Abstract
Purpose
To explore the possibility of determining majority via a morphology-based examination of the epiphyseal-diaphyseal fusion by 3.0 T magnetic resonance imaging (MRI), a prospective cross-sectional study developing and applying a new stage classification was conducted.
Materials and methods
344 male and 350 female volunteers of German nationality between the ages of 12-24 years were scanned between May 2013 and June 2015. A 3.0 T MRI scanner was used, acquiring a T1-weighted (T1-w) turbo spin-echo sequence (TSE) and a T2-weighted (T2-w) TSE sequence with fat suppression by spectral pre-saturation with inversion recovery (SPIR). The gathered information was sifted and a five-stage classification was formulated as a hypothesis. The images were then assessed using this classification. The relevant statistics were defined, the intra- and interobserver agreements were determined, and the differences between the sexes were analysed.
Results
The application of the new classification made it possible to correctly assess majority in both sexes by the examination of the epiphyses of the knee joint. The intra- and interobserver agreement levels were very good (κ > 0.80). The Mann-Whitney-U Test implied significant sex-related differences for most stages.
Conclusion
Applying the presented MRI classification, it is possible to determine the completion of the 18th year of life in either sex by 3.0 T MRI of the knee joint.
Key points
• Based on prospective referential data a new MRI classification was formulated.
• The setting allows assessment of the age of an individual’s skeletal development.
• The classification scheme allows the reliable determination of majority in both sexes.
• The staging shows a high reproducibility for instructed and trained professional personnel.
• The proposed classification is likely to be adaptable to other long bone epiphyses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In applied forensic age assessment in the living, one key interest lies in the reliable proof of an individual’s majority [1]. In most European countries majority is attained by the completion of the 18th year of life [2]. Over the course of a human’s skeletal development, age-related morphological changes take place. These changes can be and have been examined by multiple modalities [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. The current recommendations by the Study Group on Forensic Age Diagnostics (AGFAD) [28] are based on ionising radiation, namely orthopantomograms, plain x-rays of the hand and, if need be, computed tomographies of the medial clavicular epiphyses [28], following the “minimum-age concept” [1].
This means that in judicial proceedings, in which it is to be proved with the highest level of certainty that the legally relevant age threshold is surpassed, the chronological age of the youngest individual of the referential study showing the same developmental stage as the examined individual is assumed to be the individual’s minimum age. This is to prevent a falsely overestimated chronological age and will almost always lead to a lower estimated age than the actual age. Therefore, minimum ages of morphological characteristics are of the utmost importance to examinations in forensic age assessment.
Magnetic resonance imaging (MRI) has not been implemented into the AGFAD’s recommendations, despite the fact that the general feasibility has already been shown [20]. Even several original staging systems have already been proposed [16, 17] and adaptions of preexisting classifications have been attempted [18, 19]. So far, all these approaches failed to meet the requirements of the AGFAD’s recommendations.
Therefore, our scientific working group decided to conduct a prospective cross-sectional study with a sufficient cohort to explore the possibility of determining majority via a morphology-based examination of the epiphyseal-diaphyseal fusion of the knee-joint by 3.0 T MRI. In a first step, a most precise depiction of the osseous structures was achieved with a T1-weighted (T1-w) turbo spin-echo sequence (TSE). This provided an anatomical image of the bony structures [29], with which it was possible to rule out general atypia or pathological changes to the bone itself. In case of a finished epiphyseal-diaphyseal bony fusion, it then served as a template on which to locate the exact position of the former growth plate. In a second step, a T2-weighted (T2-w) TSE sequence with fat suppression by spectral pre-saturation with inversion recovery (SPIR) provided information about stationary watery components within the previously depicted osseous structures and/or in cartilaginous parts, while discriminating other soft tissues and especially fat. This was done under the assumption that previously found T2-hyperintense signals [16, 17] could resemble a waning watery residuum of the fusion’s process. It was also expected that the sole acquisition of the specific T1-w sequence would not suffice as could be derived from earlier studies [27].
