Abstract
Objectives
To estimate the impact of endoaortic stents/mechanical heart valves on the output of an automatic exposure control (AEC) system and CT radiation dose.
Methods
In this phantom study, seven stents and two valves were scanned with varying tube voltage (80/100/120 kVp), AEC activation (enabled/disabled) and prosthesis (present/absent), for a total of 540 scans. For each prosthesis, the dose-length product (DLP) was compared between scans with the AEC enabled and disabled. Percentage confidence levels for differences due to the prosthesis were calculated.
Results
Differences between results with the AEC enabled and disabled were not statistically significant (p ≥ 0.059). In the comparison with and without the prosthesis, DLP was unchanged at 80 kVp and 100 kVp, while a slight increase was observed at 120 kVp. The radiation dose varied from 1.8 mGy to 2.4 mGy without the prosthesis and from 1.8 mGy to 2.5 mGy with the prosthesis (confidence level 37–100%).
Conclusions
The effect of the prosthesis on the AEC system was negligible and not clinically relevant. Therefore, disabling the AEC system when scanning these patients is not likely to provide a benefit.
Key points
• CT-AEC system is not impaired in patients with endoaortic prostheses/heart valves.
• Negligible differences may be observed only at 120 kVp.
• Disabling the AEC system in these patients is not recommended.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Various methods have been used to minimize radiation dose to patients, in keeping with the ALARA principle (As Low As Reasonably Achievable) [1–3], including optimization techniques used in computed tomography (CT), such as the automatic exposure control (AEC) system. These systems are typically based on automatic adaptation of the x-ray tube current according to the electron density of the scanned region, as represented in localizer radiographs [4, 5]. In practice, tube current varies to accommodate differences in x-ray attenuation due to patient anatomy, shape, and size.
A substantial increase in tube load could thus be expected due to the presence of a metal prosthesis in a patient’s body. Indeed, Rizzo et al. [6] demonstrated a 34% increase in the mean tube load for abdominal-pelvic CT in patients with an orthopedic prosthesis. Therefore, as a preventive measure to reduce the risk of radiation exposure, radiologists and technicians have reasons to turn off the AEC system when scanning these patients.
Endoaortic stent grafts such those used for endovascular aortic aneurism repair (EVAR) are characterized by a certain metal component that may cause the AEC system to increase the tube load. This metal component also varies among stent types (thoracic, abdominal, iliac, etc.) and manufacturers. Although the metal content of endoaortic stents is lower than that of orthopedic prostheses, we hypothesized that the presence of these stents would be associated with a non-negligible increase in radiation dose.
Radioprotection is a peculiar dimension of evidence-based radiology [7], and is even more relevant for patients treated with EVAR. In fact, these patients typically undergo CT not only before treatment (to optimize the selection of the proper stent size and characteristics), but also many follow-up CT scans, with a tight time schedule [6]. Considering the lifelong surveillance required, patients treated with EVAR may absorb a substantial cumulative radiation dose.
Mechanical heart valves implanted in patients with valve disease are another type of prosthesis, with an overall metal content comparable to that of endoaortic stents. Thus, the above-mentioned radioprotection issues apply to them as well.
The aim of this phantom study was to estimate the impact of endoaortic stents or heart valves on the output of an AEC system and radiation dose using a multislice CT scanner.
Methods
Preparation of the phantoms
Ethics committee approval was not needed for this phantom study. We used two different phantoms in combination with nine different prostheses, which included endoaortic stent grafts (n = 7) and mechanical heart valves (n = 2), whose characteristics are detailed below.
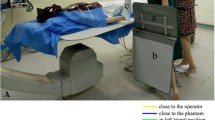
The first phantom consisted of a 20-cm-long plastic container filled with water, with the prosthesis completely immersed (Fig. 1a). The second phantom was set up using a plastic cylinder with an internal cavity into which one endoaortic stent graft for iliac bifurcation was placed (Fig. 2a). This phantom measured 30 cm in length, and it was used only for radiation dose measurements, performed using thermoluminescent dosimeters (TLDs), as further explained below.
Cylindrical phantom with the stent positioned in the internal cavity (a) and experimental setup for radiation dose measurements (b): cylindrical phantom and 10 thermoluminescent dosimeters positioned close to the sections with higher metal content. Each dosimeter position is labeled with numbers from 1 to 10. The dashed line represents the profile of the prosthesis
Prosthesis characteristics
The seven endoaortic stents were Zenith Flex endovascular grafts (Cook Medical, Bloomington, IN, USA), and each differed in terms of length, diameter, and metal composition. The graft modules were constructed of full-thickness woven polyester fabric sewn to self-expanding stainless steel stents (specific technical details are under copyright). These stents were intended for use in thoracic and abdominal EVAR procedures. They are all illustrated in Fig. 3.
