Abstract
Objectives
To investigate the usefulness of Simultaneous Non-contrast Angiography and intraPlaque haemorrhage (SNAP) imaging in characterising carotid intraplaque haemorrhage (IPH) compared with magnetisation-prepared rapid acquisition gradient-echo (MP-RAGE) sequence.
Methods
Fifty-four symptomatic patients (mean age: 63.1 ± 5.7 years, 38 males) with carotid atherosclerosis were recruited and underwent carotid MR imaging. The presence and area of IPH on SNAP and MP-RAGE images were determined. The agreement in identifying IPH and its area between SNAP and MP-RAGE was analysed.
Results
Of 1368 slices with acceptable image quality in 54 patients, 13% and 22.6% were found to have IPH on MP-RAGE and SNAP images, respectively. There was moderate agreement between MP-RAGE and SNAP sequences in identifying IPH (κ = 0.511, p = 0.029). The area of IPH on SNAP images was significantly larger than that on MP-RAGE images (17.9 ± 18.2 mm2 vs. 9.2 ± 10.5 mm2, p < 0.001). For IPHs detected by SNAP imaging, the area of IPHs also detected by the MP-RAGE sequence was significantly larger than that of IPHs not detected by the MP-RAGE sequence (17.9 ± 19.2 mm2 vs. 6.4 ± 6.2 mm2, p < 0.001).
Conclusion
Compared with the MP-RAGE sequence, SNAP imaging detects more IPHs, particularly for smaller IPHs, suggesting that SNAP imaging might be a more sensitive tool for identification of carotid haemorrhagic plaques.
Key Points
• Moderate agreement was found between SNAP and MP-RAGE in identification of IPH
• SNAP imaging might be a more sensitive tool to detect carotid IPHs
• Compared with the MP-RAGE sequence, SNAP imaging can detect carotid IPHs with smaller size
• SNAP imaging can help clinicians to optimise the treatment strategy
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It has been shown that intraplaque haemorrhage (IPH) of carotid artery atherosclerotic plaque, as one of the key vulnerable features, can accelerate plaque progression [1, 2] and increase the risk of ischaemic cerebrovascular events [2,3,4,5,6]. A study by Noguchi et al. showed that high intensity in carotid plaques on magnetisation-prepared rapid acquisition gradient-echo (MP-RAGE) imaging, which may represent IPH, was significantly associated with cardiac events [7]. In addition, investigators found that, for patients with carotid plaques with IPH, the stenting therapy group developed more new ipsilateral silent ischaemic lesions than the carotid endarterectomy (CEA) group, suggesting CEA might be a better treatment for carotid plaques with IPH [8]. Therefore, accurate identification of carotid IPH prior to onset of cerebrovascular events is important for prevention of stroke and optimising the treatment strategy.
Currently, the assessment of carotid IPH is mainly based on the T1-weighted magnetic resonance (MR) imaging techniques, such as time of flight (TOF), T1-weighted (T1W) and MP-RAGE imaging sequences. Among these T1-weighted sequences, MP-RAGE has been demonstrated to have the highest sensitivity and accuracy in detecting carotid IPH validated by histology [9]. Most recently, Yao et al. utilised apparent diffusion coefficient (ADC) measurement on diffusion-weighted imaging to detect carotid artery IPH without histological validation [10]. Recently, Wang et al. proposed the Simultaneous Non-contrast Angiography and intraPlaque haemorrhage (SNAP) imaging sequence, which allows non-contrast MR angiography and identification of IPH simultaneously [11]. The feasibility of SNAP imaging in evaluating carotid IPH has been preliminarily reported [11]. However, the usefulness of SNAP imaging in identification of carotid IPH has not been validated in vivo. This study sought to investigate the usefulness of SNAP imaging in characterising carotid IPH by comparing it with MP-RAGE sequence.