Materials and methods
The study uses a data set that was acquired by the European study “Age estimation in unaccompanied minors by means of MRI”. Multiple regions of interest of several hundreds of volunteers were scanned and are currently being analysed. Due to this, parts of the cohort’s results have already been reported concerning different approaches or other regions of interest [20, 27, 30, 31].
After the responsible ethics committee had voted in favour of the study, the recruitment of the volunteers via advertisement in local media and the internet was begun. Each of the volunteers or their legal guardian, in case of minors, had signed a written consent prior to their examination.
Between May 2013 and June 2015 344 male and 350 female volunteers of German nationality in the age bracket 12-24 years were prospectively scanned (see Table 1). Noted characteristics of the volunteers included sex, proven age, known illnesses as well as past and present medication. The participants were to be distributed evenly across groups of up to 25 per year of age and sex. Exclusion criteria for the recruitment and the examination were the presence of skeletal development-relevant diseases and/or disorders, trauma to the knee joint area and the common contra-indications of magnetic resonance imaging, especially incorporated metal elements, recent surgical procedures, freshly implanted vascular clips, claustrophobia and potential pregnancy. The knee joint was chosen as the region of interest (ROI) since earlier studies suggested its epiphyses to possibly show relevant morphological changes around the 18th year of life in both sexes [16,17,18,19, 32]. In case of exclusion criteria concerning the left knee joint (e.g. trauma or implant) there was the possibility to switch to the right knee joint.
The MRI scans were performed on a 3.0 T scanner (Philips 3.0 T Achieva, gradients 80 mT/m; Philips Medical Systems, Amsterdam, The Netherlands) using a high-resolution surface coil (Sense Flex M, Philips Medical Systems, Amsterdam, The Netherlands). The scans were repeated in case of motion artefacts.
A T1-w TSE sequence in coronal orientation was used (TR 633 ms; TE 20 ms; flip angle 90 degree; duration 3:51 min; measured voxel size 0.6 × 0.77 × 3 mm; reconstructed voxel size 0.31 × 0.31 × 3 mm). Furthermore, an additional T2-w TSE SPIR sequence in coronal orientation was used (TR shortest; TE 65 ms; flip angle 90 degree; duration 3:08 min; measured voxel size 0.6 × 0.76 × 3 mm; reconstructed voxel size 0.31 × 0.31 × 3 mm).
The images were viewed on a PACS workstation, first evaluated by an examiner with experience in musculoskeletal MRI diagnostics (C.O.) and sifted for characteristic morphological landmarks on which to base a classification.
The formulated classification then was applied to the acquired images to evaluate its capabilities in assessing majority. For determining the intraobserver agreement a re-evaluation of 100 randomly chosen cases was performed after a lapse of 2 months to prevent a recall bias. A second examiner with more than 20 years of experience in musculoskeletal MRI diagnostics (V.V.) also evaluated the same group of 100 cases for determining the interobserver agreement. The evaluations were performed without knowledge of sex, age and earlier evaluations of the examined volunteers.
Statistical analyses were conducted using IBM SPSS Statistics 24 (Build 1.0.0.407) for Mac OS X (release 15/03/2016, IBM Corp.). Minimum, maximum, mean ± standard deviation and median with lower and upper quartiles were defined for each stage of the classification to find the minimum ages of onset. Intra- and interobserver agreements were determined by calculating the kappa coefficients. Sex-related differences in the stage assessment across the ages were analysed using the Mann-Whitney-U Test to determine their statistical relevance (p < 0.05, exact, two-tailed).
Results
In all cases, the setting provided evaluable images. Especially motion artefacts did not pose a problem.
Cohort
We prospectively scanned and evaluated 694 volunteers, ranging from 12.05 years to 24.98 years of age. For male volunteers the median age at the point of examination was 18.80 years, while the median age for female volunteers was 18.97 years. In a total of 14 cases the examination was performed on the right knee (2.02%) because of the presence of contra-indications concerning the left knee joint.
