Abstract
Background
Serial chest CT is the standard of care to establish treatment success in invasive pulmonary aspergillosis (IPA). Data are lacking how response should be defined.
Methods
Digital CT images from a clinical trial on treatment of IPA were re-evaluated and compared with available biomarkers. Total volume of pneumonia was added up after manual measurement of each lesion, followed by statistical analysis.
Results
One-hundred and ninety CT scans and 309 follow-up datasets from 40 patients were available for analysis. Thirty-one were neutropenic. Baseline galactomannan (OR 4.06, 95%CI: 1.08–15.31) and lesion volume (OR 3.14, 95%CI: 0.73–13.52) were predictive of death. Lesion volume at d7 and trend between d7 and d14 were strong predictors of death (OR 20.01, 95%CI: 1.42–282.00 and OR 15.97, 95%CI: 1.62–157.32) and treatment being rated as unsuccessful (OR 4.75, 95%CI: 0.94–24.05 and OR 40.69, 95%CI: 2.55–649.03), which was confirmed by a Cox proportional hazards model using time-dependent covariates.
Conclusion
Any increase in CT lesion volume between day 7 and day 14 was a sensitive marker of a lethal outcome (>50%), supporting a CT rescan each one and 2 weeks after initial detection of IPA. The predictive value exceeded all other biomarkers. Further CT follow-up after response at day 14 was of low additional value.
Key Points
• CT evaluation offers good prediction of outcome for invasive pulmonary aspergillosis.
• Predictive capability exceeds galactomannan, blood counts, and lesion count.
• Any progression between day 7 and day 14 constitutes a high-risk scenario.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Invasive pulmonary aspergillosis (IPA) is a frequent and severe complication of immunocompromised patients, especially those with prolonged neutropenia after receiving anti-cancer chemotherapy [1–3]. Thin-section computed tomography (CT) imaging of the lungs has become a diagnostic standard of care for early diagnosis of invasive mould infections [4, 5]. In recent years, new antifungal drugs have improved treatment results, although treatment failure rates remain close to 50% [6, 7]. For most patients, the underlying immunosuppression cannot be stopped at onset of IPA; cancer patients often need further chemotherapy or allogeneic stem cell transplantation. For these reasons, there is great uncertainty on the optimal treatment duration for IPA [8, 9].
There are currently no biomarkers allowing reliable monitoring of IPA disease activity. For patients with positive baseline result, decline of serum galactomannan during the course of therapy seems to be associated with favourable outcome [10–12]. However, no clinical studies have demonstrated the safety of treatment discontinuation based on galactomannan levels thus far. In the current situation, response assessment and decision making is typically based on serial CT imaging, unspecific markers of inflammation (C-reactive protein, fever) and patient immune status. Previous studies have demonstrated slow regression of pulmonary lesions associated with IPA [9, 13, 14]. In one study reporting on CT findings in 46 patients with IPA, the mean mass reduction after 21 days was 57% [14]. Another study showed a reduction to 0.76-fold the initial volume in 30 patients with IPA receiving serial CT imaging for a median follow-up of 17 days [13]. A frequently reported finding is the paradoxical increase of lesion volume after 1 week of antifungal treatment [13–15], which can make interpretation of CT results difficult. This is often attributed to recovery of immune function, especially an increase of neutrophil counts in formerly neutropenic patients. The optimum timing of follow-up imaging and reliable radiological predictors of treatment failure remain unknown.
We assessed serial CT images from a recent clinical trial on treatment of IPA by manual three-dimensional volume measurement to define the optimal cut-off for assessment of treatment success and survival [16].
Methods
Background
Digital CT images from a recently completed clinical trial [17, 18] were re-evaluated in detail for volumetric analysis of all pulmonary lesions and full count of all pulmonary lesions. Pulmonary lesion was defined as pathological finding of any size of potential infectious origin, excluding lung pathologies clearly attributable to other conditions, e.g. tumour lesions or atelectasis. In brief, patients were included into the study if they had proven or probable IPA by modified criteria of the European Organization for Research and Treatment of Cancer and the Mycoses Study Group (EORTC/MSG), as described previously [7]. The trial investigated safety and pharmacokinetics of escalating doses of caspofungin as the primary treatment for IPA. Trial medication was given for a maximum of 28 days; follow-up information was collected until 12 weeks after end-of-treatment (EOT). The study was registered with the European Union Drug Regulating Authorities Clinical Trials website (EudraCT 2006-001936-30) and on ClinicalTrials.gov (NCT00404092). All patients gave informed consent and the trial was approved by ethics committees and/or institutional review boards at all study sites.
