Abstract
Objective
To evaluate the feasibility of image subtraction in late iodine enhancement CT (LIE-CT) for assessment of myocardial infarction (MI).
Methods
A comprehensive cardiac CT protocol and late gadolinium enhancement MRI (LGE-MRI) was used to assess coronary artery disease in 27 patients. LIE-CT was performed after stress CT perfusion (CTP) and CT angiography. Subtraction LIE-CT was created by subtracting the mask volume of the left ventricle (LV) cavity from the original LIE-CT using CTP dataset. The %MI volume was quantified as the ratio of LIE to entire LV volume, and transmural extent (TME) of LIE was classified as 0%, 1–24%, 25–49%, 50–74% or 75–100%. These results were compared with LGE-MRI using the Spearman rank test, Bland-Altman method and chi-square test.
Results
One hundred twenty-five (29%) of 432 segments were positive on LGE-MRI. Correlation coefficients for original and subtraction LIE-CT to LGE-MRI were 0.79 and 0.85 for %MI volume. Concordances of the 5-point grading scale between original and subtraction LIE-CT with LGE-MRI were 75% and 84% for TME; concordance was significantly improved using the subtraction technique (p <0.05).
Conclusion
Subtraction LIE-CT allowed more accurate assessment of MI extent than the original LIE-CT.
Key Points
• Subtraction LIE-CT allows for accurate assessment of the extent of myocardial infarction.
• Subtraction LIE-CT shows a close correlation with LGE-MRI in %MI volume.
• Subtraction LIE-CT has significantly higher concordance with TME assessment than original LIE-CT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Late gadolinium enhancement (LGE) with magnetic resonance imaging (MRI) correlates well with histopathological findings in infarcted myocardium, and is used as a gold standard for assessment of myocardial infarction (MI) [1]. The global extent of MI on LGE-MRI is closely related to left ventricle (LV) dysfunction, cardiac mortality and poor outcomes [2, 3]. Previous studies have reported that the transmural extent (TME) of MI on LGE-MRI indicates potential improvement of LV contractility after revascularisation [4, 5]. However, LGE-MRI is contraindicated for patients with a metal implant or an implantable cardioverter defibrillator and for those with claustrophobia.
Late iodine enhancement computed tomography (LIE-CT) has been investigated in several studies for its ability to detect MI [6, 7]. Because iodine has pharmacokinetics similar to those of gadolinium [8], the extent of MI seen on LIE-CT, as on LGE-MRI, is also closely related to recovery of cardiac function and clinical outcomes [9, 10]. However, unlike LGE-MRI, LIE-CT has the disadvantages of lower image contrast in infarcted myocardium [11, 12] and difficulty in differentiation between myocardium and the LV cavity because of interference with enhancement in the LV cavity after administration of contrast medium (CM) [8, 13, 14]. Post-processing subtraction techniques are now widely used in CT. CT-based subtraction angiography has been shown to be an effective diagnostic imaging tool in cerebrovascular disease [15, 16]. In this study, we applied the image subtraction technique for LIE-CT and evaluated its feasibility for assessment of the extent of MI using LGE-MRI as the reference.
Methods
Study population
This retrospective study was approved by the ethics committee at our institution, and informed consent was obtained from all patients. This study is registered on the UMIN Clinical Trials Registry (approval number UMIN 000027484). The study included 27 patients who had undergone a comprehensive cardiac CT protocol and LGE-MRI between March 2015 and May 2017. All patients were clinically known or suspected to have coronary artery disease (CAD), and the indications for cardiac CT and MRI were determined by the attending physician. Patients with acute MI, cardiomyopathy, severe LV dysfunction (ejection fraction < 20%), chronic atrial fibrillation or greater than first-degree atrioventricular block were excluded. Patients who underwent revascularisation and/or experienced cardiovascular events such as MI or worsening heart failure during the imaging session and those with an incomplete LIE-CT dataset because of inadequate coverage (a truncation issue) or incomplete subtraction post-processing because of misregistration were also excluded to ensure reasonable evaluation of CT and MRI.