Examination
A T1-w TSE sequence was utilised to acquire images aiming for osseous structures. An additional T2-w TSE SPIR sequence was utilised to acquire images aiming at stationary watery components within the previously depicted osseous structures and/or in cartilaginous parts. The examinations took approximately 10 to 15 min to complete, including positioning.
MRI classification
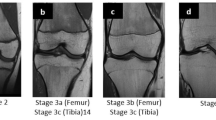
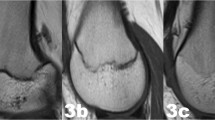
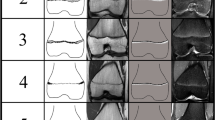
The examinations of the volunteers were sorted separately for both sexes from youngest to oldest. In a first step, all slices of each examination were then looked through to get an overview of the general morphology of the growth plate and the process of its ossification. This was done without knowledge of the exact age of the volunteers. In a second step, sketches of the found characteristics and changes of the growth plate were drawn for both sequences. Here, the separation by sex was dropped since unlike the chronology of the ossification the morphology of the growth plate showed no sex-related differences. Only characteristics that could be found alike in most cases of similar age were taken into account. At this point a distinct continuum of the growth plate’s development could be recognised. The sketches were then sifted for easily identifiable changes, i.e. the presence or absence of certain features. These morphological features were then put into an ascending order and the following staging system was formulated as a hypothesis (see Figs. 1 and 2 for the corresponding schematics and examples; see Table 2 for the shortened version):
Schematic drawings for the stages of the T2 TSE SPIR sequence and examples (3.0 T; non-contrast enhanced; coronal slice orientation); from left to right: male 13 years, male 14 years, female 16 years, male 20 years, female 22 years. MRI magnetic resonance imaging; TSE turbo spin echo; SPIR signal pre-saturation with inversion recovery
Stage 2: In the T1-w sequence a continuous band of intermediate signal intensity is visible, walled by serrated lines of low to no signal intensity towards the epiphysis and the diaphysis.
In the T2-w sequence the epiphysis is demarcated by a serrated line of low to no signal intensity. The metaphysis shows two serrated lines of high signal intensity. Both lines can be continuous or discontinuous.
Stage 3: In the T1-w sequence a discontinuous band of intermediate signal intensity is visible. The band is walled by serrated lines of low to no signal intensity towards the epiphysis and the diaphysis that sporadically convene and interrupt the band, forming a single serrated line with no signal intensity.
In the T2-w sequence the metaphysis shows two serrated lines of high signal intensity that sporadically convene, forming a single thin and serrated line of high signal intensity.
Stage 4: In the T1-w sequence a discontinuous thin and serrated line of intermediate signal intensity between the epiphysis and the diaphysis is visible. In the continuity of the line, thicker sections with no signal intensity can be seen.
In the T2-w sequence a thin single, discontinuous or dotted line of hyperintense signal is visible in the same position as the described thin line of the corresponding T1-w sequence. In the continuity of the line, thicker hyperintense sections can be seen.
Stage 5: In the T1-w sequence a continuous thin line of intermediate signal intensity between the epiphysis and the diaphysis is visible.
The T2-w sequence shows a single thin, discontinuous or dotted line of hyperintense signal in the same position as the described thin line of the corresponding T1-w sequence.
Stage 6: In the T1-w sequence a continuous thin line of intermediate signal intensity between the epiphysis and the diaphysis is visible.
The T2-w sequence shows no hyperintense signal in the same position as the described thin line of the corresponding T1-w sequence.
The stages are based on the presence or absence of certain landmarks of the epiphyseal-diaphyseal fusion’s morphological appearance, mainly drawn from the T1-w sequence. In the T1-w sequence they are characterised by the presence of a continuous band-like morphology of the growth plate (stage 2), the presence of a discontinuous band-like morphology (stage 3), the beginning thin-lined demarcation of the fusion’s “scar” (stage 4), the completely demarcated fusion’s “scar” with a corresponding T2-hyperintense signal (stage 5) and finally without a corresponding T2-hyperintense signal (stage 6). Stages 5 and 6 have the exact same appearance in the T1-w sequence and cannot be further differentiated without the T2-w sequence.