Computed tomography was not part of the study intervention, but all CT studies performed as part of clinical routine were submitted to one site (CPH) for centralized radiological assessment of study inclusion criteria and treatment response blinded to clinical information. The role of contrasting agents for IPA is not fully established, and investigators were encouraged to perform native CTs for IPA diagnosis and follow-up.
Data processing and analysis
Total volume of pneumonia was added up after manual measurement of each pulmonary lesion at a PACS workstation (synapse 4.2, FUJI, Düsseldorf, Germany), using a window/level setting of 1600/-600 H.U. Axial images were screened for pulmonary lesions, which were then measured in all three axes using axial and coronal images. Coronal images were reconstructed if not already part of the dataset. Volume was calculated by multiplying size in three dimensions multiplied with Pi divided by 6, as described elsewhere [13]. All analyses were performed using the thinnest slices available, i.e. 1 or 2 mm thickness.
To allow comparison of CT studies from different patients during follow-up, all time-dependent covariates were allocated to follow-up time slots: 0 ± 3 days, 7 ± 3 days, 14 ± 3 days, 21 ± 3 days, 28 ± 3 days, 56 ± 13 days, 84 ± 13 days, 112 ± 13 days, and 140 ± 13 days, with day 0 as the first day of study treatment. Patients with fewer than two CTs with available image data were excluded from analysis. If more than one CT was performed during one follow-up slot, the CT closer to the intended follow-up was chosen. If more than one CT was performed with the same interval from follow-up, the first one was used for the evaluation.
The same allocation was performed with absolute neutrophil counts (ANC), white blood cell counts (WBC), and galactomannan; however, these values were only available until end of treatment (EOT), which was maximum 28 days. Linear regression was used to impute relative trends for all scaled values at each follow-up in the time series. Trends were used for imputation of inlying missing values.
Response was defined as clinically meaningful improvement of attributable symptoms, signs, and radiographic (≥50% decrease) or bronchoscopic abnormalities, if present at enrolment. Failure was defined as deterioration of attributable clinical or radiographic abnormalities necessitating alternative antifungal therapy or resulting in death.
Statistical analysis
The primary goal of this post hoc analysis was to identify predictors of outcome by an exploratory analysis of available covariates. We added time series data on lesion volume, lesion count, galactomannan, leukocyte count, and neutrophil count to the dataset of the clinical trial and calculated trends between points of the timeline. As this study looked at a subset of another study, we performed a new descriptive analysis of patient demographics and baseline condition.
To predict which patients would ultimately have treatment success and which patients would survive, we performed further analyses: we used Mann-Whitney U test to compare differences between outcome groups at different points of the timeline, Spearman’s rho for correlation analysis between covariates, and binary logistic regression to identify independent predictors of outcome. To confirm or refute findings from the normalized time-line (see above), we fitted a Cox proportional hazards model with time-dependent covariates using full available temporal granularity (date of assessment). Finally, we used receiver-operating characteristics (ROC) to suggest a cut-off for future evaluation.
Results
Six patients were excluded from the study due to insufficient records. Exclusion was associated either with study centres only submitting printed copies (two patients) or early study termination (four patients). The two patients with printed copies were considered to be missing completely at random and, therefore, ignorable. For the four patients with early termination, missingness was not at random, but associated with treatment failure; however, as patients with severe deterioration before first follow-up assessment inherently could not benefit from standardized follow-up assessment, we decided to omit data from these patients as well. Therefore, inference of the following analyses is limited to patients without severe deterioration within the first week of treatment.
Absolute measurements of WBC, ANC, galactomannan, lesion volume, and lesion count showed high skewness and kurtosis and were logarithmically transformed. Because of high overlap of ANC and WBC and sporadic missingness of ANC values, the ANC was dropped from all logistic regression analyses.
