Abstract
Objectives
To determine the negative predictive value (NPV) of non-specific benign results from cone-beam CT (CBCT)-guided transthoracic core-needle biopsy (TTNB) and identify predicting factors for false-negative for malignancies.
Methods
From January 2009–December 2011, 1,108 consecutive patients with 1,116 lung lesions underwent CBCT-guided TTNB using an 18-gauge coaxial cutting needle. Among them, 226 patients with 226 TTNBs, initially diagnosed as non-specific benign, were included in this study. The medical charts, radiological or pathological follow-ups were reviewed to classify false-negative and true-negative results and to identify which variables were associated with false-negatives.
Results
Of 226 lesions, 24 (10.6%) were finally confirmed as malignancies and 202 (89.4%) as benign, of which the NPV was 89.4% (202/226). Multivariate analysis revealed that part-solid nodule (PSN) (odds ratio (OR), 3.95; P = 0.022), a biopsy result of ‘granulomatous inflammation’ (OR, 0.04; P = 0.022), and exact location of needle tip within targets (OR, 0.37; P = 0.045) were significantly associated with false-negatives among initial non-specific benign biopsy results.
Conclusion
The NPV of the non-specific benign biopsy was 89.4%. PSN was a significant positive indicator, but a biopsy result of ‘granulomatous inflammation’ and exact location of needle tip within targets were significant negative indicators for false-negatives.
Key Points
• The negative predictive value of the non-specific benign biopsy was 89.4%.
• A part-solid nodule is a significant predictor for false-negative biopsy (OR = 3.95).
• Pathological diagnosis of granulomatous inflammation is a robust indicator for ‘true-negatives’.
• Identifying needle tip within target lesions is a significant predictor for ‘true-negatives’.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Transthoracic core-needle biopsy (TTNB) has been proven to be a safe, highly accurate and minimally invasive approach to determining the benign or malignant nature of pulmonary lesions, and is considered to be the standard tissue sampling method for peripheral lung lesions [1–3]. Indeed, for lung cancer, TTNB has an approximately 90% chance of providing an accurate confirmation of the diagnosis [4]. Therefore, taken together with its extremely low false-positive rate (0.0–0.02%) [5], these positive biopsy results for malignancies using TTNB can have a direct impact on clinical decision making.
Establishing a specific benign diagnosis such as tuberculosis, fungal infection, or hamartoma via TTNB is also quite valuable as it enables patients with suspicious pulmonary lesions to avoid unnecessary surgery [4]. In this regard, core-needle biopsy greatly improved the ability to provide a specific diagnosis for benign lesions, whereas the specific diagnoses of benign conditions made through fine needle aspiration (FNA) has varied between 12% and 50% [6–8].
Despite excellent diagnostic accuracy for malignancies with TTNB and use of the core-needle biopsy technique, non-specific benign results for malignancies from lung biopsies still remain a diagnostic challenge, and have led to a key clinical dilemma. Whether or not a lesion is adequately sampled, if it is adequately sampled, can we be certain that the lesion is truly negative; what should be the next best diagnostic work-up without ‘specific’ results [9]? Due to the substantial false-negative rate of TTNBs, ranging from 5% to 12% [10–13], non-specific benign biopsy results cannot necessarily guarantee a lesion’s benignity and, therefore, cannot preclude further work-ups, including more invasive diagnostic procedures (such as surgery). In fact, there is no established consensus at present as to which diagnostic work-up should be included when initial lung biopsies show non-specific benign results, although there have been several differing options including repeated TTNB (on the same target), a biopsy at another site (if available), follow-ups or other imaging examinations (such as 18F-FDG PET) [4].
Therefore, the purpose of this study was to determine the negative predictive value (NPV) of non-specific benign results from cone-beam CT (CBCT)-guided TTNB and to identify the predicting factors of false-negatives among these non-specific benign biopsy results.
Materials and methods
This retrospective study was approved by the Institutional Review Board of Seoul National University Hospital with a waiver of the requirement for patient informed consent.
Subjects
From January 2009 to December 2011, 1,153 consecutive CBCT-guided TTNBs were performed on 1,116 lung lesions (mean (± SD) size, 2.7 ± 1.7 cm) in 1,108 patients (633 male, 475 female; mean (± SD) age, 62.4 ± 12.3 years) at our hospital. Out of 1,108 patients, eight underwent TTNBs twice for different pulmonary lesions. Thirty-seven repeat TTNB procedures were excluded in this study (as this study dealt solely with the initial TTNBs).