The radiation dose was calculated from the dose-length product in a dose report (conversion factor 0.014) [17].
Cardiac CT protocol and post-processing
We used a 256-slice CT scanner (Brilliance iCT, Philips Healthcare, Cleveland, OH, USA) and an automatic dual injector (Stellant DualFlow; Nihon Medrad KK, Osaka, Japan). The LIE-CT scan was performed as a part of a comprehensive cardiac CT protocol after stress dynamic myocardial CT perfusion (CTP) and coronary CT angiography (CTA) [18]. In brief, using iodinated CM (Iopamiron-370 [iopamidol 370 mg iodine/ml]; Bayer Yakuhin, Ltd, Osaka, Japan), pharmacological stress dynamic CTP (CM 30–50 ml, and 10–20 ml for timing bolus scan) was performed, and coronary CTA (CM 30–70 ml) were performed 5 min after CTP. Data acquisition for LIE-CT was performed 5 min after coronary CTA without additional administration of CM using the same prospective electrocardiogram-gated scan mode targeting end-systolic cardiac phase (40% of the RR interval) as for CTP. Scan parameters for CTP were as follows: tube voltage 80 kV; tube current 75–110 mAs; gantry rotation speed 0.27 s/rotation; detector collimation 128 × 0.625 mm; coverage 8 cm (detector-length, scanning under a single breath-hold in the expiration position); and 3600 reconstruction. Scan parameters for LIE-CT were as follows: tube voltage 80 kV; tube current 130–210 mAs; gantry rotation speed 0.27 s/rotation; detector collimation 128 × 0.625 mm; coverage 8 cm (detector-length, scanning under a single breath-hold in the expiration position); and 3600 reconstruction.
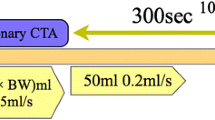
The axial images for CTP and LIE-CT with a 1.25-mm thickness were reconstructed using knowledge-based iterative model reconstruction (Body Routine Level 1, Philips Healthcare) [18]. The subtraction LIE-CT image was created using a dedicated workstation (Ziostation2, Ziosoft, Inc., Tokyo, Japan) using the following procedure (Fig. 1). A single-phase image at the time of peak enhancement of the LV cavity was selected from a series of dynamic CTP images and three-dimensionally superimposed on the original LIE-CT image using deformable registration. The mask volume of the LV cavity was created from the CTP image, and the subtraction LIE-CT image was then created by subtracting the image signal of the LV cavity from the original LIE-CT image using the mask volume from the CTP image.
Post-processing of subtraction LIE-CT. Subtraction LIE-CT was created by subtracting the image signal of the LV cavity from the original LIE-CT image using one phase of dynamic CTP images at the time of peak enhancement of the LV cavity. LIE-CT late iodine enhancement computed tomography, CTP computed tomography perfusion, LV left ventricle
MRI protocol and image analysis
We used a 3-Tesla MRI scanner (Achieva 3.0 T Quasar Dual; Philips Healthcare, Best, The Netherlands). Following a previously described established protocol [19], LGE-MRI images were obtained 10 min after injection of 0.2 mmol/kg gadopentetate dimeglumine (Magnevist; Bayer Healthcare Pharmaceuticals, Whippany, NJ, USA) using three-dimensional inversion recovery sequences. The image acquisition parameters were as follows: maximum gradient amplitude, 80 mT/m; slew rate, 200 mT/m/ms; repetition time, 3.5 ms; echo time, 2.3 ms; flip angle, 15°; field of view, 320 × 320 mm2; matrix, 256 × 256; and slice thickness, 5 mm.