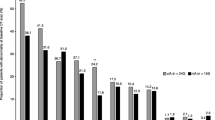
Forty patients with 190 CT scans and 309 follow-up records from three centres were included into the analysis set. Caspofungin dose was 70 mg in seven patients (18%), 100 mg in eight patients (20%), 150 mg in nine patients (23%), and 200 mg in 16 patients (40%). Eighteen patients (45%) were female. At treatment start, 31 patients (78%) were neutropenic and 12 (43%) had a positive galactomannan. Underlying diseases were acute leukaemia in 20 patients (50%), chronic lymphocytic leukaemia in five (13%), high-grade lymphoma in six (15%), low-grade lymphoma in three (8%), and other diseases in six (14%). At EOT, one patient (3%) had achieved complete response (CR), 23 (58%) partial response (PR), four (10%) stable disease, while 10 (25%) experienced progressive disease, and two (5%) developed a relapse after initial response. Eleven patients died during follow-up. Lesion size and outcome over time are demonstrated in (Fig. 1).
Baseline
At baseline, 1136 pulmonary lesions were measured, 516 of which were ≥ 1 cm large in at least one dimension and 269 of which were ≥ 1 cm3. Patients who died during the study period demonstrated a higher baseline galactomannan compared to survivors, while otherwise showing similar baseline risk factors according to lesion volume, lesion count, WBC, and ANC (Table 1). There was considerable variance in all baseline parameters, especially lesion count, which ranged from five to 131. Stepwise backward logistic regression of baseline parameters allowed some prediction of survival (Cox & Snell R2 0.165, 73% prediction accuracy) by log10 of lesion volume (OR 3.14, 95% CI: 0.73 – 13.52) and log10 of galactomannan (OR 4.06, 95% CI: 1.08 – 15.31).
Day 7
At day 7, 24 (60%) patients were still neutropenic and eight (20%) had a positive galactomannan. Lesion volume declined in patients who were ultimately rated as having treatment success (-17,453 mm3), while it increased in patients with treatment failure (+508,192 mm3), although given broad confidence intervals, this difference was not significant. Univariate analysis showed that patients who died during the study period had a higher relative increase of lesion volume and a larger absolute lesion volume at day 7 (Table 1). However, even survivors showed increasing lesion volumes between day 0 and day 7. Stepwise backward logistic regression allowed an improved model for treatment outcome (Cox & Snell R2 0.374, 80.6% prediction accuracy) using change of volume between day 7 and day 14 (OR 40.69, 95% CI: 2.55 – 649.03) and log10 of lesion volume (OR 4.75, 95% CI: 0.94 – 24.05). The same model was able to predict death (Cox & Snell R2 0.336, 83.3%) with OR 20.01 (95% CI: 1.42 – 282.00) for log10 of lesion volume and OR 15.97 (95% CI: 1.62 – 157.32) for relative change of volume between day 7 and 14.
Day 14
After 2 weeks, 37 patients (93%) remained under observation. Of these, 15 (41%) were still neutropenic and seven (19%) had a positive galactomannan. Lesion volume declined both in patients responding (-112,530 mm3) and not responding (-74,651 mm3), while lesion count remained relatively stable.
Univariate analysis showed a strong correlation between relative change of lesion volume between days 7 to 14 and survival (Table 2). Again, a lower lesion volume was associated with better survival (Table 2).
Further correlations
Statistical analysis by Spearman’s rho showed no significant correlations between galactomannan, WBC, lesion volume, and lesion count over the period from day 0 to day 28. To exclude lag-time bias, all correlations were retested after introducing one follow-up step of lag-time to each variable in turn. For follow-up visits at day 21 and day 28, no additional correlations between outcome/survival were found by univariate and multivariate analysis.
A Cox proportional hazards model with time-dependent covariates measured between baseline and day 28 was calculated for treatment failure and survival. For failure, only log10 of lesion volume was a significant predictor (HR 6.04, 95% CI: 1.60 – 22.78), while for death, both log10 of lesion volume (HR 14.72, 95% CI 3.67 – 59.08) and log10 of galactomannan (HR 4.58, 95% CI: 2.00 – 10.51) were predictive. These results were robust to separate analysis of risk factors (ANC, WBC) and variables used for response assessment (lesion count, lesion volume, galactomannan).
Finally, we screened predictors of survival using decision-tree analysis with Chi-Squared Automatic Interaction Detector and receiver operating characteristics for simple cut-offs that would be applicable at bedside (statistics not shown). We identified the trend between day 7 and day 14 as the most promising single indicator in our dataset. Patients with any increase in lesion volume between day 7 and day 14 had a 50% risk of death and a 52% risk of treatment failure (Fig. 2). This method detected 83% of deaths and 69% of treatment failures.