It was possible to assess a respective stage of the distal femoral epiphysis and the proximal tibial epiphysis in all cases (694). The evaluation took below 1 min per case.
Observed minimum ages in the distal femoral epiphysis
In males, the minimum ages for stages 2, 3, 4, 5 and 6 of the epiphyseal-diaphyseal fusion of the distal femoral epiphysis were 12.05, 12.13, 15.49, 15.71 and 21.24 years, respectively (compare Table 3).
In females, the minimum ages for stages 2, 3, 4, 5 and 6 of the epiphyseal-diaphyseal fusion of the distal femoral epiphysis were 12.11, 12.16, 14.33, 14.82 and 20.65 years, respectively (compare Table 4).
Observed minimum ages in the proximal tibial epiphysis
In males, the minimum ages for stages 2, 3, 4, 5 and 6 of the epiphyseal-diaphyseal fusion of the proximal tibial epiphysis were 12.05, 12.13, 14.68, 15.71 and 19.85 years, respectively (compare Table 5).
In females, the minimum ages for stages 2, 3, 4, 5 and 6 of the epiphyseal-diaphyseal fusion of the proximal tibial epiphysis were 12.56, 12.11, 12.48, 14.44 and 17.65 years, respectively (compare Table 6).
Intra- and interobserver agreement
After calculating Cohen’s kappa, we found a very good intraobserver agreement level concerning the distal femoral epiphysis (κ = 0.914) and the proximal tibial epiphysis (κ = 0.893).
After calculating Cohen’s kappa, we found a very good interobserver-agreement level concerning the distal femoral epiphysis (κ = 0.913) and the proximal tibial epiphysis (κ = 0.847).
Statistical differences of the sexes
The performed Mann-Whitney-U Test concerning the distal femoral epiphysis implies significant sex-related differences for most stages: stage 2 (p < 0.02), stage 3 (p < 0.01), stage 4 (p < 0.01), stage 5 (p < 0.01) and stage 6 (p = 0.46).
The performed Mann-Whitney-U Test concerning the proximal tibial epiphysis implies significant sex-related differences for most stages: stage 2 (p = 0.92), stage 3 (p < 0.01), stage 4 (p < 0.01), stage 5 (p < 0.02) and stage 6 (p < 0.03).
Discussion
The new classification enables us to determine majority in both sexes. Following the “minimum-age concept” [1], this is possible in males via stage 6 of the distal femoral epiphysis, as the youngest volunteer in this stage was 21.24 years old. Likewise, it is possible via stage 6 of the proximal tibial epiphysis, as the youngest male volunteer in this stage was 19.85 years old. In females, we can determine majority via stage 6 of the distal femoral epiphysis, since the youngest volunteer in this stage was 20.65 years old. However, the majority cannot be determined in females via the evaluation of the proximal tibial epiphysis, because the youngest volunteer in the final stage 6 was 17.65 years old.
The results also show a harmonic increase of ages of onset for stages 4-6 across all examined epiphyses, which is in concordance with comparable studies [16,17,18,19, 27]. This implicates that the classification in fact captures the progression of epiphyseal-diaphyseal ossification and fusion as expected.
However, the results also show a statistical bias, i.e. a selection bias, concerning some minimum and maximum ages. The maximum ages for stages 5 and 6 of both epiphyses in both sexes are superimposed, in that the maximum age of stage 6 lies below that of stage 5. This is most likely due to the cohort’s chosen upper cut-off of 24 years of life in combination with the low number of cases displaying stage 6. Bearing in mind that a continuum of ossification and fusion was observed, the actual maximum age of stage 6 will lie above that of stage 5. Nonetheless, it can be assumed that the lower extremes of stage 6 were captured.