An independent observer (with 7 years of experience in cardiac imaging) semi-automatically quantified the global extent of MI on LGE-MRI using the Ziostation2 software with a signal intensity threshold of more than 6 standard deviations (SDs) above the remote myocardium, as previously described [19]. The %MI volume was quantified as the ratio of LGE to entire LV volume in LGE-MRI. Two independent observers (with 9 and 13 years of experience in cardiac imaging) blinded to any other clinical and imaging data visually evaluated the TME of LGE as regional MI extent, according to the 16-segment model excluding the apex [20]. All myocardial segments were classified as 0%, 1–24%, 25–49%, 50–74% or 75–100%. Any discrepancy in evaluations made by the two observers was resolved by consensus.
LIE-CT image analysis
Multi-planar reformatted cardiac short-axis images were created for original and subtraction LIE-CT. Slice thickness was set at gapless 5 mm to match the MRI datasets. The original LIE-CT image and the subtraction LIE-CT image were independently displayed and assessed with a basic window level of 140 Hounsfield units (HU) and a window width of 220 HU. Readers independently adjusted the optimal window setting in each case as needed. We performed separate analysis of two series of LIE-CT images in random order, which were spaced at 2-week intervals to minimise recall bias.
An independent blinded observer (with 4 years of experience in cardiac imaging) manually quantified the global extent of MI on LIE-CT using the Ziostation2 software. Myocardium that was more highly attenuated than the remote myocardium was defined as MI for LIE-CT and manually quantified by drawing a region of interest. The volume of LIE was calculated by multiplying the summed area by the slice thickness. The %MI volume was quantified as the ratio of LIE to entire LV volume in LIE-CT. Two independent observers (with 9 and 17 years of experience, respectively, in cardiac imaging), who were blinded to any other clinical and imaging data, visually evaluated the TME of LIE as regional MI in the original and subtraction LIE-CT datasets according to the 16-segment model, excluding the apex [20]. Segmental TME of LIE was classified into the aforementioned five groups, as was LGE-MRI.
Statistical analysis
Continuous data are expressed as the mean ± SD or the median (interquartile range) according to the underlying distribution. Agreement between two observers on visual assessment of LGE-MRI and LIE-CT was evaluated using Cohen’s kappa (κ) statistic. The relationship between LGE-MRI and LIE-CT with regard to the %MI volume was assessed using the Spearman rank correlation test and the Bland-Altman method. For segment-based analysis, we adjusted for the clustered nature of the data using logistic generalised estimating equations [21]. Ordinal and binary assessments of TME were compared between original and subtraction LIE-CT using the chi-squared test. The statistical analyses were performed using JMP 12 software (SAS Institute, Cary, NC, USA), and the Stata software, version 11.2 (StataCorp LP, College Station, TX, USA). In all tests, a p-value of <0.05 was considered to be statistically significant.
Results
Study population
The patients’ demographic and clinical characteristics are shown in Table 1. None of the 27 patients had undergone revascularisation therapy, had cardiovascular events, or had an incomplete LIE-CT dataset. Post-processing of the subtraction LIE-CT image was performed successfully in all cases. The mean effective radiation dose was 7.8 ± 3.4 mSv for dynamic CTP, 5.7 ± 2.2 mSv for coronary CTA and 0.4 ± 0.2 mSv for LIE-CT. The average total amount of CM administered was 114.7 ± 23.7 ml.
Cardiac MRI-based diagnosis of myocardial segments
Of 432 segments, 125 (29%) were diagnosed with MI by LGE-MRI; TME of LGE was 0% in 307 segments, 1–24% in 26, 25–49% in 49, 50–74% in 31, and 75–100% in 19. The interobserver agreement for qualitative assessment of LGE-MRI was 0.87, which indicates satisfactory reliability (κ >0.70).
LGE-MRI versus LIE-CT for assessment of MI
The correlation charts and Bland-Altman plots for %MI volume between LGE-MRI and original and subtraction LIE-CT are shown in Fig. 2A–D. The %MI volumes for original and subtraction LIE-CT correlated significantly with the %MI volume for LGE-MRI (both p <0.05). The Spearman’s correlation coefficient was 0.79 for original LIE-CT and 0.85 for subtraction LIE-CT. The Bland-Altman plots show a mean difference of -3.4% (95% limits of agreement: -14.3 to 7.5) for original LIE-CT and -1.3% (95% limits of agreement: -10.7 to 8.2) for subtraction LIE-CT.