Pathological results of TTNBs in this study were classified into one of the following four groups: (1) diagnosis or suspicion of malignancy; (2) specific benign; (3) non-specific benign; or (4) non-diagnostic. ‘Specific benign’ results were defined as benign tumours (e.g. hamartoma and leiomyoma) or infectious diseases with identified pathogens, including fungal, bacterial or mycobacterial infections that could explain the radiological findings [14]. ‘Non-specific benign’ results were defined as the presence of benign pathological features, such as inflammatory cells or fibrosis, but not enough to render a specific diagnosis. Non-diagnostic biopsies were defined when adequate specimens were not obtained through biopsy or when the obtained specimens were not pathologically adequate for diagnosis.
Based on the clinical suspicion for malignancy, underlying co-morbidity of the patients and patient preferences, the attending physician comprehensively decided the protocol for the follow-up (e.g. surgical resection, repeat biopsy, imaging follow-up) of non-specific benign results from TTNB.
The final diagnosis of cases with non-specific benign biopsy results was determined to be true negative when: (1) a specific benign diagnosis was established from a subsequent surgical resection or repeat biopsy, (2) the lesion regressed with medical (or conservative) treatment, or (3) the lesion remained stable in size for at least 2 years [15]. False-negative biopsies were defined as those in which the diagnosis of malignancy was established from a subsequent surgical or repeat biopsy [14].
In this study, 720 and 102 lesions were confirmed as malignant or specific benign lesions, respectively. Nine biopsies were non-diagnostic. A total of 285 biopsies were classified as non-specific benign. However, 59 of these cases were lost to follow-up or had insufficient data for follow-up imaging surveillance. Therefore, we excluded 59 non-specific benign lesions for which a final diagnosis was not established. Finally, 226 initial non-specific benign biopsies in 226 patients (mean (± SD) age, 59.5 ± 12.2 years; age range, 21–86) were included in this study. There were 119 male patients (mean (± SD) age, 61.6 ± 11.8 years; age range, 21–86) and 107 female patients (mean (± SD) age, 57.1 ± 12.2 years; age range, 22–83 years). The mean (± SD) size of the lesions was 2.4 cm ± 1.5 (range, 0.6–9.4 cm).
CBCT-guided TTNB technique
All 1,116 consecutive TTNBs were performed by, or under the supervision of, a primary attending operator (C.M.P., with 7 years of experience in image-guided TTNB). TTNBs were performed by using two different CBCTs (Axiom Artis dTA/VB30 flat-panel detector with a 2,048 × 1,538 element, Siemens, Erlangen, Germany; Allura Xper FD20 flat-panel detector with a 2,480 × 1,920 element, Philips Healthcare, Best, The Netherlands). On pre-procedural CBCT images, operators determined the most effective, and safest, needle pathways to their targets. After a 17-gauge coaxial introducer was inserted into the target, procedural CBCT was performed to identify the proper location of the coaxial needle tip. Subsequently, an 18-gauge cutting needle (Stericut®, TSK Laboratory, Tochigi-Ken, Japan) was advanced into the target through a coaxial introducer and biopsies were performed until sufficient specimens were obtained.
After the removal of the coaxial introducer, post-procedural CBCT was performed to identify immediate procedure-related complications. An erect chest radiograph was routinely obtained 3 h after biopsy to evaluate the presence of pneumothorax. If patients developed substantial chest pain, dyspnoea or hypoxaemia, an erect or supine chest radiograph was immediately obtained.
Data collection: Procedure and clinical information
After each TTNB, patient demographics (age and sex), target lesion characteristics (size, location, nodule type) and procedural information (patient position, pleura-to-target distance (PTD), number of pleural passages, number of tissue samplings) were routinely recorded at our institution. Lesion size, defined as the longest diameter of the lesion, was measured on diagnostic chest CT images and the target lesion type was categorized into either solid or part-solid nodules (PSNs) according to their CT morphologies. The presence of procedure-related complications (pneumothorax, haemoptysis, air-embolism or procedure-related mortality) was also recorded. A retrospective chart review was performed for relevant assessment of clinical information, which included smoking status, number of pack years, history of previous malignancy or tuberculosis, and the patient’s immune status.
