Abstract
Objectives
To quantitatively assess carpal tunnel syndrome (CTS) with DTI by evaluating two approaches to determine cut-off values.
Methods
In forty patients with CTS diagnosis confirmed by nerve conduction studies (NCs) and 14 healthy subjects (mean age 58.54 and 57.8 years), cross-sectional area (CSA), apparent diffusion coefficient (ADC) and fractional anisotropy (FA) at single and multiple levels with intraobserver agreement were evaluated.
Results
Maximum and mean CSA and FA showed significant differences between healthy subjects and patients (12.85 mm2 vs. 28.18 mm2, p < 0.001, and 0.613 vs. 0.524, p=0.007, respectively) (10.12 mm2 vs. 19.9 mm2, p<0.001 and 0.617 vs. 0.54, p=0.003, respectively), but not maximum and mean ADC (p > 0.05). For cut-off values, mean and maximum CSA showed the same sensitivity and specificity (93.3 %). However, mean FA showed better sensitivity than maximum FA (82.6 % vs. 73.9 %), but lower specificity (66.7 % vs. 80 %), and significant correlation for maximum CSA, 97 % (p < 0.01), with good correlation for maximum ADC and FA, 84.5 % (p < 0.01) and 62 % (p=0.056), respectively.
Conclusions
CSA and FA showed significant differences between healthy subjects and patients. Single measurement at maximum CSA is suitable for FA determination.
Key Points
• DTI showed that FA is stronger than ADC for CTS diagnosis.
• Single- and multiple-level approaches were compared to determine FA and ADC.
• Single-level evaluation at the thickest MN cross-sectional area is sufficient.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carpal tunnel syndrome (CTS) is the most common entrapment neuropathy of the upper limb. Diagnosis is based mainly on clinical signs and symptoms and confirmed by nerve conduction studies (NCS).
Ultrasound (US) and magnetic resonance imaging (MRI) showed feasibility in diagnosing and grading CTS depending mainly on monitoring morphological changes of the median nerve (MN) as enlargement and flattening ratio, palmar bowing and increased signal in MRI [1,2,3,4]; however, MRI showed low sensitivity (23–96 %) and specificity (38–87 %) upon using these parameters [2].
Diffusion tensor imaging (DTI) based on MRI is a method that can provide information about tissue microstructure and architectural organisation by monitoring the random motion of water molecules in tissues [5,6,7,8,9].
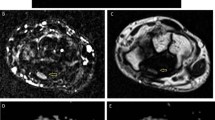
Quantitative assessment of DTI is feasible by calculating multiple parameters as apparent diffusion coefficient (ADC) that reflects molecular diffusivity under restriction and fractional anisotropy (FA) as a quantitative index used to characterise directional variability in diffusion Figs. 1 and 2.
Axial cut section of the wrist in a carpal tunnel syndrome patient. (a) Axial cut section of the median nerve (MN) (white arrows) at the entrance of the carpal tunnel. (b) Axial cut section of MN within the carpal tunnel (white arrows) under the flexor retinaculum (white triangles). (c) Axial RESOLVE TRACE image where regions of interest were drawn inside the MN, which is seen as a hyperintense signal ovoid shape structure (white arrows). Axial apparent diffusion coefficient (ADC) map (d) and fractional anisotropy (FA) map (e) showing MN (white arrows)
DTI was first applied for central nervous system white matter [10], then Skropil et al. [11] did the first application of DTI on peripheral nerves in a study conducted on three healthy volunteers using a 1.5-T scanner to image the sciatic nerve. The first application on peripheral nerves using a 3-T scanner was done by Hiltunen et al. [12].
Following these successful trials, multiple studies were published evaluating the MN in healthy subjects and CTS patients. However, there is lack of consensus about normal and abnormal values of DTI parameters in MN evaluation [13,14,15,16].
We aimed in the current study to emphasise the differences between CTS patients and healthy subjects by quantitative evaluation of DTI parameters in order to define cut-off values that may aid in the diagnosis using two different ways of data evaluation at a single compared to multiple levels.