Materials and methods
Study population
In total, 64 patients [mean age, 68 years ± 9.5 (SD); age range, 29-82 years] with recent cerebrovascular symptoms (< 2 weeks) and carotid atherosclerotic disease (> 30% stenosis) determined by ultrasound from November 2012 to April 2016 were recruited and underwent MR imaging for carotid arteries. The exclusion criteria include carotid occlusion and contraindications to MR imaging examination. The clinical characteristics such as age, gender, history of smoking, hypertension, diabetes, and stroke were collected from the clinical records. The levels of high-density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol (TC) and triglycerides (TG) were also recorded. The study protocol was approved by the institutional review board and a written consent form was obtained from all subjects.
Carotid MR imaging
Carotid MR imaging was performed on a whole-body 3.0-T MR scanner (Achieva TX, Philips Healthcare, Best, The Netherlands) with dedicated eight-channel carotid coil. The carotid arteries were imaged using a multicontrast vessel wall imaging protocol with the following parameters: (1) three-dimensional time of flight (3D TOF): fast field echo (FFE), TR/TE 20/4 ms, flip angle 20°; (2) quadruple-inversion-recovery T1-weighted imaging (T1W QIR): turbo spin echo (TSE), TR/TE 800/10 ms, flip angle 20°; (3) multislice double inversion-recovery T2-weighted imaging (T2W MDIR): TSE, TR/TE 4800/50 ms, flip angle 90°: (4) MP-RAGE: fast field echo (FFE), TR/TE 8.8/5.3 ms, flip angle 15°; (5) SNAP: fast field echo (FFE), TR/TE 89.9/4.8 ms, flip angle 11/15°. The TOF, T1W, T2W, and MP-RAGE images were acquired cross-sectionally centred at the bifurcation of index carotid arteries with a field of view (FOV) 140 × 140 mm2. The index carotid artery was defined as arteries associated with clinical symptoms. The slice thickness was 2 mm for T1W and T2W imaging and 1 mm for TOF and MR-RAGE imaging, respectively. The longitudinal coverage was 32 mm for T1W, T2W, TOF, and MP-RAGE imaging. The SNAP images were acquired coronally with FOV of 140 × 140 mm2. The corrected real images derived from SNAP imaging were re-sliced cross-sectionally with slice thickness of 2 mm centred to the bifurcation of index carotid arteries.
Image analysis
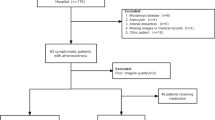
All carotid MR images were interpreted by three radiologists with more than 5 years’ experience in vascular imaging blinded to clinical information. A custom-designed software of CASCADE [12] was utilised to trace the boundaries of lumen and outer wall and intraplaque haemorrhage compositions. The work flow of image review was as follows (Fig. 1): (1) one reviewer outlined the lumen and wall boundaries on multi-contrast MR images including TOF, T1W, T2W, MP-RAGE, and SNAP images; (2) the presence and size of the IPH were determined by the two other reviewers on a single MP-RAGE imaging sequence after hiding all other contrast-weighted images with consensus; (3) after 1 month time interval for minimising the bias of memory, the presence and size of the IPH were determined by the two reviewers on a single SNAP imaging sequence after hiding all other contrast-weighted images with consensus. The presence of IPH on MP-RAGE or SNAP images was defined as hyperintense compared to adjacent muscle (signal intensity ratio ≥ 1.5:1). The area of IPH on MP-RAGE and SNAP images for each axial location was measured. The image quality (IQ) of MR images was evaluated using a 4-point scale (from poor to excellent) and the images with poor IQ (IQ = 1) were excluded [13].
Statistical analysis
Continuous variables are described as mean ± standard deviation (SD) and the percentage is used to describe the categorical variables. Cohen’s kappa (κ) was analysed to determine the agreement between SNAP and MP-RAGE in identification of IPH. Pearson correlation coefficients (r) were evaluated to assess the correlations of IPH area between SNAP and MP-RAGE sequences. The paired t test was used to compare the difference in IPH area between MP-RAGE and SNAP images when IPH was present on both imaging sequences. A p value of p < 0.05 was considered as statistically significant. All statistical analyses were performed using SPSS 16.0 (SSPS Inc. Chicago, IL, USA).
