bile duct), renal lesion [18, 19] and retroperitoneal fibrosis.
Laboratory data
The serum concentrations of immunoglobulin G (IgG), immunoglobulin G fraction 4 (IgG4) and carbohydrate antigen 19-9 (CA19-9), if available, were recorded.
Statistical analysis
Receiver operating characteristic (ROC) analysis was used to compare the diagnostic performance of CT and MRI, for each rater, in differentiating AIP from PDA. The sensitivity of CT and MRI for the diagnosis of AIP and PDA, as determined by each rater, was compared using McNemar’s test. To measure the agreement between the two raters in terms of confidence ratings for differentiating AIP from PDA and the diagnosis based on the confidence ratings, linear weighted κ values were calculated. A κ value greater than 0 indicated a positive correlation (κ<0.21, poor; κ=0.21–0.40, fair; κ=0.41–0.60, moderate; κ=0.61–0.80, good; and κ>0.80, excellent agreement) [20]. The frequency of the key imaging features for differentiating AIP from PDA were compared between CT and MRI using McNemar’s or the chi-squared test, as appropriate. P<0.05 was considered statistically significant. SPSS for Windows version 21.0 (IBM Corp.) and MedCalc for Windows version 12.5.0.0 (MedCalc) were used for the statistical analyses.
Results
Diagnostic performances of CT and MRI
The diagnostic performances of CT and MRI, including the results of ROC analysis and sensitivity for the diagnosis of AIP and PDA, are presented in Table 1. The area under the ROC curve (Az value) was significantly greater for MRI (0.993–0.995) than for CT (0.953–0.976) for both raters (p≤0.035). The sensitivities of MRI were higher than those of CT for the diagnosis of AIP (88.5–90.2% vs. 77–80.3%, p≤0.07) and PDA (97.5–99.2% vs. 91.8–94.3%, p≤0.031) for both raters, although the difference for AIP was statistically marginal (p=0.07) for rater 1.
The two raters agreed on the confidence ratings for differentiating AIP from PDA using MRI and CT in 79.2% (145/183) and 61.7% (113/183) of the cases, respectively, and the corresponding linear weighted κ value was higher for MRI (κ=0.858, excellent agreement) than for CT (κ=0.714, good agreement). The two raters agreed on the diagnosis of AIP and PDA using MRI and CT in 95.6% (175/183) and 86.9% (159/183) of the cases, respectively, and the corresponding linear weighted κ value for MRI (κ=0.923, excellent agreement) was also higher than that for CT (κ=0.806, excellent agreement).
Key imaging features on CT and MRI
The comparison of CT and MRI for the presence of key imaging features to differentiate between AIP and PDA is summarized in Table 2. For AIP, multiple pancreatic masses (39.3% vs. 21.3%), delayed homogeneous enhancement of the pancreatic mass (93.4% vs. 73.8%), MPD stricture (85.2% vs. 54.1%), and multiple MPD strictures (47.5% vs. 11.5%) were observed significantly more frequently using MRI than CT (p≤0.008). The frequencies of halo sign, MPD stricture pattern and other organ involvement in AIP did not significantly differ between CT and MRI (p≥0.317). For PDA, discrete pancreatic mass (87.7% vs. 75.4%), MPD stricture (86.1% vs. 78.7%) and upstream pancreatic atrophy (57.4% vs. 50.8%) were observed significantly more frequently using MRI than CT (p≤0.039). The frequencies of MPD stricture pattern and marked upstream MPD dilatation in PDA did not significantly differ between CT and MRI (p≥0.18). Representative cases are presented in Figs. 2 and 3.
A 63-year-old man with AIP. Arterial-phase CT (a–c) and pre-contrast T1-weighted MR (d–f) images show ill-defined mass-like swelling (arrows in a and d) in the pancreatic tail. The MR images demonstrate two small hypointense nodules (arrowheads) in the pancreatic body or tail (e) and head (f), which are not detectable on CT
A 62-year-old man with AIP. Arterial-phase CT (a–c) and pre-contrast T1-weighted MR (d–e) images show multifocal hypodense/hypointense lesions (arrows) in the pancreatic body and tail. The lesions appear more conspicuous on MRI than CT. The slightly dilated MPD (arrowhead) is barely visible on CT (b). Conversely, heavily T2-weighted axial MR image (f) and MRP image (g) demonstrate the unrevealed (on CT) signal of the slightly dilated MPD (arrowheads), indicating the multifocal strictures extremely well. Arterial (h) and delayed (i) phase MR images show delayed homogeneous enhancement in the pancreatic lesions (arrows)
Laboratory data
The serum concentrations of IgG (> 1,800 mg/dl), IgG4 (> 135 mg/dl) and CA 19-9 (> 37 U/ml) were elevated above the normal limit in 17.5% (10/57), 38.6 % (22/57) and 16.7% (10/61) of the patients with AIP, respectively. The serum CA19-9 level was elevated in 70.5% (86/122) of the patients with PDA. None of the patients with PDA exhibited elevated serum IgG (0/47) and IgG4 (0/46) levels.