One board-certificated radiologist (H.K., with 3 years of experience in chest radiology), reviewed procedural CBCT images retrospectively to determine the location of a coaxial needle tip within various targets. It was designated as ‘needle tip within target’ when a coaxial needle tip was present within the target lesions on procedural CBCT images. The reviewer also collected information about the maximum standardized uptake values (SUVmax) of the target lesions (where available).
Statistical analysis
Among cases with non-specific benign results on TTNBs, comparison of false-negative cases versus true-negative cases was performed using the independent-samples t-test, Pearson’s chi-square test, Fisher’s exact test (in terms of patient or lesion characteristics) and biopsy procedural variables, as appropriate. For normality of distribution and equality of variances, continuous data were tested using Levene’s test. A p-value of less than 0.05 indicated a significant difference.
Subsequently, multivariate logistic regression analysis was performed to determine the distinguishing features of false-negative malignancies from those of true benign. Variables with a p-value of less than 0.10 in univariate analyses were used as input variables for multivariate analysis. Due to the complete separation phenomenon, the penalized maximum likelihood estimation method was used instead of classic logistic regression analysis to determine the individual distinguishing features of false-negative cases from true-negative TTNB cases [16].
All statistical analyses were conducted using SPSS (version 18.0 for Windows, SPSS, Chicago, IL, USA) and STATA software (version 10, Stata Corp, College Station, TX, USA).
Results
Of the 226 cases, 24 (10.6%) were ultimately determined to be malignant and 202 (89.4%) to be benign. The negative predictive value (NPV) of non-specific benign pathologies upon initial TTNB was 89.4% (202/226; confidence interval (CI), 84.4– 92.9).
Final diagnosis of non-specific benign biopsies
The final diagnoses of the 24 false-negatives included 20 primary lung cancers (14 adenocarcinomas, one large cell lung carcinoma, three squamous cell carcinomas, one non-small cell lung cancer and one mucoepidermoid carcinoma) and four metastases (three from colon cancer and one from thyroid cancer). The final diagnoses of these 24 false-negatives were made through surgical resection (n = 13), repeat TTNBs (n = 9) and bronchoscopic biopsies (n = 2). The median diagnostic delay in these 24 malignancies was 0.7 months, ranging from 1 day to 32.3 months.
The final diagnoses of 202 true negatives were rendered on the basis of surgical resection (n = 19), repeat TTNBs (n = 6), positive acid-fast bacilli (AFB) sputum smear with anti-tuberculosis treatment response (n = 2) and shrinkage upon follow-up or stability in size for at least 2 years (n = 175). Of the 19 surgical cases, non-specific inflammation without identifiable organisms (n = 9), organized pneumonia (n = 2), pulmonary tuberculosis (n = 2), cryptococcosis (n = 2), aspergillosis (n = 3) or hamartoma (n = 1) were their final diagnoses. Of the six repeat TTNBs, five pulmonary tuberculosis and one sclerosing hemangioma were finally diagnosed.
False-negatives versus true-negatives: Predictors of final false-negatives
Table 1 summarizes the demographic, clinical and biopsy procedural features of ‘false-negatives’ and ‘true negatives’. On univariate analyses, there were significant differences between the two groups in terms of patient age 50 years or older (95.8% vs. 79.2%, p = 0.022), part-solid nodule (25% vs. 5.4%, p = 0.001), needle tip within targets (62.5% vs. 84.7%, p = 0.007) and biopsy results of ‘granulomatous inflammation’ (0% vs. 40.1%, p = 0.000). There were no significant differences between the two groups in terms of biopsy-related complications and clinical features of patients such as history of malignancy or immune status (compromised vs. competent).
Multiple logistic regression analyses revealed that PSN (adjusted odds ratio [OR] = 3.95, p = 0.022) was a significant independent positive indicator for false-negatives (Fig. 1). A biopsy result of ‘granulomatous inflammation’ (OR = 0.04, p = 0.022) and needle tip within target (OR = 0.37, p = 0.045) were significant independent indicators for true-negatives (Table 2) (Fig. 2).