Results
Of 64 recruited patients, 10 were excluded because of poor image quality. Of the remaining 54 patients (mean age: 63.1 ± 5.7 years old), 70% (38/54) were male, 64.8% (35/54) had hypertension, 16.7% (9/54) had a history of smoking, 29.6% (16/54) had diabetes, and 42.6% (23/54) had stroke. The clinical characteristics of this study population are detailed in Table 1.
Agreement between SNAP and MP-RAGE
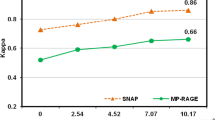
In the 108 carotid arteries of 54 patients, 1368 slices had acceptable image quality for analysis. Of 1368 slices, 13% (178/1368) and 22.6% (309/1368) were found to have IPH on MP-RAGE and SNAP images, respectively. The IPH was found in 10.5% (144/1368) slices on both SNAP and MP-RAGE images. The kappa value of κ was 0.511 (p = 0.029, Table 2) between SNAP and MP-RAGE images in the identification of IPH. Pearson correlation analysis revealed that there was strong correlation between SNAP and MP-RAGE in measuring the IPH area (r = 0.816, p < 0.001).
Comparison of IPH area between SNAP and MP-RAGE
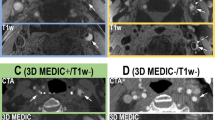
In all 144 slices having IPH on both SNAP and MP-RAGE images, the area of IPH on SNAP was significantly larger than that on MP-RAGE (17.9 ± 18.2 mm2 vs. 9.2 ± 10.5 mm2, p < 0.001, Fig. 2). Similarly, the volume of IPH on SNAP images was larger than that on MP-RAGE images (165.2 ± 227.9 mm3 vs. 74.2 ± 116.9 mm3, p < 0.001). For IPHs detected by SNAP imaging, the area of IPHs also detected by MP-RAGE was significantly larger than that of IPHs not detected by MP-RAGE (17.9 ± 18.2 mm2 vs. 6.4 ± 6.2 mm2, p < 0.001). Figure 3 represents examples showing IPH on both MP-RAGE and SNAP images with different size (a, b), and IPH was detected by SNAP imaging but not detected by MP-RAGE imaging (c, d).
SNAP imaging has different performances in evaluating carotid IPH compared to the MP-RAGE sequence. The a and b images are from the same carotid artery location of one patient. SNAP imaging shows a larger IPH than MP-RAGE (a and b, arrows). The c and d images are from the same carotid artery location of another patient. SNAP imaging detects IPH (d, arrows) but MP-RAGE does not
Discussion
In this study, we evaluated the performance of SNAP imaging in the identification of carotid IPH compared with MP-RAGE imaging sequences. We found that SNAP imaging detected 70% more IPHs than MP-RAGE sequences, indicating the SNAP sequence might be a more sensitive imaging tool for identification of carotid IPH. In addition, we found the size of IPH measured by SNAP imaging was significantly larger than that measured by MP-RAGE imaging and SNAP imaging can detect IPHs with smaller size. Our findings suggest that SNAP imaging might be a sensitive tool for identification of carotid IPH, particularly for small IPHs.