The interobserver agreement for the qualitative assessment was 0.75 (95% confidence interval: 0.69–0.81) for original LIE-CT and 0.85 (95% confidence interval: 0.80–0.89) for subtraction LIE-CT. These results indicate satisfactory reliability for each (κ >0.70). A comparison of LGE-MRI and LIE-CT with regard to TME is shown in Table 2. The complete concordance ratio according to the 5-point grading scale was 75% for original LIE-CT and 84% for subtraction LIE-CT, there was a significant difference in the concordance ratio between original and subtraction LIE-CT (p <0.05). In the binary assessment of TME ≥50% and ≥75%, using the LV subtraction technique, the concordance ratios were significantly improved from 90% to 96% and from 96% to 99%, respectively (p <0.05). A representative case is shown in Fig. 3.
A 71-year-old woman with an old myocardial infarction. Cardiac short-axial LGE-MRI image shows myocardial infarction as LGE in the inferior wall at basal level (arrowhead) (A). The original LIE-CT also shows myocardial infarction as LIE in the inferior wall at basal level (arrowhead) (B). The subtraction LIE-CT shows it more clearly than the original LIE-CT (arrowhead) (C). LGE-MRI late gadolinium enhancement magnetic resonance imaging, LIE-CT late iodine enhancement computed tomography
Discussion
Our study shows that subtraction LIE-CT allowed for more accurate quantification of %MI volume than original LIE-CT, and that the concordance ratio for TME of MI on subtraction LIE-CT was significantly improved in comparison with original LIE-CT.
The %MI volume is a strong prognostic predictor of cardiac death and major adverse cardiovascular events [3]; thus, accurate assessment of the extent of MI is important. The lower image contrast of LIE-CT has remained a fundamental limitation [11, 12], and potentially affecting quantification of the extent of MI because of the difficulty in differentiating between the LV cavity, remote myocardium and infarcted myocardium [8, 13, 14]. The scan timing of LIE-CT is also an important factor in assessment of MI. Previous studies have applied LIE-CT data acquisition 5–15 min after injection of CM [14, 22, 23], which leads to the low contrast between the LV cavity and myocardium because of remnants of CM in the LV cavity, especially in patients with a high body weight. Langer et al. proposed that LIE-CT required a higher contrast dose (2.0 ml/kg, 700 mg iodine/kg) in clinical practice to achieve acceptable contrast of the LV cavity allowing for accurate LV volumetry [14]. Some investigators have proposed solutions such as using the spatial frequency filtration and image averaging on repeated axial scans [24], dual-energy imaging [25] and the dedicated noise-reduction filter in a single axial scan [7]. In this study, we used post-processing subtraction of the LV cavity to delineate the LV myocardium objectively in quantification of MI without impairment of contrast between normal and infarcted myocardium. Although a total amount of CM (mean 114.7 ± 23.7 ml, 673.3 ± 157.3 mg iodine/kg) was less than the amount of CM previously proposed [17], the post-processing subtraction technique helped to reduce the quantification errors of %MI volume despite the lesser amount of CM. In the visual assessment of TME, the interobserver agreement was increased from 0.75 in original LIE-CT to 0.85 in subtracted LIE-CT, although it was not significant. In the quantification of LGE-MRI size to the LV mass, some studies have also indicated that visual assessment provides comparable results to the threshold-based segmentation using 5 and more SD of signal intensity in remote myocardium [26, 27]. Aikawa et al. recently reported the feasibility of a threshold-based segmentation in LIE-CT in patients with cardiac sarcoidosis [28]. However, some difficulties in the quantification of LIE-CT mass remained in this study because of several reasons, such as: (1) the time difference in cardiac phase (CT vs. MR), (2) individual difference in attenuation of LIE-CT, (3) the inherent heterogeneity of MI for quantification of LIE volume, and (4) lower contrast-to-noise ratio in LIE-CT, and so on. Further studies will be needed for exploring the optimal threshold of CT attenuation in LIE-CT including iodine dose, scan parameters, reconstruction and image post-processing.