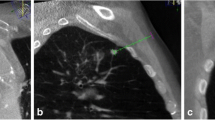
A false-negative part-solid nodule for which the biopsy result was benign non-specific pathology. (a) A diagnostic chest CT image in a 66-year-old man shows a 2.4-cm part-solid nodule (arrow) in the right lower lobe and lung cancer was highly suspected. (b) On the transverse procedural CBCT image, the coaxial needle tip abutted the mass. After the biopsy, the lesion was diagnosed as intra-alveolar macrophages and fibrins. Taking into consideration the discrepancy between the biopsy results and the CT findings, he performed a wedge resection through video-assisted thoracic surgery (VATS). Finally, the lesion was confirmed as adenocarcinoma
A mass for which the biopsy result was a chronic granulomatous inflammation. (a) A diagnostic chest CT image in a 71-year-old woman showed a 4.7-cm irregular mass (arrow) in the right upper lobe. (b) The maximum standardized uptake value (SUV) of the mass was 8.1 on FDG-PET/CT scan. (c) On the transverse procedural CBCT image, the exact location of the needle tip in the mass was noted. There was a minimal amount of pneumothorax. A chronic granulomatous inflammation with negative polymerase chain reaction for Mycobacterium tuberculosis (TB-PCR) was reported on the biopsy specimen. (D) After 6 months with conservative management, the lesion was almost completely resolved on follow-up CT scan
True negatives: Chronic granulomatous inflammation
The most common pathological result from non-specific benign biopsy was granulomatous inflammation (n = 81). The final diagnosis was determined for eight chronic granulomatous inflammatory lesions on the basis of surgical resection (n = 6) and repeat biopsy (n = 2) revealing four cases of pulmonary tuberculosis, two of granulomatous inflammation without identifiable organisms, one unexplained vasculitis and one case of cryptococcosis. The majority of remaining cases with a diagnosis of granulomatous inflammation (n = 52) had shrunk (or resolved) on subsequent chest radiograph or chest CT scans, and 21 lesions remained stable in size for more than 2 years. It should be noted that there were no false-negatives out of the 81 cases of granulomatous inflammations.
Discussion
We found that 10.6% (24 out of 224) of non-specific benign biopsy results from CBCT-guided TTNBs were falsely negative for malignancies. Surprisingly, there were no false-negatives in the 81 cases of granulomatous inflammations after TTNBs. Granulomatous inflammation is pathologically characterized by the recruitment and organization of activated macrophages and lymphocytes in discrete lesions [17]. Granuloma formation is a type of protective response to chronic infection, but it can also be elicited by non-infectious agents [17, 18]. The causes of granulomatous lung disease include infection, sarcoidosis, autoimmune disease, hypersensitivity pneumonitis, aspiration pneumonia, talc granulomatosis, etc. [19]. However, a majority of granulomas in the lung are caused by mycobacterial or fungal infection, although organisms were identified by special stains in only one-third [20].
Few granulomas have been observed in association with carcinomas, carcinoid tumours and various forms of Hodgkin’s and non-Hodgkin’s lymphoma [21]. However, there were actually no false-negative cases among the 81 granulomatous inflammations in this study. Furthermore, in a study by Mukhopadhyay et al.[22], only two cases (lymphoma or lymphoid interstitial pneumonia) were identified among 500 cases of granulomatous lung disease. If anything, granulomatous inflammation is one of the most common sources of false-positive diagnoses in fine-needle lung aspirates rather than false-negatives [23]. Therefore, a granulomatous inflammation from a benign biopsy result can assure the patient and physician that it is indicative of a benign process and can preempt the need for more invasive diagnostic procedures in order to avoid false-negative results.
Comparison of the biopsy-related parameters showed that false-negatives occurred in more biopsies in which the needle tip was not verified to be within the established targets (37.5% vs. 15.3%, p = 0.007). Confirmation of the final needle location within targets can guarantee that the tissue sampling would be successful and reduce sampling error or technical failure of TTNBs. The main explanation for false-negatives can be that they are mostly a result of tissue sampling errors or failure to sample sufficient biological specimen for pathological diagnosis [9]. Hence, it may be important for operators performing TTNBs to confirm the exact position of the needle tip within targets.