Discussion
Our study shows that MRI provides better diagnostic performance than CT in differentiating non-diffuse-type AIP from PDA. The better diagnostic performance of MRI was due to the superiority of MRI over CT in demonstrating several key distinguishing features of the two diseases, particularly for AIP: multiple pancreatic masses, delayed homogeneous enhancement of the pancreatic mass and multiple MPD strictures. It is important to detect multiple pancreatic masses for differentiating AIP from PDA because this finding is highly specific for AIP, as demonstrated in our study as well as in the literature [5, 6]. The pancreatic mass observed in AIP usually appears as a mass-like swelling, without significant difference in the degree of contrast enhancement compared with the normal pancreas, unlike the discrete hypoenhancing mass observed in PDA. Moreover, CT inherently has poor soft-tissue contrast, and thus detection of subtle mass, particularly small lesion, in AIP is relatively difficult. Conversely, MRI has excellent soft-tissue contrast, and fat-suppressed pre-contrast T1-weighted images have outstanding ability to detect pancreatic mass, even small lesions. These factors may contribute to the large difference observed in this study between MRI (39.3%) and CT (21.3%) in demonstrating multiple pancreatic masses in AIP. In previous studies reporting CT or MRI findings of AIP, the multiplicity was observed more frequently on MRI (33–44%) [5, 6] than CT (6%) [4], which supports our results.
The diagnostic value of evaluating enhancement patterns using MRI in AIP was addressed in a previous study by Rehnitz et al. [7]. In their study, MRI was found to be significantly better than CT at revealing delayed enhancement of the pancreatic mass in AIP, and similar results were obtained in our study. These results may be attributed to the higher soft-tissue contrast of MRI, as suggested in the previous study. However, in our study, delayed homogeneous enhancement of the pancreatic mass on CT might presumably be slightly underestimated due to the lack of a 3-min delayed phase, unlike MRI.
The considerable difference between MRI (47.5%) and CT (11.5%) in evaluating multiple MPD strictures in AIP is a noteworthy result of our study. The CT images seem to have inherent limitations in depicting the MPD stricture in AIP, which mostly manifests as tapered rather than abrupt narrowing, with upstream MPD dilatation being absent or mild, considerably dissimilar to that in PDA, as shown in this study. Thus, it is not easy to detect MPD stricture itself using CT. Moreover, it may be even more difficult to detect the slightly dilated MPD between subtle masses in multifocal type AIP using CT. Conversely, on MRI, especially heavily T2-weighted cross-sectional or MRP images, MPD narrowing or non-visualization in strictured segment as well as the dilated upstream MPD, even if mild, would be easily perceivable because water or fluid is remarkably accentuated as a very bright signal, whereas the other structures appear hypointense and dark. The large difference in the detection of MPD stricture in AIP between MRI (85.2%) and CT (54.1%) can be understood in the same context. MRI, including MRP, is frequently used in the diagnosis of AIP since its various strengths have been demonstrated in many studies [5,6,7,8,9,10,11, 21]. Moreover, as a recent study showed promising results for MRP at high field strength, utilization of MRP in the diagnostic evaluation of AIP as an alternative to invasive ERP may increase further in the future [22].
Another importrant advantage of MRI revealed in our study was the higher inter-rater agreement in differentiating non-diffuse-type AIP and PDA than that for CT. We speculated that the relatively low inter-rater agreement of CT was most likely due to the inferiority in demonstrating the aforementioned key imaging features, which may make image interpretation more subjective than with MRI. In previous studies, inter-rater agreements with regard to the CT interpretation of AIP were modest and varied (κ=0.14–0.75), which supports our conjecture [23, 24]. On the other hand, there may be only a few radiologists who are highly experienced in the diagnosis of AIP as this disease is very rare, particularly the non-diffuse-type. Hence, inter-rater variability regarding the image interpretation of non-diffuse-type AIP may be unavoidable to some degree. Given the results of our study, nevertheless, such inter-rater variability is expected to be reduced by appropriate utilization of MRI with MRP in daily practice. Further, the potential benefit of diffusion-weighted MRI (DWI) in differentiating AIP from PDA has recently been highlighted, as the apparent diffusion coefficient values for AIP were consistently lower than those for PDA [5, 6, 10]. Therefore, a combination of conventional MRI with DWI may enhance its diagnostic capability.