Materials and methods
Patients
Our Institutional Review Board and Ethics Committee approved the study. Written informed consent was obtained from all subjects including 40 patients (28 females and 12 males; mean age 58.45 years; range: 38–76) with CTS and 14 healthy subjects (ten females and four males; mean age 57.8 years; range: 39–84). All patients were diagnosed for CTS by clinical examination and confirmed by nerve conduction studies (NCS). They had the characteristic clinical symptoms of CTS, e.g. localised pain and complaint at the palmar aspect of the hand that was localised to the medial three and a half fingers with pain aggravation at night-time and associated numbness and tingling. Patients complained about CTS symptoms for a mean duration of 7 months (range 1–12 months) with positive electrophysiological tests just 1–2 days before the examination time according to the modified scoring system of Padua et al. [17]. Exclusion criteria included pregnancy, systemic diseases, treatment with drugs that may affect nerve conduction, previous surgery or trauma of the examined hand, and general contraindications to MRI. All healthy subjects had neither a history of systemic diseases nor CTS symptoms. They had no history of hand trauma or surgery and did not undergo NCS.
MRI examination
Examination protocol
MRI was performed on a 3-T whole body scanner (MAGNETOM Skyra, Siemens, Erlangen, Germany) using a 15-channel hand-wrist coil. All subjects were scanned feet first in the supine position. The examined hand in the dedicated wrist coil was placed at the side of the patient in a neutral position midway between supination and pronation. The palm was facing the body and the thumb pointed upwards.
The examination protocol included the following conventional sequences for anatomical evaluation of the MN and diagnosis of any associated pathology: T1-weighted 3D gradient echo sequence (VIBE; coronal plane, frequency selective water excitation, TR=12 ms, TE=5.27 ms, flip angle=25°, voxel size: 0.3 mm × 0.3 mm × 0.3 mm) and T2-weighted inversion recovery turbo-spin echo sequence (T2tirm) (transversal orientation, TR=4,000 ms, TI=200 ms, TE=51 ms, turbo factor: 24, number of averages: 2 voxel size: 0.3 mm × 0.3 mm × 3 mm). For DTI a readout-segmented echo-planar imaging sequence (RESOLVE) was used [18] in transverse plane with the following imaging parameters: TR=3,400 ms, TE=93 ms, b-factors: 0 and 1,000 s/mm2, 12 diffusion directions, voxel-size: 0.9 mm × 0.9 mm × 3 mm
Image analysis
Data analysis was performed by a MSK radiologist (MA) with 10 years’ experience blinded to clinical diagnosis and NCS results using the free software ImageJ (Wayne Rasband, National Institutes of Health, Bethesda, MD, USA).
Conventional sequences assessment
T2tirm images were analysed for nerve anatomy and morphology. In addition, the cross-sectional area (CSA) was measured by manually tracing its outline in all transverse sections along the whole course of the examined MN segment and calculating the maximum CSA and mean CSA for each patient. The region of interest (ROI) was drawn carefully around the margin of the nerve just inside the perineurium.
DTI parameters
ADC and FA were evaluated for the MN starting at the level of the distal edge of the pronator quadratus muscle including a segment of about 3.5–4 cm towards the carpal tunnel. The evaluation included ROI placement in every slice (slice thickness = 3 mm) in a total of 13 slices of T2tirm (CSA), ADC and FA maps (which means 39 ROIs per patient). The mean of CSA, ADC and FA of the whole nerve segment (mean CSA, mean ADC and mean FA) were calculated and compared to ADC and FA values at the location of maximum CSA (max CSA, max ADC and max FA).
Statistical analysis
The statistical calculations were performed using R Project for Statistical Computing [19]. To compare values between patients and healthy subjects an unpaired Wilcox-Test was used. To assess intraobserver agreement evaluation of DTI and CSA parameters was repeated for ten randomly selected examinations (six patients and four healthy subjects) by the same examiner after 5 months. The respective ROIs were newly placed. To determine the agreement between the two repeats the Pearson correlation coefficient was calculated. Results were considered significant for p-values less than 0.05. For ROC analysis the pROC package for R was applied [20].
Results
Data were evaluated according to the different calculation approaches.