Previous studies reported that target size, lower lobe location, fewer adjustments of the needle, lack of positive cultures and the occurrence of a pneumothorax were associated with false-negatives (or inconclusive results) of CT-guided TTNBs [14, 15, 24, 25]. In this study, there were no significant correlations between false-negative results of benign non-specific biopsies and any of the following factors: patient sex, lesion size, location or procedure-related features such as patient’s position, pleura-to-target distance, number of pleural passages or number of tissue samples. However, the proportion of part-solid nodules was significantly higher in the false-negative group than in the true-positive group (25% vs. 5.4%, p = 0.001). Importantly, the malignancy rate for persistent PSNs was 63% and, actually, their malignancy probability was significantly higher than that for solid lung nodules (p = 0.03) [26]. Lu et al. [27] revealed that stromal invasion of subsolid nodule was underestimated in 43.5% of CT-guided core biopsy specimens compared to the surgical pathology, and Kim et al. [28] also reported an underestimation of the pathological grade of subsolid nodules on biopsy. Given the substantially higher probability of malignancy and some limitations for the pathological confirmation of PSNs on biopsy specimens, a non-specific benign result from a PSN biopsy should prompt immediate additional evaluation (or surgical resection) in order to exclude false-negative malignancy.
Our study is limited by its retrospective design. The final confirmation of their benignity could not always be recorded unless patients were referred for re-evaluation to our department by their attending physicians. Furthermore, as a relatively large proportion of our initial population did not undergo follow-up (59 cases), there may have been selection bias of the study population. Thus, the negative predictive value of non-specific benign results of TTNBs may not have been exactly estimated. Second, we did not review pathological specimens to determine whether false-negative cases resulted from sampling or interpretive errors (or both). However, since ‘the confirmation of needle tip’ position within targets was thought to reduce sampling errors and ‘the non-visualization of needle tip’ within targets could reasonably be considered a significant predictive factor for false-negative biopsies [29], sampling errors might be a major contributor to false-negative biopsies.
In our study of 226 initial non-specific benign TTNBs, we found that a cancer diagnosis was missed in 10.6% of the non-specific benign biopsy results. PSN was a significant associated factor of a false-negative malignancies and a biopsy result of ‘granulomatous inflammation’ and needle tip within targets were significant predictors of true-benign lesions.
Abbreviations
- CBCT:
-
Cone-beam computed tomography
- FDG-PET:
-
Fluorodeoxyglucose (18F-FDG) positron emission tomography
- NPV:
-
Negative predictive value
- PSN:
-
Part-solid nodule
- TTNB:
-
Transthoracic core-needle biopsy
References
Libby DM, Smith JP, Altorki NK, Pasmantier MW, Yankelevitz D, Henschke CI (2004) Managing the small pulmonary nodule discovered by CT. Chest 125:1522–1529
Manhire A, Charig M, Clelland C et al (2003) Guidelines for radiologically guided lung biopsy. Thorax 58:920
Fontaine-Delaruelle C, Souquet P-J, Gamondes D et al (2015) Negative predictive value of transthoracic core-needle biopsy: a multicenter study. Chest 148:472–480
Rivera MP, Mehta AC, Wahidi MM (2013) Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143:e142S–e165S
Schreiber G, McCrory DC (2003) Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest 123:115S–128S
Gong Y, Sneige N, Guo M, Hicks ME, Moran CA (2006) Transthoracic Fine-Needle Aspiration vs Concurrent Core Needle Biopsy in Diagnosis of Intrathoracic Lesions A Retrospective Comparison of Diagnostic Accuracy. Am J Clin Pathol 125:438–444
Greif J, Marmor S, Schwarz Y, Staroselsky AN (1999) Percutaneous core needle biopsy vs. fine needle aspiration in diagnosing benign lung lesions. Acta Cytol 43:756–760
Klein JS, Salomon G, Stewart EA (1996) Transthoracic needle biopsy with a coaxially placed 20-gauge automated cutting needle: results in 122 patients. Radiology 198:715–720
Minot DM, Gilman EA, Aubry MC et al (2014) An investigation into false-negative transthoracic fine needle aspiration and core biopsy specimens. Diagn Cytopathol 42:1063–1068