Our results suggest that serum concentrations of IgG and IgG4 are also helpful for the differential diagnosis of AIP and PDA as they were not elevated in any of the patients with PDA. However, elevated serum concentrations of IgG (17.5%) and IgG4 (38.6%) were not sensitive for AIP, consistent with previous study results [25, 26]. Moreover, elevation of serum CA 19-9 was not specific for PDA as it was elevated in 16.7% of the patients with AIP. These limitations of laboratory tests make it even more crucial for the imaging modalities to accurately differentiate AIP from PDA.
Our study has a few limitations. Firstly, the blinded raters had participated in patient diagnosis in daily practice; hence, recall bias might have occurred. Secondly, there was a discrepancy in the study period between the two patient groups, i.e. 10 years for the AIP group versus 3 years for the PDA group, which may have caused variations in the quality of imaging examinations for the AIP group. The imaging quality of CT might presumably be affected more than that of MRI for the older cases, which may increase the difference in diagnostic performance between these two modalities. However, a long study period for the patients with AIP was inevitable due to the rarity of this disease. Thirdly, 25 patients (nine with AIP and 16 with PDA) underwent intervention procedures, including biliary stenting, diagnostic ERP, endoscopic ultrasonography-guided biopsy of the pancreatic mass and endoscopic nasobiliary drainage, between the CT and MRI examinations. However, we found no definite procedure/device-related changes/artifacts or only very minor changes, not affecting image interpretation. Therefore, there is very little chance that the study results were affected by the intervention procedures. Fourthly, as the two raters were restricted to selecting only between non-diffuse AIP and PDA, the difference in the diagnostic performances of CT and MRI reported in this study might be an over-representation of the real-world scenario where non-diffuse-type AIP is very rare and overlaps with other entities, such as non-AIP pancreatitis.
In conclusion, MRI has a better diagnostic performance than CT in differentiating non-diffuse-type AIP from PDA, which is due to the superiority of MRI over CT in demonstrating the key distinguishing features of both diseases, including multiple pancreatic masses, delayed homogeneous enhancement of the pancreatic mass and multiple MPD strictures.
Abbreviations
- AIP:
-
Autoimmune pancreatitis
- IgG:
-
Immunoglobulin G
- MPD:
-
Main pancreatic duct
- MRP:
-
Magnetic resonance pancreatography
- PDA:
-
Pancreatic ductal adenocarcinoma
References
Finkelberg DL, Sahani D, Deshpande V, Brugge WR (2006) Autoimmune pancreatitis. N Engl J Med 355:2670–2676
Chari ST, Takahashi N, Levy MJ et al (2009) A diagnostic strategy to distinguish autoimmune pancreatitis from pancreatic cancer. Clin Gastroenterol Hepatol 7:1097–1103
Kamisawa T, Imai M, Yui Chen P et al (2008) Strategy for differentiating autoimmune pancreatitis from pancreatic cancer. Pancreas 37:e62–e67
Kim JH, Kim MH, Byun JH et al (2012) Diagnostic Strategy for Differentiating Autoimmune Pancreatitis From Pancreatic Cancer: Is an Endoscopic Retrograde Pancreatography Essential? Pancreas 41:639–647
Choi SY, Kim SH, Kang TW, Song KD, Park HJ, Choi YH (2016) Differentiating Mass-Forming Autoimmune Pancreatitis From Pancreatic Ductal Adenocarcinoma on the Basis of Contrast-Enhanced MRI and DWI Findings. AJR Am J Roentgenol 206:291–300
Hur BY, Lee JM, Lee JE et al (2012) Magnetic resonance imaging findings of the mass-forming type of autoimmune pancreatitis: Comparison with pancreatic adenocarcinoma. J Magn Reson Imaging 36:188–197
Rehnitz C, Klauss M, Singer R et al (2011) Morphologic Patterns of Autoimmune Pancreatitis in CT and MRI. Pancreatology 11:240–251
Kim HJ, Kim YK, Jeong WK, Lee WJ, Choi D (2015) Pancreatic duct "Icicle sign" on MRI for distinguishing autoimmune pancreatitis from pancreatic ductal adenocarcinoma in the proximal pancreas. Eur Radiol 25:1551–1560