The seven stents used in this study were Zenith Flex endovascular grafts (Cook Medical, Bloomington, IN, USA), constructed of full-thickness woven polyester fabric sewn to self-expanding stainless steel stents. The two mechanical cardiac valves were Bicarbon Overline aortic 24 LOV and Carbomedics Reduced aortic R5-025 (Sorin, Saluggia, Italy)
Two mechanical heart valves were also used for comparison (Fig. 3): the Bicarbon Overline aortic 24 LOV and the Carbomedics Reduced aortic R5-025 (Sorin, Saluggia, Italy). These valves consist of two semicircular leaflets made of pyrolytic carbon that can rotate about struts attached to the valve housing (titanium alloy Ti6A14V). This design is mounted within a suture ring made of polyester.
Computed tomography measurements
Measurements were performed using a CT scanner (SOMATOM Sensation 64, Siemens Medical Solutions, Erlangen, Germany) equipped with a CARE Dose 4D AEC system.
The scan configurations performed for each of the nine prostheses are presented in Table 1, including all possible combinations obtainable by varying the tube voltage (80 kVp, 100 kVp, or 120 kVp), activation of CARE Dose 4D (enabled or disabled), and the presence of the prosthesis (present or absent), for a total of 12 (configurations) × 9 (prostheses) × 5 (scans per configuration) = 540 scans. To compare measurements obtained with CARE Dose 4D enabled and disabled, the mean tube load (automatically calculated by the scanner when using CARE Dose 4D) was entered as a fixed value when CARE Dose 4D was disabled.
To simulate clinical practice, prostheses intended for thoracic procedures (nos. 1, 8, and 9 in Fig. 3) were studied using the imaging protocol typically employed for this segment, and a similar process was conducted for prostheses intended for abdominal procedures (nos. 2 through 7 in Fig. 3).
CT scan parameters were as follows: collimation 64 × 0.6 mm, slice thickness 5 mm, slice increment 5 mm, for a pitch of 1.0; the scan length was adapted to the phantom. To increase precision, five scans were performed for each configuration in Table 1, and the mean was calculated.
Radiation dose measurements
Radiation dose local measurements were carried out using TLDs. Upon heating, the TLD re-emits the energy previously absorbed from ionizing radiation in the form of light. The TLDs were chips of 3.2 × 3.2 × 0.9 mm3 made of LiF:Mg,Ti (TLD-100). They were supplied and read by an accredited calibration laboratory (Nuclear Engineering Department, Politecnico di Milano, Milan, Italy).
Measurements were carried out using only one stent graft (no. 7 in Fig. 3). Twenty TLDs were positioned around the cylindrical phantom, with two TLDs for each of the ten positions depicted in Fig. 2b. These positions were close to the stent components with higher metal content.
Five scans were acquired first without the stent, and subsequent scans were obtained with the stent placed in the phantom. The x-ray tube voltage was 120 kVp and CARE Dose 4D was active.
Data analysis
Data from the five scans for each configuration were averaged.
With the CARE Dose 4D enabled, the x-ray tube load of each single slice was recorded for only one stent at different voltages, in order to obtain a profile along the phantom length and to correlate it with the various stent components.
The impact of a prosthesis on the output of CARE Dose 4D was determined by comparing the dose-length product (DLP) obtained with CARE Dose 4D enabled and disabled (Wilcoxon signed-rank test for paired data). To evaluate the impact of the prosthesis on radiation dose with the CARE Dose 4D enabled, DLP and TLD measurements obtained with and without the prosthesis were compared. The difference was tested using the confidence level, which represents the probability that the observed difference was by chance.
Results
Table 2 shows the influence of the presence of a prosthesis on the output of the CARE Dose 4D. As expected, the DLP increased with increasing tube voltage, from 19 mGy*cm to 73 mGy*cm for the abdominal protocol and from 12 mGy*cm to 48 mGy*cm for the thoracic protocol at 80 kVp and 120 kVp, respectively. However, the difference in the DLP obtained with CARE Dose 4D enabled vs. disabled was not statistically significant (p ≥ 0.059).
Table 3 presents the DLP obtained with CARE Dose 4D enabled for scans with and without the prosthesis and for each of the three different tube voltages. As the table shows, in all but one case, there was no change in DLP between scans without the prosthesis (“No” in Table 3) and those with the prosthesis at 80 kVp or 100 kVp. A slight DLP increase due to the presence of the prosthesis was observed only at 120 kVp, with increases in percentage ranging from 0.0 to 2.1% compared to scans without the prosthesis.