The first approach for evaluation of maximum (max) CSA, max ADC and max FA
The max CSA of the MN was significantly lower in healthy subjects compared to CTS patients (12.85 mm2, range: 9–20 mm2 vs. 28.18 mm2, range: 12.8–87.9, respectively, p<0.001). A good discriminating ability was found for this parameter by applying ROC analysis (AUC=0.969; 95 % CI: 0.933–1). Thereby a cut-off value of 17.02 mm2 was found, leading to a sensitivity of 93.3 % (95 % CI: 84.4–100) and specificity of 93.3 % (95 % CI: 80–100).
The max ADC was 1.57 × 10-3 mm2/s (range: 1.1–1.94 × 10-3 mm2/s) in healthy volunteers and 1.54 × 10-3 mm2/s (range: 0.92–2.57 × 10-3 mm2/s) in CTS patients, with no significant difference between the two groups (p=0.563).
The FA evaluated at the site of max CSA showed significantly lower values (p=0.007) in CTS patients (max FA = 0.524; range: 0.317–0.854) compared to healthy subjects (max FA = 0.613, range: 0.4–0.77). ROC analysis resulted in a cut-off value of 0.575, with sensitivity and specificity of 73.9 % (95 % CI: 60.9–87) and 80 % (95 % CI: 60–100), respectively (AUC=0.735, 95 % CI: 0.577–0.893).
The second approach for evaluation of mean CSA, mean ADC and mean FA along the examined MN segment
The mean CSA of the whole examined MN segments showed highly significant lower values in healthy subjects (10.12 mm2; range: 7.23–16.13) compared to CTS patients (19.9 mm2; range: 11.01–31.94) (p<0.001).
No significant differences between mean ADC (of the whole examined MN segments) in CTS patients (1.48 × 10-3 mm2/s; range: 1.13–1.79 × 10-3 mm2/s) and healthy subjects (1.47 × 10-3 mm2/s; range: 1.22–1.75 × 10-3 mm2/s) was found (p = 0.645).
The mean FA (of the whole examined MN segment) was 0.617 (range: 0.45–0.73) in healthy subjects versus 0.54 (range: 0.33–0.69) in CTS patients and the difference between the two groups was found to be significant (p=0.003).
ROC analysis revealed good discrimination between healthy subjects and CTS patients for both CSA (cut-off: 13.92 mm2, AUC=0.974, 95 % CI: 0.943–1) and FA (cut-off: 0.623, AUC=0.754, 95 % CI: 0.6–0.91); however, no discriminating ability was detected for mean ADC (AUC=0.472).
With the above cut-off values, sensitivity and specificity were found to be 93.3 % (95 % CI: 84.4–100) and 93.3 % (95 % CI: 80–100), respectively, for mean CSA and 82.6 % (95 % CI: 71.7–93.5) and 66.7 % (95 % CI: 40–93.3), respectively, for mean FA.
Intraobserver agreement
The Pearson correlation coefficients for the mean ADC and mean FA were 98 % (95 % CI: 91.5–99.5, p < 0.01) and 99 % (95 % CI: 95–99.7 %, p < 0.01), respectively.
Although mean CSA showed a lower correlation coefficient of 70 % (95 % CI 13.2–93.2, p = 0.023), the slice position of maximum median nerve CSA showed a significantly high correlation coefficient of 97 % (95 % CI: 87–99, p < 0.01). Correspondingly the ADC and FA at the site of maximum CSA showed also good correlation coefficients of 84.5 % (95 % CI: 46–96.2, p < 0.01) and 62 % (95 % CI: 1–90, p=0.056), respectively.
Discussion
Quantitative evaluation of DTI in CTS patients has already been shown to be feasible by calculating two commonly used parameters, ADC and FA, in attempting to obtain values for CTS patients and healthy subjects, with wide diversity shown in different studies [14, 21,22,23,24,25]. In the literature, ADC values ranging between 0.99 × 10-3 mm2/s [23] and 1.866 × 10-3 mm2/s [26] and FA values ranging between 0.359 [26] and 0.64 [23] were shown for CTS patients. Our calculated values for CTS patients fall within these ranges: 1.482 × 10-3 mm2/s and 0.545 for mean ADC and mean FA and 1.54 × 10-3 mm2/s and 0.524 for max ADC and max FA, respectively.