Furthermore, the minimum ages of stages 2 and 3 that lie close to 12 years are most likely determined by the cohort’s chosen lower cut-off of 12 years of life. These should therefore be considered artificial and the actual minimum ages likely lie below the documented range. Additionally, the age groups of 12 years and 14 years in males and of 12 years and 13 years in females could not be filled sufficiently. Since these age groups would presumably have displayed stages 2 and 3 a recruitment bias must also be suspected here. In general, the minimum ages bordering 12 years of life and the maximum ages bordering 24 years of life as well as derived data of those values are most likely influenced by the cohort’s age range and/or the recruitment.
The benefit of the “minimum-age concept” is that it does not take the artificial upper limits of stages 5 and 6 into account. The only relevant values are the minimum ages of the respective stages.
The practical application showed high reproducibility for instructed and trained professional personnel, with very good intra- and interobserver agreement levels.
Overall, female individuals displayed an earlier onset of the progression of the skeletal development, which is in concordance with similar studies [16,17,18,19].
The reasoning behind a side preference of the knee joint was to get the best possible visual comparability within the cohort. Cases in which the right knee joint was additionally scanned showed no differences in the analyses.
The classification itself has to be compared to those of Jopp et al. [17] and Dedouit et al. [16] and to the adaption of the classification by Schmeling et al. [27]. The one by Jopp et al. is based solely on the epiphyseal-diaphyseal osseous fusion. It is derived from only a small, retrospectively analysed sample with incomplete knowledge of the circumstances of image acquisition, as Jopp et al. stated themselves [17], and thereby unverified. The approach by Dedouit et al. is mainly based on the absolute measurable thickness of the growth plate layer(s). As the physical dimensions of anatomical structures are associated with an individual’s body height, they are however unsuited for absolute measurement. The classification of Schmeling et al. [23] with the additional sub-stages of Kellinghaus et al. [25] can be applied to the specific T1-w sequence that was used in this study, but it does not take into account the watery components and soft tissues of the osseous structures. Thereby its application does not allow the determination of majority in either sex [27].
The new classification starts with stage 2 as the chosen cut-off of the age brackets (12-24 years) and does not cover the earliest process of epiphyseal ossification. Therefore, room was left for earlier stages that might be observed over the course of additional studies.
However, while the features of stages 2-4 can be attributed to the commonly known anatomical structures of the growth plate, the origin of the faint hyperintense signal in the T2-w SPIR sequence remains unclear. Further anatomical studies are needed to find the precise physical correlate.
Concerning limitations, the restriction of the cohort to volunteers of German nationality implies a limited applicability of the results. However, from earlier studies we know that the Western Caucasian ethnicity with high socio-economic status shows the fastest progression through the ossification process [33, 34]. This means that the found minimum ages will rather lead to an underestimation of age when applied to other ethnicities, especially to those of lower socio-economic status.
Due to the dependence of MRI on the setting, the classification is limited to 3-T scanners and the given sequences.
Abbreviations
- MRI:
-
Magnetic resonance imaging
- SPIR:
-
Signal pre-saturation with inversion recovery
- T1-w:
-
T1-weighted
- T2-w:
-
T2-weighted
- TSE:
-
Turbo spin-echo
References
Schmeling A, Dettmeyer R, Rudolf E, Vieth V, Geserick G (2016) Forensic age estimation. Dtsch Arztebl Int 113:44–50