In identifying carotid IPH, in the present study, we found that SNAP imaging showed moderate agreement and can detect 70% more IPHs compared with MP-RAGE. In a previous study, Wang et al. [11] demonstrated that the SNAP sequence significantly improved the intraplaque haemorrhage-wall contrast-to-noise ratio (p <0.01) and blood suppression efficiency (p < 0.01) using optimised parameters with phase-sensitive inversion-recovery reconstruction. With increased IPH-wall contrast, SNAP may detect plaques with smaller IPH that may not detectable by MP-RAGE. In the present study, the area of IPHs detected by both SNAP and MP-RAGE imaging was found to be significantly larger than that of IPHs detected by SNAP only. This finding indicates that SNAP imaging can detect IPHs with smaller size compared with MP-RAGE. A previous study has shown that the sensitivity and the agreement of MP-RAGE with histology were decreased when detecting IPH with small size or coexisting heavy calcification [9]. Investigators believed that it is more difficult or even impossible to detect calcified IPH with coexisting signal trends (an increase due to T1 shortening and a decrease due to the susceptibility effect) at 3.0 T [9]. In contrast, for the higher detection of T1 hyperintensity within the carotid wall on SNAP, there might be a possibility of overestimation of SNAP imaging in identification of IPH. Our results further compel the evidence that SNAP imaging may be a more sensitive tool for identification of carotid IPH compared with MP-RAGE.
In the present study, for IPH detected by both SNAP and MP-RAGE imaging, its size on SNAP images was significantly larger than that on MP-RAGE images. The IPH was quantified manually when hyperintense signal was present on MP-RAGE or SNAP images. The contour of IPH delineated on SNAP images might be larger than that on MP-RAGE images because of the larger dynamic range and higher IPH-wall contrast-to-noise ratio (CNR) of SNAP than MP-RAGE [14]. Moreover, MP-RAGE might underestimate IPH size because of the insufficient IPH-wall CNR. The criteria for IPH quantification are critical for IPH area measurement. Recently, a histology-verified intensity threshold for IPH detection by MP-RAGE has been optimised as 1.6 times the adjacent muscle intensity, similar to our criteria [15]. However, the criteria for IPH quantification by SNAP are still lacking and should be determined in future studies.
SNAP imaging not only provides vessel wall images that can be used for detecting IPH, but also simultaneously generates non-contrast MR angiography for measuring arterial luminal stenosis. In a previous study, Wang et al. [11] showed that the lumen area of carotid arteries measured by SNAP MRA was significantly correlated with that measured by TOF MRA (intraclass correlation coefficient = 0.96, p < 0.001). In measuring intracranial and carotid artery stenosis, SNAP MRA has been reported to have excellent agreement with TOF MRA (κ = 0.79-0.82) [16, 17]. However, the accuracy and agreement for evaluating luminal stenosis of the carotid by using the SNAP sequence is still lacking validation compared with the clinical “gold standard”, such as CE-MRA and DSA.
This study has several limitations. First, in the present study, MR-RAGE was considered as reference to determine the usefulness of SNAP imaging in the identification of carotid IPH. It is suggested to perform studies with histology as the gold standard to validate the ability of both MP-RAGE and SNAP imaging in identifying IPHs with different sizes in the future. Second, the SNAP images were acquired coronally and all the images were re-sliced cross-sectionally for comparison, which may lead to possible mismatch with 2D multicontrast MR images. Future studies with axial acquisition of SNAP images are suggested. Third, the image interpretation by reviewers may yield subjective bias and the signal intensity threshold for identifying IPH is still lacking. Automatically detecting IPH with an optimised algorithm may minimise subjective bias.
In conclusion, compared with the MP-RAGE sequence, SNAP imaging detects more intraplaque haemorrhages, particularly for haemorrhages with smaller size, suggesting that SNAP imaging might be a more sensitive non-invasive tool for identification of carotid haemorrhagic plaques.