Nerve compression may lead to oedema of the nerve, which can extend for a variable distance in addition to the variability of the site of compression, either proximal or distal within the carpal tunnel. Most of the studies measured CSA of MN at the proximal carpal tunnel, but the nerve may be enlarged at other sites such as proximal to the flexor retinaculum or even in the distal carpal tunnel [27]. Other studies depended upon measuring the apparent largest portion of MN with in the carpal tunnel [3, 4]. To the best of our knowledge all the previously published studies considered ROI drawing and MN evaluation at single or multiple particularly anatomically determined levels (e.g. pisiform level and proximal to the flexor retinaculum) [1, 24, 25, 28]; however, based on those reasons we considered involving the whole examined nerve segment in our evaluation to prove ROIs at each axial cut section in order to get a mean value of the whole examined nerve segment, which may help in avoiding the unintended missing of a possible pathological segment. In addition, we evaluated the max CSA of MN segment irrespective of its anatomical location to determine both ADC and FA values at this particular level, which might be supposed to represent the site of maximum pathology within the examined MN segment (max ADC and max FA). Finally, both approaches were compared to determine which might be better for CTS diagnosis.
Both max CSA and mean CSA were significantly higher in CTS patients compared to healthy subjects (p<0.001), which may emphasise the proposed diffuse swelling of the examined nerve segment and the presence of oedema and structural changes.
Koh et al. [28] studied 42 CTS patients and 42 age-matched controls using a 3-T MRI scanner evaluating and comparing the value of CSA and DTI in diagnosing CTS. They detected the largest area under the curve (AUC) for CSA at a P1 level (1 cm proximal to pisiform level) among all the used anatomical parameters with a significant difference between CTS patients and healthy subjects (p=0.0001), showing values of 12.77 mm2 for control subjects versus 17.54 mm2 for CTS patients. This is in concordance with our results, where a significant difference was found between both groups when using max CSA, showing 12.85 mm2 for healthy subjects and 28.18 mm2 for CTS patients.
Our results are also in accord with Guggenberger et al. [29], who showed differences in CSA of the MN between healthy subjects and CTS patients at three different levels of measurement, with values of 11.99 cm2 and 14.89 cm2 for healthy subjects and CTS patients, respectively, at pisiform level.
Our study detected significant differences between CTS patients and healthy subjects by using either mean FA or max FA (p=0.003 and 0.007, respectively) and not for mean and max ADC (p>0.05). A significant decrease of mean FA was noted (0.617 and 0.54 for healthy subjects and CTS patients, respectively). Also, max FA showed a significant decrease in CTS patients compared to healthy subjects (0.524 vs. 0.613, respectively). Our results are in accord with Khalil et al. [30] who showed in 13 CTS patients and 13 healthy volunteers significantly lower FA values, but no significant difference with regard to ADC. They claimed that the significant reduction in FA (p=0.03) may be due to chronic compression, which may lead to histological changes within the nerve; they also suggested that the non-significant change in ADC (p>0.05) was due to the low number of subjects included in their study, which we showed in all of our 40 patients.
On the contrary, our results regarding mean ADC are not in accord with Guggenberger et al. [29] and Stein et al. [21], who showed a significant increase in ADC values in CTS patients compared to healthy subjects. This may be attributed to the difference in the examination protocol, where we used a RESOLVE sequence for the DTI approach instead of echo planar imaging sequence (EPI). RESOLVE is less prone to susceptibility artefacts and distortions than conventional EPI sequences [18], which is why we chose to use it in the current study. The difference in the post-processing approach may be another reason for this difference, in that we used the entire examined MN segment in our post-processing analysis; however, this was also noted upon applying max CSA, which may be attributable to the difference in the sample population and the relatively low number of healthy subjects in our study. However, both approaches, despite being more time-consuming, showed no significant differences between healthy subjects and CTS patients. Furthermore, both approaches emphasised the important role of FA in differentiation between healthy subjects and CTS patients, but questioned the role of ADC. Although we hypothesised that this new approach to data evaluation, calculating for mean ADC and mean FA, may compensate for the differences in ADC and FA parameters that may be caused by position differences, we did not notice significant differences between the two approaches regarding the obtained values.