Yang W, Sun W, Li Q et al (2015) Diagnostic Accuracy of CT-Guided Transthoracic Needle Biopsy for Solitary Pulmonary Nodules. PLoS One 10:e0131373
Yamagami T, Yoshimatsu R, Miura H et al (2013) Diagnostic performance of percutaneous lung biopsy using automated biopsy needles under CT-fluoroscopic guidance for ground-glass opacity lesions. Br J Radiol 86:20120447
Yaffe D, Koslow M, Haskiya H, Shitrit D (2015) A novel technique for CT-guided transthoracic biopsy of lung lesions: improved biopsy accuracy and safety. Eur Radiol 25:3354–3360
Floridi C, Muollo A, Fontana F et al (2014) C-arm cone-beam computed tomography needle path overlay for percutaneous biopsy of pulmonary nodules. Radiol Med 119:820–827
Gelbman BD, Cham MD, Kim W et al (2012) Radiographic and clinical characterization of false negative results from CT-guided needle biopsies of lung nodules. J Thorac Oncol 7:815–820
Hiraki T, Mimura H, Gobara H et al (2009) CT Fluoroscopy-Guided Biopsy of 1,000 Pulmonary Lesions Performed With 20-Gauge Coaxial Cutting NeedlesDiagnostic Yield and Risk Factors for Diagnostic Failure. Chest 136:1612–1617
Heinze G, Schemper M (2002) A solution to the problem of separation in logistic regression. Stat Med 21:2409–2419
Perez RL, Rivera-Marrero CA, Roman J (2003) Pulmonary granulomatous inflammation: From sarcoidosis to tuberculosisSemin Respir Infect, pp 23-32
Silverman JF (1995) Inflammatory and neoplastic processes of the lung: differential diagnosis and pitfalls in FNA biopsies. Diagn Cytopathol 13:448–462
Mukhopadhyay S, Gal AA (2010) Granulomatous lung disease: an approach to the differential diagnosis. Arch Pathol Lab Med 134:667–690
Doxtader EE, Mukhopadhyay S, Katzenstein A-LA (2010) Core needle biopsy in benign lung lesions: pathologic findings in 159 cases. Hum Pathol 41:1530–1535
Myers JL, Tazelaar HD (2008) Challenges in pulmonary fibrosis: 6--Problematic granulomatous lung disease. Thorax 63:78–84
Mukhopadhyay S, Farver CF, Vaszar LT et al (2012) Causes of pulmonary granulomas: a retrospective study of 500 cases from seven countries. J Clin Pathol 65:51–57
Auger M, Moriarty AT, Laucirica R et al (2010) Granulomatous inflammation-an underestimated cause of false-positive diagnoses in lung fine-needle aspirates: observations from the college of american pathologists nongynecologic cytopathology interlaboratory comparison program. Arch Pathol Lab Med 134:1793–1796
Lee YJ, Hwang YI, Kim TJ et al (2011) Inconclusive result from CT guided transthoracic needle aspiration and biopsy: affecting factors and final outcome. J Lung Cancer 10:94–101
Montaudon M, Latrabe V, Pariente A, Corneloup O, Begueret H, Laurent F (2004) Factors influencing accuracy of CT-guided percutaneous biopsies of pulmonary lesions. Eur Radiol 14:1234–1240
Henschke CI, Yankelevitz DF, Mirtcheva R, McGuinness G, McCauley D, Miettinen OS (2002) CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol 178:1053–1057
Lu C-H, Hsiao C-H, Chang Y-C et al (2012) Percutaneous computed tomography-guided coaxial core biopsy for small pulmonary lesions with ground-glass attenuation. J Thorac Oncol 7:143–150
Kim TJ, Lee J-H, Lee C-T et al (2008) Diagnostic accuracy of CT-guided core biopsy of ground-glass opacity pulmonary lesions. AJR Am J Roentgenol 190:234–239
Saad RS, Silverman JF (2010) Respiratory cytology: differential diagnosis and pitfalls. Diagn Cytopathol 38:297–307
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Chang Min Park.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Funding
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (grant number : HC15C3390).
Statistics and biometry
No complex statistical methods were necessary for this paper.
Ethical approval
Institutional Review Board approval was obtained.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported (Kim JI, Park CM, Lee SM, Goo JM (2015) Rapid needle-out patient-rollover approach after cone beam CT-guided lung biopsy: effect on pneumothorax rate in 1,191 consecutive patients. Eur Radiol 25:1845–1853)
Methodology
• retrospective
• cross-sectional study
• performed at one institution
Rights and permissions
About this article
Cite this article
Kim, J.I., Park, C.M., Kim, H. et al. Non-specific benign pathological results on transthoracic core-needle biopsy: how to differentiate false-negatives?. Eur Radiol 27, 3888–3895 (2017). https://doi.org/10.1007/s00330-017-4766-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-4766-3