The TME of MI is required for decision-making with regard to revascularisation in patients with CAD because identification of viable myocardium is important to predict improvement of LV function after revascularisation [4, 29]. In the present study, LIE-CT was obtained as a part of a comprehensive cardiac CT protocol that has been proposed to be useful in patients with a high pre-test probability of CAD [30]. A previous study showed that use of fully iterative model reconstruction improved the image quality and diagnostic performance of LIE-CT for detecting MI, but it was inadequate for quantification of MI [18]. Some studies have also indicated that LIE-CT has a tendency to overestimate TME assessment in comparison with LGE-MRI [25, 31]. According to the present results, the subtraction technique could reduce overestimation of TME and improve the accuracy of assessment of the regional extent of MI. However, large-scale prospective studies are now required to evaluate the clinical feasibility of subtraction LIE-CT for assessment of myocardial viability before revascularisation.
This study has several limitations. First, it is a retrospective, single-centre study with a relatively small number of patients included. Second, LIE-CT was acquired at the end-systolic phase, which might affect the TME assessment to a certain extent because of thickening of the remaining viable rim in the cardiac cycle. Matsumoto et al. found no significant difference in the assessment of TME between the diastolic phase and the systolic phase [32]. However, careful attention has to be paid for the assessment of TME in systolic phase especially in patients with hypertrabeculations, LV hypertrophy and cardiomyopathy. In addition, not only the phasic change in the appearance of myocardial trabeculations during cardiac cycle, but also the robustness of the subtraction post-processing and the spatial resolution of CT should be considered for the LIE-CT assessment. Third, the visual quantification of MI in LIE-CT was individually performed with a basic setting of window level and window width. More objective segmentation is preferable using a threshold as LGE-MRI [28]. In this present study, because of the above-mentioned reasons and small sample size to determine the cut-off value of SD above the mean attenuation of the remote myocardium, we adopted the present post-processing. Forth, the present study evaluated only for MI in the LV because of the time-difference in peak enhancement of the right ventricle and the LV cavity. The assessment of MI in the right ventricle will also be important for clinical practice [33]. Further studies will be needed to enhance the feasibility for the assessment of right ventricular MI in LIE-CT. Finally, we used CTP data in a comprehensive cardiac CT protocol for subtraction of the LV cavity. CTA data may also be used for subtraction of the LV cavity if the data acquisition was in the same cardiac phase as the LIE-CT. However, a further evaluation study is needed to investigate the feasibility of subtraction LIE-CT with CTA.
In conclusion, subtraction LIE-CT is helpful for improving the accuracy of assessment of global and regional extent of MI in comparison with that of the original LIE-CT. Subtraction LIE-CT may be feasible for prediction of clinical outcome and myocardial viability in patients with CAD.