Barcelo et al. [31] evaluated DTI of MN in 15 CTS patients and 20 healthy subjects using 3-T MRI in a similar study using EPI sequence for DTI. They considered a mean measurement of ADC and FA resulting from three different levels. A significant reduction of mean FA was noted in CTS patients compared to healthy volunteers (p=0.01), but no significant change was noted regarding mean ADC. These result corroborate our results as they emphasise the important role of FA and the insignificance of ADC in the diagnosis of CTS patients; however, according to our results, a single measurement at max CSA is sufficient.
In another study by Hiltunen et al. [14] they evaluated DTI of the MN in CTS patients both before and after surgery. They did not show a significant difference in either ADC or FA in CTS patients and the age-matched control group. A significant difference was found between pre-operative CTS patients and both the young control group and post-operative CTS patients, respectively. The difference in examination protocol, subject age, post-processing evaluation and multiple other parameters between our study and their study might have impacted on the results, and be the reason for this difference.
To the best of our knowledge, Guggenberger et al. [29] was the only study that could determine cut-off values for both FA and ADC. They showed 0.47 as a cut-off value for FA with a sensitivity and specificity of 83 % and 67 %, respectively and 1.05 × 10-3 mm2/s as a cut off value for ADC, with 83 % and 54 % sensitivity and specificity, respectively. In our study 0.62 was found to be the cut-off value for mean FA with 82.6 % sensitivity and 66.7 % specificity and 0.58 the cut-off value for max FA, with 73.9 % sensitivity and 80 % specificity. Therefore, according to our results measurement of max FA offers better results in the evaluation of CTS.
Our study confirmed the range of values for both FA and ADC and the significant reduction of FA in CTS patients compared to healthy subjects with cut-off value determination, which may be helpful for obtaining a more robust CTS diagnosis. We used two different approaches for data evaluation, using single- and multiple-level assessment. We even obtained a mildly higher specificity by using the single-level approach, which is of course more time saving.
Our study did have some limitations, with the relatively low number of healthy subjects included being the first and most important of them, but obtaining normative values for healthy subjects was beyond the scope of our study, as it had been studied intensively in the literature.
Second, only one reader evaluated the results with no interobserver evaluation, which might have affected the results. In our study, we adopted a long post-processing evaluation protocol that included drawing ROIs for all cut sections of MN in three different evaluated sequences (T2 tirm, ADC map and FA map). Such a process required a minimum of 90 min of complete meticulous work followed by registration and evaluation of the corresponding values. Unfortunately, in our study we could not repeat the same process with another examiner. However, all the post-processing was performed by an experienced radiologist with 10 years of experience in MSK radiology. For robustness of the data, two examiners did a preliminary trial evaluating three patients and three volunteers. The first examiner, who was the same examiner that performed data evaluation in this study, evaluated those subjects followed by another more experienced examiner (AK), who was a radiologist with 15 years of MSK radiology experience. The results obtained in this pre-study test showed good interobserver correlation between the two examiners. The results of those subjects were not included in this study.
In addition, our results showed a good intraobserver correlation, despite the time window of 5 months between the two evaluations. Our results showed a better correlation for mean ADC and mean FA, which may emphasise the reproducibility of the two parameters more than mean CSA. A high correlation for the slice position of max CSA with good correlation of corresponding max ADC and max FA supports our suggestion of using the single approach evaluation rather than a multiple-level evaluation.
In our study, we considered evaluation of certain specific parameters to evaluate MN; however, there are some other parameters that were not considered, such as ratio calculation of max CSA/CSA. In previous publications that were conducted on CTS patients, Δ-CSA and ratio showed efficiency in diagnosing CTS and grading of its severity [3, 4]. As the aim was directed mainly to functional evaluation of DTI using both ADC and FA, we did not include such parameters for initial data evaluation. However, a larger population to correlate DTI with severity grading will be of interest for further studies. US has already been proved in a study conducted on 427 patients to correlate with NLG severity a significant difference between mild and moderate CTS severity and moderate and severe CTS severity grades (p<0.001) [4]. Delta and ratio parameters are currently representative of routine US evaluation, which was not the target in our study.