Abbreviations
- IPH:
-
intraplaque haemorrhage
- MP-RAGE:
-
Magnetisation-prepared rapid acquisition gradient-echo
- SNAP:
-
Simultaneous non-contrast angiography and intraplaque haemorrhage
- MR:
-
Magnetic resonance
- MRA:
-
Magnetic resonance angiography
References
Takaya N, Yuan C, Chu B et al (2005) Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: a high-resolution magnetic resonance imaging study. Circulation 111:2768–2775
Underhill HR, Yuan C, Yarnykh VL et al (2009) Arterial remodeling in the subclinical carotid artery disease. JACC Cardiovasc Imaging 2:1381–1389
WH X, Li ML, Gao S et al (2012) Middle cerebral artery intraplaque hemorrhage: prevalence and clinical relevance. Ann Neurol 71:195–198
Singh N, Moody AR, Gladstone DJ et al (2009) Moderate carotid artery stenosis: MR imaging-depicted intraplaque hemorrhage predicts risk of cerebrovascular ischemic events in asymptomatic men. Radiology 252:502–508
Altaf N, Daniels L, Morgan PS et al (2008) Detection of intraplaque hemorrhage by magnetic resonance imaging in symptomatic patients with mild to moderate carotid stenosis predicts recurrent neurological events. J Vasc Surg 47:337–342
Takaya N, Yuan C, Chu B et al (2006) Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI--initial results. Stroke 37:818–823
Noguchi T, Yamada N, Higashi M et al (2011) High-intensity signals in carotid plaques on T1-weighted magnetic resonance imaging predict coronary events in patients with coronary artery disease. JACC Cardiovasc Imaging 58:416–422
Yamada K, Yoshimura S, Kawasaki M et al (2011) Embolic complications after carotid artery stenting or carotid endarterectomy are associated with tissue characteristics of carotid plaques evaluated by magnetic resonance imaging. Atherosclerosis 215:399–404
Ota H, Yarnykh VL, Ferguson MS et al (2010) Carotid intraplaque hemorrhage imaging at 3.0-T MR imaging: comparison of the diagnostic performance of three T1-weighted sequences. Radiology 254:551–563
Yao B, Yang L, Wang G et al (2016) Diffusion measurement of intraplaque hemorrhage and intramural hematoma using diffusion weighted MRI at 3T in cervical artery. Eur Radiol 26:3737–3743
Wang J, Börnert P, Zhao H et al (2013) Simultaneous non-contrast angiography and intraplaque hemorrhage (SNAP) imaging for carotid atherosclerotic disease evaluation. Magn Reson Med 69:337–345
Kerwin W, Xu D, Liu F et al (2007) Magnetic resonance imaging of carotid atherosclerosis: plaque analysis. Top Magn Reson imaging 18:371–378
Underhill HR, Yuan C, Terry JG et al (2008) Differences in carotid arterial morphology and composition between individuals with and without obstructive coronary artery disease: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 10:1–11
Wang J, Ferguson MS, Balu N et al (2010) Improved carotid intraplaque hemorrhage imaging using a slab-selective phase-sensitive inversion-recovery (SPI) sequence. Magn Reson Med 64:1332–1340
Liu J, Balu N, Hippe DS et al (2016) Semi-automatic carotid intraplaque hemorrhage detection and quantification on magnetization-prepared rapid acquisition gradient-echo (MP-RAGE) with optimized threshold selection. J Cardiovasc Magn Reson 18:41
Li Q, Wang J, Chen H et al (2015) Characterization of craniocervical artery dissection by simultaneous MR noncontrast angiography and intraplaque hemorrhage imaging at 3T. AJNR Am J Neuroradiol 36:1769–1775
Wang J, Guan M, Yamada K et al (2016) In vivo validation of simultaneous non- contrast angiography and intraPlaque hemorrhage (SNAP) magnetic resonance angiography: An intracranial artery study. Plos One 11:e0149130
Funding
This study is funded by the grants from the National Natural Science Foundation of China (81771825, 81361120402) and Beijing Municipal Science and Technology Project (D131100002313002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Xihai Zhao MD, PhD.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Methodology
• Retrospective
• Observational
• Performed at one institution
Rights and permissions
About this article
Cite this article
Li, D., Zhao, H., Chen, X. et al. Identification of intraplaque haemorrhage in carotid artery by simultaneous non-contrast angiography and intraPlaque haemorrhage (SNAP) imaging: a magnetic resonance vessel wall imaging study. Eur Radiol 28, 1681–1686 (2018). https://doi.org/10.1007/s00330-017-5096-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5096-1