Discussion
Using comprehensive manual measurement on serial CT images of the lung in IPA patients, our study allowed a detailed quantitative analysis of pulmonary lesions associated with IPA. We used the temporal granularity of our dataset for a detailed analysis of previously described and new indicators of treatment success.
To our knowledge, our study is the first to demonstrate a strong association between radiographic response at day 14 and treatment outcome. From this point in time onwards, lesion volume significantly diverged between patients surviving and not surviving, without reuniting during the follow-up period. This observation may allow early identification of patients failing treatment, which could be used as breakpoint in future clinical trials and help physicians to select patients who could potentially benefit from earlier salvage treatment. As a simple clinical rule, any positive trend in CT lesion volume between day 7 and day 14 marked a high risk of adverse outcomes with rates for death and treatment failure of 50%. Thus, after detection of IPA, a follow-up CT scan after 1 and 2 weeks appears to be attractive for outcome estimation.
Our study confirms the previous observation of an initial increase in lesion volume between day 0 and day 7 [13–15]. As observed by Caillot et al., this increase was not generally connected to adverse outcomes and although a larger lesion volume was associated with overall mortality, there was no clear cut-off to predict survival between day 0 and day 7 [13]. Lesion count and lesion volume at baseline were also not predictive of treatment results. Although galactomannan was numerically associated with beneficial outcomes, as described before [10–12], the association was less pronounced than with radiographic imaging and did not explain the residual variance. A low baseline galactomannan was indicative of survival; however, the clinical trial did allow inclusion of patients with possible IPA as per EORTC/MSG criteria [19]. Patients with high baseline galactomannan would qualify as probable IPA, which has already been associated with worse outcome in subgroup analyses of large clinical trials [20, 21]. Interestingly, the lesion count remained stable in most patients, irrespective of their treatment response.
Early assessment of treatment failure is essential to optimize treatment strategies. Outside of clinical trials, most patients receive primary treatment with a triazole class antifungal [22, 23]. Recent reports have raised concern of increasing multi-triazole resistance in clinical Aspergillus spp. isolates [24, 25]. If treatment fails, it must also be considered that diagnosis relies on surrogate markers, i.e. radiological and serological findings in the presence of acknowledged host factors, in >90% of all cases [19]. Such tests come with considerable risk of being falsely positive, and high rates of alternate diagnoses have been reported in patients failing antifungal treatment [26]. Early detection of potentially fatal treatment failure will allow adapting the treatment strategy while ruling out important differential diagnoses, e.g. mucormycosis or non-infectious lesions.
We performed our analysis with the expectation of detecting independent beneficial effects of neutrophil recovery early during the treatment [27, 28]. This was not the case. Even after eliminating all covariates with possible interactions from multivariate analysis, a Cox proportional hazards model showed no significant association between outcome and ANC and/or WBC as time-dependent covariates.
Our study is limited by not enforcing the intended follow-up schedule with the result of missing data and need for imputation. We have focused on overall survival over treatment failure, as rating of treatment failure intrinsically interacts with the observation of the pulmonary lesions over time. Mortality attributable to IPA was deliberately not assessed during the trial as attribution of causes of death in this severely sick patient group is virtually impossible. All patients not surviving were still on antifungal treatment at the time of death. Another limitation is the relatively small number of patients, leading to considerable variance. This should, therefore, be considered as an exploratory study that needs confirmation using a larger dataset. Of note, only a part of the overall variance was explained by the observed covariates, and future studies should try to elucidate further determinants of treatment success and survival. Finally, all patients in this study received caspofungin at four different dose groups. Treatment with other antifungal drug classes may induce different response kinetics.
As a conclusion, we hypothesize that CT evaluation by volumetry of pulmonary lesions at baseline, day 7, and day 14 offers good prediction of treatment success and, more importantly, survival. In our trial, the predictive capability of this measurement exceeded those of serial galactomannan determination or lesion count on chest CT. As a bedside rule, any progression between day 7 and day 14 constitutes a high-risk scenario of a fatal outcome, possibly defining a patient group in need of intervention, e.g. further diagnostics or change of treatment strategy. On the other hand, for patients showing response between day 7 and day 14, we did not find additional value for further CT follow-ups after day 14. Further studies including antifungal standard regimens are warranted before definite conclusions can be drawn from our observation.