Negrelli R, Manfredi R, Pedrinolla B et al (2015) Pancreatic duct abnormalities in focal autoimmune pancreatitis: MR/MRCP imaging findings. Eur Radiol 25:359–367
Muhi A, Ichikawa T, Motosugi U et al (2012) Mass-forming autoimmune pancreatitis and pancreatic carcinoma: Differential diagnosis on the basis of computed tomography and magnetic resonance cholangiopancreatography, and diffusion-weighted imaging findings. J Magn Reson Imaging 35:827–836
Kim M, Jang KM, Kim JH et al (2017) Differentiation of mass-forming focal pancreatitis from pancreatic ductal adenocarcinoma: value of characterizing dynamic enhancement patterns on contrast-enhanced MR images by adding signal intensity color mapping. Eur Radiol 27:1722–1732
Furuhashi N, Suzuki K, Sakurai Y, Ikeda M, Kawai Y, Naganawa S (2015) Differentiation of focal-type autoimmune pancreatitis from pancreatic carcinoma: assessment by multiphase contrast-enhanced CT. Eur Radiol 25:1366–1374
Otsuki M, Chung JB, Okazaki K et al (2008) Asian diagnostic criteria for autoimmune pancreatitis: consensus of the Japan-Korea Symposium on Autoimmune Pancreatitis. J Gastroenterol 43:403–408
Chari ST, Smyrk TC, Levy MJ et al (2006) Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol 4:1010–1016 quiz 1934
Shimosegawa T, Chari ST, Frulloni L et al (2011) International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas 40:352–358
Carbognin G, Girardi V, Biasiutti C et al (2009) Autoimmune pancreatitis: imaging findings on contrast-enhanced MR, MRCP and dynamic secretin-enhanced MRCP. Radiol Med 114:1214–1231
Manfredi R, Frulloni L, Mantovani W, Bonatti M, Graziani R, Mucelli RP (2011) Autoimmune Pancreatitis: Pancreatic and Extrapancreatic MR Imaging-MR Cholangiopancreatography Findings at Diagnosis, after Steroid Therapy, and at Recurrence. Radiology 260:428–436
Kim B, Kim JH, Byun JH et al (2014) IgG4-related kidney disease: MRI findings with emphasis on the usefulness of diffusion-weighted imaging. Eur J Radiol 83:1057–1062
Seo N, Kim JH, Byun JH, Lee SS, Kim HJ, Lee MG (2015) Immunoglobulin G4-Related Kidney Disease: A Comprehensive Pictorial Review of the Imaging Spectrum, Mimickers, and Clinicopathological Characteristics. Korean J Radiol 16:1056–1067
Kundel HL, Polansky M (2003) Measurement of observer agreement. Radiology 228:303–308
Park SH, Kim MH, Kim SY et al (2010) Magnetic resonance cholangiopancreatography for the diagnostic evaluation of autoimmune pancreatitis. Pancreas 39:1191–1198
Kim JH, Byun JH, Kim MH et al (2017) Pancreatic Duct in Autoimmune Pancreatitis: Intraindividual Comparison of Magnetic Resonance Pancreatography at 1.5 T and 3.0 T. Pancreas 46:921–926
Takahashi N, Fletcher JG, Fidler JL, Hough DM, Kawashima A, Chari ST (2008) Dual-phase CT of autoimmune pancreatitis: a multireader study. AJR Am J Roentgenol 190:280–286
Zaheer A, Singh VK, Akshintala VS et al (2014) Differentiating autoimmune pancreatitis from pancreatic adenocarcinoma using dual-phase computed tomography. J Comput Assist Tomogr 38:146–152
Frulloni L, Scattolini C, Falconi M et al (2009) Autoimmune pancreatitis: differences between the focal and diffuse forms in 87 patients. Am J Gastroenterol 104:2288–2294
Song TJ, Kim MH, Moon SH et al (2010) The combined measurement of total serum IgG and IgG4 may increase diagnostic sensitivity for autoimmune pancreatitis without sacrificing specificity, compared with IgG4 alone. Am J Gastroenterol 105:1655–1660
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Jin Hee Kim.
Conflict of interest
The authors of this article declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Rights and permissions
About this article
Cite this article
Lee, S., Kim, J.H., Kim, S.Y. et al. Comparison of diagnostic performance between CT and MRI in differentiating non-diffuse-type autoimmune pancreatitis from pancreatic ductal adenocarcinoma. Eur Radiol 28, 5267–5274 (2018). https://doi.org/10.1007/s00330-018-5565-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5565-1