Jayaraman J, Roberts GJ, Wong HM, McDonald F, King NM (2016) Ages of legal importance: Implications in relation to birth registration and age assessment practices. Med Sci Law 56:77–82
Schmidt S, Schiborr M, Pfeiffer H, Schmeling A, Schulz R (2013) Sonographic examination of the apophysis of the iliac crest for forensic age estimation in living persons. Sci Justice 53:395–401
Schmidt S, Schiborr M, Pfeiffer H, Schmeling A, Schulz R (2013) Age dependence of epiphyseal ossification of the distal radius in ultrasound diagnostics. Int J Legal Med 127:831–838
Schulz R, Schiborr M, Pfeiffer H, Schmidt S, Schmeling A (2013) Sonographic examination on the time frame of ossification of the distal fibula epiphysis. Arch Kriminol 231:156–165
Schulz R, Schiborr M, Pfeiffer H, Schmidt S, Schmeling A (2013) Sonographic assessment of the ossification of the medial clavicular epiphysis in 616 individuals. Forensic Sci Med Pathol 9:351–357
Schulz R, Schiborr M, Pfeiffer H, Schmidt S, Schmeling A (2014) Forensic age estimation in living subjects based on ultrasound examination of the ossification of the olecranon. J Forensic Leg Med 22:68–72
Schulz R, Zwiesigk P, Schiborr M, Schmidt S, Schmeling A (2008) Ultrasound studies on the time course of clavicular ossification. Int J Legal Med 122:163–167
Schmidt S, Muhler M, Schmeling A, Reisinger W, Schulz R (2007) Magnetic resonance imaging of the clavicular ossification. Int J Legal Med 121:321–324
Hillewig E, Degroote J, Van der Paelt T et al (2013) Magnetic resonance imaging of the sternal extremity of the clavicle in forensic age estimation: towards more sound age estimates. Int J Legal Med 127:677–689
Hillewig E, De Tobel J, Cuche O, Vandemaele P, Piette M, Verstraete K (2011) Magnetic resonance imaging of the medial extremity of the clavicle in forensic bone age determination: a new four-minute approach. Eur Radiol 21:757–767
Vieth V, Schulz R, Brinkmeier P, Dvorak J, Schmeling A (2014) Age estimation in U-20 football players using 3.0 Tesla MRI of the clavicle. Forensic Sci Int 241:118–122
Dvorak J, George J, Junge A, Hodler J (2007) Age determination by magnetic resonance imaging of the wrist in adolescent male football players. Br J Sports Med 41:45–52
Dvorak J, George J, Junge A, Hodler J (2007) Application of MRI of the wrist for age determination in international U-17 soccer competitions. Br J Sports Med 41:497–500
Wittschieber D, Vieth V, Timme M, Dvorak J, Schmeling A (2014) Magnetic resonance imaging of the iliac crest: age estimation in under-20 soccer players. Forensic Sci Med Pathol 10:198–202
Dedouit F, Auriol J, Rousseau H, Rouge D, Crubezy E, Telmon N (2012) Age assessment by magnetic resonance imaging of the knee: a preliminary study. Forensic Sci Int 217(232):e231–e237
Jopp ESI, Maas R, Adam G, Püschel K (2010) Proximale Tibiaepiphyse im Magnetresonanztomogramm. Neue Möglichkeit zur Altersbestimmung bei Lebenden? [Proximal tibial epiphysis in magnetic resonance imaging. New possibility for age estimation of living persons?]. Rechtsmedizin 20:464–468
Kramer JA, Schmidt S, Jurgens KU, Lentschig M, Schmeling A, Vieth V (2014) The use of magnetic resonance imaging to examine ossification of the proximal tibial epiphysis for forensic age estimation in living individuals. Forensic Sci Med Pathol 10:306–313
Kramer JA, Schmidt S, Jurgens KU, Lentschig M, Schmeling A, Vieth V (2014) Forensic age estimation in living individuals using 3.0 T MRI of the distal femur. Int J Legal Med 128:509–514
Ottow C, Krämer JA, Olze A et al (2014) Magnetresonanztomographiestudie zur Altersschätzung von unbegleiteten minderjährigen Flüchtlingen [Magnetic resonance tomography studies on age estimation of unaccompanied minor refugees]. Rechtsmedizin 25:12–20
Tangmose S, Jensen KE, Villa C, Lynnerup N (2014) Forensic age estimation from the clavicle using 1.0T MRI--preliminary results. Forensic Sci Int 234:7–12
Terada Y, Kono S, Tamada D et al (2013) Skeletal age assessment in children using an open compact MRI system. Magn Reson Med 69:1697–1702