References
Patterson TF, Kirkpatrick WR, White M et al (2000) Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. I3 Aspergillus Study Group. Medicine (Baltimore) 79:250–260
Gerson SL, Talbot GH, Hurwitz S, Strom BL, Lusk EJ, Cassileth PA (1984) Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med 100:345–351
Kontoyiannis DP, Marr KA, Park BJ et al (2010) Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 50:1091–1100
Heussel CP, Kauczor HU, Heussel G, Fischer B, Mildenberger P, Thelen M (1997) Early detection of pneumonia in febrile neutropenic patients: use of thin-section CT. AJR Am J Roentgenol 169:1347–1353
Heussel CP, Kauczor HU, Heussel GE et al (1999) Pneumonia in febrile neutropenic patients and in bone marrow and blood stem-cell transplant recipients: use of high-resolution computed tomography. J Clin Oncol 17:796–805
Herbrecht R, Denning DW, Patterson TF et al (2002) Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 347:408–415
Cornely OA, Maertens J, Bresnik M et al (2007) Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial). Clin Infect Dis 44:1289–1297
Ruping MJ, Vehreschild JJ, Cornely OA (2008) Patients at high risk of invasive fungal infections: when and how to treat. Drugs 68:1941–1962
Ruping MJ, Vehreschild JJ, Groll A et al (2011) Current issues in the clinical management of invasive aspergillosis--the AGIHO, DMykG, OGMM and PEG web-based survey and expert consensus conference 2009. Mycoses 54:e557–e568
Koo S, Bryar JM, Baden LR, Marty FM (2010) Prognostic features of galactomannan antigenemia in galactomannan-positive invasive aspergillosis. J Clin Microbiol 48:1255–1260
Chai LYA, Kullberg BJ, Johnson EM et al (2012) Early serum galactomannan trend as a predictor of outcome of invasive aspergillosis. J Clin Microbiol 50:2330–2336
Woods G, Miceli MH, Grazziutti ML, Zhao WZ, Barlogie B, Anaissie E (2007) Serum aspergillus galactomannan antigen values strongly correlate with outcome of invasive aspergillosis - a study of 56 patients with hematologic cancer. Cancer 110:830–834
Caillot D, Latrabe V, Thiebaut A et al (2010) Computer tomography in pulmonary invasive aspergillosis in hematological patients with neutropenia: an useful tool for diagnosis and assessment of outcome in clinical trials. Eur J Radiol 74:E173–E176
Lim C, Seo JB, Park SY et al (2012) Analysis of initial and follow-up CT findings in patients with invasive pulmonary aspergillosis after solid organ transplantation. Clin Radiol 67:1179–1186
Brodoefel H, Vogel M, Hebart H et al (2006) Long-term CT follow-up in 40 non-HIV immunocompromised patients with invasive pulmonary aspergillosis: kinetics of CT morphology and correlation with clinical findings and outcome. Am J Roentgenol 187:404–413
Cornely O, Vehreschild JJ, Ruping M et al (2010) A phase II dose escalation study of caspofungin for invasive aspergillosis. Bone Marrow Transplant 45:S218–S219
Cornely OA, Vehreschild JJ, Vehreschild MJ et al (2011) Phase II dose escalation study of caspofungin for invasive Aspergillosis. Antimicrob Agents Chemother 55:5798–5803
Wurthwein G, Cornely OA, Trame MN et al (2013) Population pharmacokinetics of escalating doses of caspofungin in a phase II study of patients with invasive aspergillosis. Antimicrob Agents Chemother 57:1664–1671
De Pauw B, Walsh TJ, Donnelly JP et al (2008) Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46:1813–1821
Greene RE, Schlamm HT, Oestmann JW et al (2007) Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis 44:373–379
Cornely OA, Maertens J, Bresnik M et al (2011) Efficacy outcomes in a randomised trial of liposomal amphotericin B based on revised EORTC/MSG 2008 definitions of invasive mould disease. Mycoses 54:e449–e455
Castagna L, Bramanti S, Sarina B, Todisco E, Ibatici A, Santoro A (2012) ECIL 3-2009 update guidelines for antifungal management. Bone Marrow Transplant 47:866
Maertens J, Marchetti O, Herbrecht R et al (2011) European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3–2009 update. Bone Marrow Transplant 46:709–718