Abbreviations
- CAD:
-
Coronary artery disease
- CM:
-
Contrast medium
- CT:
-
Computed tomography
- CTA:
-
Computed tomography angiography
- CTP:
-
Computed tomography perfusion
- HU :
-
Hounsfield units
- LGE:
-
Late gadolinium enhancement
- LIE:
-
Late iodine enhancement
- LV:
-
Left ventricle
- MI:
-
Myocardial infarction
- MRI:
-
Magnetic resonance imaging
- SD :
-
Standard deviations
- TME:
-
Transmural extent
References
Kim RJ, Fieno DS, Parrish TB et al (1999) Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 100:1992–2002
Cheong BY, Muthupillai R, Wilson JM et al (2009) Prognostic significance of delayed-enhancement magnetic resonance imaging: survival of 857 patients with and without left ventricular dysfunction. Circulation 120:2069–2076
Larose E, Rodés-Cabau J, Pibarot P et al (2010) Predicting late myocardial recovery and outcomes in the early hours of ST-segment elevation myocardial infarction traditional measures compared with microvascular obstruction, salvaged myocardium, and necrosis characteristics by cardiovascular magnetic resonance. J Am Coll Cardiol 55:2459–2469
Kim RJ, Wu E, Rafael A et al (2000) The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 343:1445–1453
Hadamitzky M, Langhans B, Hausleiter J et al (2014) Prognostic value of late gadolinium enhancement in cardiovascular magnetic resonance imaging after acute ST-elevation myocardial infarction in comparison with single-photon emission tomography using Tc99m-Sestamibi. Eur Heart J Cardiovasc Imaging 15:216–225
Goetti R, Feuchtner G, Stolzmann P et al (2011) Delayed enhancement imaging of myocardial viability: low-dose high-pitch CT versus MRI. Eur Radiol 21:2091–2099
Matsuda T, Kido T, Itoh T et al (2015) Diagnostic accuracy of late iodine enhancement on cardiac computed tomography with a denoise filter for the evaluation of myocardial infarction. Int J Card Imaging 31:177–185
Gerber BL, Belge B, Legros GJ et al (2006) Characterization of acute and chronic myocardial infarcts by multidetector computed tomography: comparison with contrast-enhanced magnetic resonance. Circulation 113:823–833
Sato A, Nozato T, Hikita H et al (2012) Prognostic value of myocardial contrast delayed enhancement with 64-slice multidetector computed tomography after acute myocardial infarction. J Am Coll Cardiol 59:730–738
Shapiro MD, Sarwar A, Nieman K, Nasir K, Brady TJ, Cury RC (2010) Cardiac computed tomography for prediction of myocardial viability after reperfused acute myocardial infarction. J Cardiovasc Comput Tomogr 4:267–273
Nieman K, Shapiro MD, Ferencik M et al (2008) Reperfused myocardial infarction: contrast-enhanced 64-Section CT in comparison to MR imaging. Radiology 247:49–56
Deseive S, Bauer RW, Lehmann R et al (2011) Dual-energy computed tomography for the detection of late enhancement in reperfused chronic infarction: a comparison to magnetic resonance imaging and histopathology in a porcine model. Investig Radiol 46:450–456
Kim RJ, Chen EL, Lima JA, Judd RM (1996) Myocardial Gd-DTPA kinetics determine MRI contrast enhancement and reflect the extent and severity of myocardial injury after acute reperfused infarction. Circulation 94:3318–3326
Langer C, Both M, Harders H et al (2015) Late enhanced computed tomography in hypertrophic cardiomyopathy enables accurate left-ventricular volumetry. Eur Radiol 25:575–584
Fujiwara H, Momoshima S, Akiyama T, Kuribayashi S (2013) Whole-brain CT digital subtraction angiography of cerebral dural arteriovenous fistula using 320-detector row CT. Neuroradiology 55:837–843
Hayashida E, Sasao A, Hirai T et al (2013) Can sufficient preoperative information of intracranial aneurysms be obtained by using 320-row detector CT angiography alone? Jpn J Radiol 31:600–607
Shrimpton PC, Hillier MC, Lewis MA, Dunn M (2006) National survey of doses from CT in the UK: 2003. Br J Radiol 79:968–980
Tanabe Y, Kido T, Kurata A, et al. (2017) Impact of knowledge-based iterative model reconstruction on myocardial late iodine enhancement in computed tomography and comparison with cardiac magnetic resonance. Int J Cardiovasc Imaging. 2017. doi: https://doi.org/10.1007/s10554-017-1137-8