In conclusion, we proved with two different approaches (single and multiple levels) that CSA and FA showed significant differences between CTS patients and healthy subjects, with cut-off values being determined. Single-level evaluation at the proposed level of maximum pathology is sufficient for FA determination with no need for more time-consuming approaches.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- CSA:
-
Cross-sectional area
- CTS:
-
Carpal tunnel syndrome
- DTI:
-
Diffusion tensor imaging
- EPI:
-
Echoplanner imaging
- FA:
-
Fractional anisotropy
- MN:
-
Median nerve
- MRI:
-
Magnetic resonance imaging
- NCS:
-
Nerve conduction studies
- ROI:
-
Region of interest
- US:
-
Ultrasound
References
Yao L, Gai N (2009) Median nerve cross-sectional area and MRI diffusion characteristics: normative values at the carpal tunnel. Skeletal Radiol 38(4):355–361
Andreisek G, Crook DW, Burg D, Marincek B, Weishaupt D (2006) Peripheral neuropathies of the median, radial, and ulnar nerves: MR imaging features. Radiographics 26(5):1267–1287
Klauser AS, Halpern EJ, De Zordo T et al (2009) Carpal tunnel syndrome assessment with US: value of additional cross-sectional area measurements of the median nerve in patients versus healthy volunteers. Radiology 250(1):171–177
Klauser AS, Abd Ellah MM, Halpern EJ et al (2015) Sonographic cross-sectional area measurement in carpal tunnel syndrome patients: can delta and ratio calculations predict severity compared to nerve conduction studies? Eur Radiol 25(8):2419–2427
Budzik JF, Balbi V, Verclytte S, Pansini V, Le Thuc V, Cotten A (2014) Diffusion tensor imaging in musculoskeletal disorders. Radiographics 34(3):E56–E72
Cotten A, Haddad F, Hayek G, Lefebvre G, Dodre E, Budzik JF (2015) Tractography: Possible Applications in Musculoskeletal Radiology. Semin Musculoskelet Radiol 19(4):387–395
Basser PJ, Jones DK (2002) Diffusion-tensor MRI: theory, experimental design and data analysis - a technical review. NMR Biomed 15(7-8):456–467
Bammer R (2003) Basic principles of diffusion-weighted imaging. Eur J Radiol 45(3):169–184
Bammer R, Acar B, Moseley ME (2003) In vivo MR tractography using diffusion imaging. Eur J Radiol 45(3):223–234
Nucifora PG, Verma R, Lee SK, Melhem ER (2007) Diffusion-tensor MR imaging and tractography: exploring brain microstructure and connectivity. Radiology 245(2):367–384
Skorpil M, Karlsson M, Nordell A (2004) Peripheral nerve diffusion tensor imaging. Magn Reson Imaging 22(5):743–745
Hiltunen J, Suortti T, Arvela S, Seppa M, Joensuu R, Hari R (2005) Diffusion tensor imaging and tractography of distal peripheral nerves at 3 T. Clin Neurophysiol 116(10):2315–2323
Wang H, Ma J, Zhao L, Wang Y, Jia X (2016) Utility of MRI Diffusion Tensor Imaging in Carpal Tunnel Syndrome: A Meta-Analysis. Med Sci Monitor 22:736–742
Hiltunen J, Kirveskari E, Numminen J, Lindfors N, Goransson H, Hari R (2012) Pre- and post-operative diffusion tensor imaging of the median nerve in carpal tunnel syndrome. Eur Radiol 22(6):1310–1319
Bulut HT, Yildirim A, Ekmekci B, Gunbey HP (2014) The diagnostic and grading value of diffusion tensor imaging in patients with carpal tunnel syndrome. Acad Radiol 21(6):767–773
Andreisek G, White LM, Kassner A, Sussman MS (2010) Evaluation of diffusion tensor imaging and fiber tractography of the median nerve: preliminary results on intrasubject variability and precision of measurements. AJR Am J Roentgenol 194(1):W65–W72
Padua L, LoMonaco M, Gregori B, Valente EM, Padua R, Tonali P (1997) Neurophysiological classification and sensitivity in 500 carpal tunnel syndrome hands. Acta Neurol Scand 96(4):211–217
Holdsworth SJ, Skare S, Newbould RD, Guzmann R, Blevins NH, Bammer R (2008) Readout-segmented EPI for rapid high resolution diffusion imaging at 3 T. Eur J Radiol 65(1):36–46
Team RDC. R (2011): A Language and Environment for Statistical Computing. R Foundation for Statistical Computing.