Schmeling A, Schulz R, Reisinger W, Muhler M, Wernecke KD, Geserick G (2004) Studies on the time frame for ossification of the medial clavicular epiphyseal cartilage in conventional radiography. Int J Legal Med 118:5–8
Demirjian A, Buschang PH, Tanguay R, Patterson DK (1985) Interrelationships among measures of somatic, skeletal, dental, and sexual maturity. Am J Orthod 88:433–438
Kellinghaus M, Schulz R, Vieth V, Schmidt S, Pfeiffer H, Schmeling A (2010) Enhanced possibilities to make statements on the ossification status of the medial clavicular epiphysis using an amplified staging scheme in evaluating thin-slice CT scans. Int J Legal Med 124:321–325
Tomei E, Sartori A, Nissman D et al (2014) Value of MRI of the hand and the wrist in evaluation of bone age: preliminary results. J Magn Reson Imaging 39:1198–1205
Ottow C, Schulz R, Pfeiffer H, Heindel W, Schmeling A, Vieth V (2017) Forensic age estimation by magnetic resonance imaging of the knee: the definite relevance in bony fusion of the distal femoral- and the proximal tibial epiphyses using closest-to-bone T1 TSE sequence. Eur Radiol. 27:5041–5048
(AGFAD) Arbeitsgemeinschaft für Forensische Altersdiagnostik. uni-muenster.de/agfad_start.html. Accessed 02.27.2017 2017
Vieth V, Link TM, Lotter A et al (2001) Does the trabecular bone structure depicted by high-resolution MRI of the calcaneus reflect the true bone structure? Invest Radiol 36:210–217
Guo Y, Olze A, Ottow C et al (2015) Dental age estimation in living individuals using 3.0 T MRI of lower third molars. Int J Legal Med 129:1265–1270
Timme M, Ottow C, Schulz R et al (2017) Magnetic resonance imaging of the distal radial epiphysis: a new criterion of maturity for determining whether the age of 18 has been completed? Int J Legal Med 131:579–584
Saint-Martin P, Rerolle C, Pucheux J, Dedouit F, Telmon N (2015) Contribution of distal femur MRI to the determination of the 18-year limit in forensic age estimation. Int J Legal Med 129:619–620
Schmeling A, Reisinger W, Loreck D, Vendura K, Markus W, Geserick G (2000) Effects of ethnicity on skeletal maturation: consequences for forensic age estimations. Int J Legal Med 113:253–258
Schmeling A, Schulz R, Danner B, Rosing FW (2006) The impact of economic progress and modernization in medicine on the ossification of hand and wrist. Int J Legal Med 120:121–126
Acknowledgements
The study was funded by the European Refugee Fund, the German Federal Office for Migration and Refugees and the Westphalian Wilhelms-University of Münster, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Andreas Schmeling.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in:
Ottow C, Krämer JA, Olze A, et al. Magnetresonanztomographiestudie zur Altersschätzung von unbegleiteten minderjährigen Flüchtlingen [Magnetic resonance tomography studies on age estimation of unaccompanied minor refugees]. Rechtsmedizin. 2014; 25(1):12-20.
Ottow C, Schulz R, Pfeiffer H, Heindel W, Schmeling A, Vieth V (2017) Forensic age estimation by magnetic resonance imaging of the knee: the definite relevance in bony fusion of the distal femoral- and the proximal tibial epiphyses using closest-to-bone T1 TSE sequence. Eur Radiol 27:5041-5048
Guo Y, Olze A, Ottow C, et al. Dental age estimation in living individuals using 3.0 T MRI of lower third molars. Int J Legal Med. 2015;129(6):1265-70.
Timme M, Ottow C, Schulz R, et al. Magnetic resonance imaging of the distal radial epiphysis: a new criterion of maturity for determining whether the age of 18 has been completed? Int J Legal Med. 2017;131(2):579-84.
Methodology
• prospective
• cross-sectional study
• performed at one institution
Rights and permissions
About this article
Cite this article
Vieth, V., Schulz, R., Heindel, W. et al. Forensic age assessment by 3.0T MRI of the knee: proposal of a new MRI classification of ossification stages. Eur Radiol 28, 3255–3262 (2018). https://doi.org/10.1007/s00330-017-5281-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5281-2