Kido T, Kido T, Nakamura M et al (2014) Three-dimensional phase-sensitive inversion recovery sequencing in the evaluation of left ventricular myocardial scars in ischemic and non-ischemic cardiomyopathy: comparison to three-dimensional inversion recovery sequencing. Eur J Radiol 83:2159–2166
Cerqueira MD, Weissman NJ, Dilsizian V, et al., American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105:539-542
Sternberg MR, Hadgu A (2001) A GEE approach to estimating sensitivity and specificity and coverage properties of the confidence intervals. Stat Med 20:1529–1539
Zhao L, Ma X, Delano MC et al (2013) Assessment of myocardial fibrosis and coronary arteries in hypertrophic cardiomyopathy using combined arterial and delayed enhanced CT: comparison with MR and coronary angiography. Eur Radiol 23:1034–1043
Shiozaki AA, Senra T, Arteaga E et al (2013) Myocardial fibrosis detected by cardiac CT predicts ventricular fibrillation/ventricular tachycardia events in patients with hypertrophic cardiomyopathy. J Cardiovasc Comput Tomogr 7:173–181
Kurobe Y, Kitagawa K, Ito T et al (2014) Myocardial delayed enhancement with dual-source CT: advantages of targeted spatial frequency filtration and image averaging over half-scan reconstruction. J Cardiovasc Comput Tomogr 8:289–298
Wichmann JL, Arbaciauskaite R, Kerl JM et al (2014) Evaluation of monoenergetic late iodine enhancement dual-energy computed tomography for imaging of chronic myocardial infarction. Eur Radiol 24:1211–1218
Bondarenko O, Beek AM, Hofman MB et al (2005) Standardizing the definition of hyperenhancement in the quantitative assessment of infarct size and myocardial viability using delayed contrast-enhanced CMR. J Cardiovasc Magn Reson 7:481–485
Spiewak M, Malek LA, Misko J et al (2010) Comparison of different quantification methods of late gadolinium enhancement in patients with hypertrophic cardiomyopathy. Eur J Radiol 74:e149–e153
Aikawa T, Oyama-Manabe N, Naya M, et al. (2017) Delayed contrast-enhanced computed tomography in patients with known or suspected cardiac sarcoidosis: A feasibility study. Eur Radiol. doi: https://doi.org/10.1007/s00330-017-4824-x
Beek AM, Kuhl HP, Bondarenko O et al (2003) Delayed contrast-enhanced magnetic resonance imaging for the prediction of regional functional improvement after acute myocardial infarction. J Am Coll Cardiol 42:895–901
Sharma RK, Arbab-Zadeh A, Kishi S et al (2015) Incremental diagnostic accuracy of computed tomography myocardial perfusion imaging over coronary angiography stratified by pre-test probability of coronary artery disease and severity of coronary artery calcification: The CORE320 study. Int J Cardiol 201:570–577
Mahnken AH, Koos R, Katoh M et al (2005) Assessment of myocardial viability in reperfused acute myocardial infarction using 16-slice computed tomography in comparison to magnetic resonance imaging. J Am Coll Cardiol 45:2042–2047
Matsumoto H, Matsuda T, Miyamoto K et al (2010) Late gadolinium-enhanced cardiovascular MRI at end-systole: feasibility study. AJR Am J Roentgenol 195:1088–1094
Masci PG, Francone M, Desmet W et al (2010) Right ventricular ischemic injury in patients with acute ST-segment elevation myocardial infarction: characterization with cardiovascular magnetic resonance. Circulation 122:1405–1412
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Teruhito Mochizuki.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors, Dr. Natsumi Yamashita, Department of Clinical Biostatistics, Section of Cancer Prevention and Epidemiology, Clinical Research Center, National Hospital Organization Shikoku Cancer Center, is an expert in statistics.
Ethical approval
Institutional Review Board approval was obtained.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Methodology
• retrospective
• observational
• performed at one institution
Rights and permissions
About this article
Cite this article
Tanabe, Y., Kido, T., Kurata, A. et al. Late iodine enhancement computed tomography with image subtraction for assessment of myocardial infarction. Eur Radiol 28, 1285–1292 (2018). https://doi.org/10.1007/s00330-017-5048-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5048-9