Fischer J, van Koningsbruggen-Rietschel S, Rietschel E et al (2014) Prevalence and molecular characterization of azole resistance in Aspergillus spp. isolates from German cystic fibrosis patients. J Antimicrob Chemother 69:1533–1536
Steinmann J, Hamprecht A, Vehreschild MJ et al (2015) Emergence of azole-resistant invasive aspergillosis in HSCT recipients in Germany. J Antimicrob Chemother 70:1522–1526
Lass-Florl C, Resch G, Nachbaur D et al (2007) The value of computed tomography-guided percutaneous lung biopsy for diagnosis of invasive fungal infection in immunocompromised patients. Clin Infect Dis 45:e101–e104
Subira M, Martino R, Franquet T et al (2002) Invasive pulmonary aspergillosis in patients with hematologic malignancies: survival and prognostic factors. Haematologica 87:528–534
Pagano L, Caira M, Candoni A et al (2010) Invasive aspergillosis in patients with acute myeloid leukemia: a SEIFEM-2008 registry study. Haematol Hematol J 95:644–650
Acknowledgements
The scientific guarantor of this publication is Jörg J. Vehreschild (joerg-janne.vehreschild@uk-koeln.de)
The authors of this manuscript declare relationships with the following companies:
JJV is supported by the German Federal Ministry of Research and Education (BMBF grant 01KI0771) and the German Centre for Infection Research, has received research grants from Astellas, Gilead Sciences, Infectopharm, Pfizer, and Essex/Schering-Plough; and served on the speakers’ bureau of Astellas, Merck Sharp Dohme/Merck, Gilead Sciences, Pfizer, and Essex/Schering-Plough.
CPH has received research funding from Siemens, Pfizer, MeVis and Boehringer Ingelheim, is a consultant to Schering-Plough, Pfizer, Basilea, Boehringer Ingelheim, Novartis, Roche, Astellas, Gilead, MSD, Lilly, Intermune and Fresenius and has received lecture honoraria from Gilead, Essex, Schering-Plough, AstraZeneca, Lilly, Roche, MSD, Pfizer, Bracco, MEDA Pharma, Intermune, Chiesi, Siemens, Covidien, Pierre Fabre, Boehringer Ingelheim, Grifols and Novartis.
AHG has served on the speaker’s bureau and as a consultant to Astellas Pharma, Cephalon, Gilead Sciences, Merck & Co., Pfizer, Schering-Plough, and Vicuron Pharmaceuticals. He has received research grants from Gilead Sciences, Merck & Co, and Pfizer.
MJGTV has served at the speakers’ bureau of Pfizer, Merck, Gilead Sciences, and Astellas Pharma, received research funding from 3M, Astellas Pharma and Gilead Sciences and is a consultant to Berlin Chemie.
GS has received research funding from MSD Sharp & Dohme, Pfizer, Gilead and Astellas and is a consultant to MSD Sharp & Dohme.
GW has no conflicts of interest to declare.
MB has no conflicts of interest to declare.
OAC is supported by the German Federal Ministry of Research and Education (BMBF grant 01KN1106), has received research grants from 3M, Actelion, Astellas, Basilea, Bayer, Celgene, Cubist, F2G, Genzyme, Gilead, GSK, Merck/MSD, Miltenyi, Optimer, Pfizer, Quintiles, and Viropharma, is a consultant to 3M, Astellas, Basilea, Cubist, F2G, Gilead, GSK, Merck/MSD, Optimer, Pfizer, Sanofi Pasteur and Summit/Vifor, and received lecture honoraria from Astellas, Gilead, Merck/MSD, and Pfizer..
This study has received funding by Merck/MSD.
One of the authors (JJV) has significant statistical expertise.
Institutional Review Board approval was obtained.
Written informed consent was obtained from all subjects (patients) in this study.
The results of the clinical trial were published in Antimicrobial Agents and Chemotherapy: "Phase II Dose Escalation Study of Caspofungin for Invasive Aspergillosis", doi:10.1128/AAC.05134-11 (2011)
Methodology:
• prospective
• randomised controlled trial
• multicenter study
• post-hoc analysis
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vehreschild, J.J., Heussel, C.P., Groll, A.H. et al. Serial assessment of pulmonary lesion volume by computed tomography allows survival prediction in invasive pulmonary aspergillosis. Eur Radiol 27, 3275–3282 (2017). https://doi.org/10.1007/s00330-016-4717-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4717-4