Robin X, Turck N, Hainard A et al (2011) pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12:77
Stein D, Neufeld A, Pasternak O et al (2009) Diffusion tensor imaging of the median nerve in healthy and carpal tunnel syndrome subjects. J Magn Reson Imaging 29(3):657–662
Naraghi A, da Gama LL, Menezes R et al (2013) Diffusion tensor imaging of the median nerve before and after carpal tunnel release in patients with carpal tunnel syndrome: feasibility study. Skelet Radiol 42(10):1403–1412
Lindberg PG, Feydy A, Le Viet D, Maier MA, Drape JL (2013) Diffusion tensor imaging of the median nerve in recurrent carpal tunnel syndrome - initial experience. Eur Radiol 23(11):3115–3123
Kabakci N, Gurses B, Firat Z et al (2007) Diffusion tensor imaging and tractography of median nerve: normative diffusion values. AJR Am J Roentgenol 189(4):923–927
Filli L, Piccirelli M, Kenkel D et al (2016) Accelerated magnetic resonance diffusion tensor imaging of the median nerve using simultaneous multi-slice echo planar imaging with blipped CAIPIRINHA. Eur Radiol 26(6):1921–1928
Brienza M, Pujia F, Colaiacomo MC et al (2014) 3T diffusion tensor imaging and electroneurography of peripheral nerve: a morphofunctional analysis in carpal tunnel syndrome. J Neuroradiol 41(2):124–130
Wong SM, Griffith JF, Hui AC, Tang A, Wong KS (2002) Discriminatory sonographic criteria for the diagnosis of carpal tunnel syndrome. Arthritis Rheum 46(7):1914–1921
Koh SH, Kwon BC, Park C, Hwang SY, Lee JW, Kim SS (2014) A comparison of the performance of anatomical MRI and DTI in diagnosing carpal tunnel syndrome. Eur J Radiol 83(11):2065–2073
Guggenberger R, Markovic D, Eppenberger P et al (2012) Assessment of median nerve with MR neurography by using diffusion-tensor imaging: normative and pathologic diffusion values. Radiology 265(1):194–203
Khalil C, Hancart C, Le Thuc V, Chantelot C, Chechin D, Cotten A (2008) Diffusion tensor imaging and tractography of the median nerve in carpal tunnel syndrome: preliminary results. Eur Radiol 18(10):2283–2291
Barcelo C, Faruch M, Lapegue F, Bayol MA, Sans N (2013) 3-T MRI with diffusion tensor imaging and tractography of the median nerve. Eur Radiol 23(11):3124–3130
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Andrea S. Klauser.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Funding
The authors state that this work has not received any funding.
Statistics and biometry
One of the authors has significant statistical expertise (Dr. Christian Kremser).
Informed consent
Written informed consent was obtained from all participants in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
No study subjects or cohorts have been previously reported.
Methodology
-
Prospective.
-
Diagnostic or prognostic study.
-
Performed at one institution
Rights and permissions
About this article
Cite this article
Klauser, A.S., Abd Ellah, M., Kremser, C. et al. Carpal tunnel syndrome assessment with diffusion tensor imaging: Value of fractional anisotropy and apparent diffusion coefficient. Eur Radiol 28, 1111–1117 (2018). https://doi.org/10.1007/s00330-017-5046-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5046-y