Examples of the tube load profile are shown in Figs. 4 and 5. Notably, the absolute difference in terms of mAs was within 1 mAs for the stent shown in Fig. 4 and within 2.5 mAs for the heart valve shown in Fig. 5.
Graphical representation of tube load modulation with respect to the slice location at 120 kVp. The black line refers to the reference scan without the prosthesis, and the gray line to the scan with endoaortic stent graft number 7 in Fig. 3
Table 4 shows the results of radiation dose measurements obtained using TLDs with and without a prosthesis in ten different positions of the phantom. The confidence level is shown as a measurement of the likelihood that the two values are statistically equivalent. The radiation dose ranged from 1.8 mGy to 2.4 mGy without the prosthesis, and from 1.8 mGy to 2.5 mGy with the prosthesis. Confidence levels ranged from 37% to 100%.
Discussion
The CARE Dose 4D system evaluated in this study employs the automatic tube current modulation technique along the z-axis using data from localizer radiographs to estimate the density, size, and shape of the anatomic region that will be scanned. We hypothesized that the presence of a prosthesis would have a non-negligible impact on the output of the CARE Dose 4D. In fact, previous research has shown that high metal content, such as that in orthopedic prostheses, causes a substantial increase in tube load, with a consequent increase in the radiation dose to the patient [6].
Although endoaortic stents and heart valves such as those used in this phantom study have much lower metal content than orthopedic prostheses, we posited that they were still capable of producing an increase in radiation. This hypothesis was assessed by comparing the DLP distributions obtained with CARE Dose 4D enabled vs. disabled by scanning a phantom containing one of the prostheses analysed in the study. As shown in Table 2, the difference between the two data sets was not statistically significant, with the DLP obtained with CARE Dose 4D enabled equal to that obtained when it was disabled, the only exception being a negligible difference for the thoracic protocol at 80 kVp, where a borderline p value was observed. Thus, our prostheses had such low metal content that they did not affect the output of the CARE Dose 4D. These data suggest that enabling CARE Dose 4D can be recommended for patients with these prostheses implanted. This conclusion differs from that reached by Rizzo et al., who studied patients with orthopedic implants with much higher metal content. Moreover, the Rizzo et al. study was performed with another equipment. CARE Dose 4D algorithmically minimizes the impact of orthopedic metallic implants. We therefore expect that also the influence of larger orthopedic implants on CARE Dose4D is much less pronounced.
Once we had demonstrated a non-detrimental impact of our prostheses on the output of CARE Dose 4D, we estimated the impact of the prosthesis itself on the DLP. Scanning in the presence of prostheses resulted in negligible to no differences in DLP compared to that measured without prostheses. As shown in Table 3, the increase (calculated only at 120 kVp) was within 1.8% for the abdominal protocol (including endoaortic stents) and within 2.1% for the thoracic protocol (including the two heart valves). Notably, the latter results were obtained with CARE Dose 4D enabled.
Although we did not have an estimate of the prosthesis metal content, the minimum DLP increase was registered for the smallest stent graft (no. 6), while the highest increase was associated with the longest stent graft (no. 1) and with the two mechanical heart valves (nos. 8 and 9), which allowed us to speculate on a proportional relationship between these two variables.
A negligible increase in radiation dose due to the prosthesis is in line with the results obtained from the analysis of the tube load profile. In fact, inspection of these profiles revealed a substantial overlap, with absolute differences within 1 mAs for the stent shown in Fig. 4 and within 2.5 mAs for the heart valve shown in Fig. 5. The slice location is matched to the shape of the prosthesis in order to determine the connection between the tube load and the metal content of the prosthesis.
A limitation of this study was that only one prosthesis manufacturer was considered and only one CT unit. However, we expect little variation among prostheses of the same type from different manufacturers.
In conclusion, the presence of an aortic stent or heart valve had a negligible effect on the output of the AEC system of the SOMATOM Sensation 64 CT scanner, with subtle differences that were not clinically relevant. Thus, our in vitro data suggest that disabling the AEC when scanning these patients provides no benefit. Increases in radiation dose due to such prostheses are within 2%, and are observable only at 120 kVp.
References
Council Directive 2013/59/EURATOM (2014) Official Journal of the European Union. https://ec.europa.eu/energy/sites/ener/files/documents/CELEX-32013L0059-EN-TXT.pdf. Accessed June 7, 2016
No authors listed (2004) Proceedings of the second ALARA conference. February 28, 2004. Houston, Texas, USA. Pediatr Radiol 34(Suppl 3):S162–S246
Prasad KN, Cole WC, Haase GM (2004) Radiation protection in humans: extending the concept of as low as reasonably achievable (ALARA) from dose to biological damage. Br J Radiol 77:97–99
Lee CH, Goo JM, Ye HJ et al (2008) Radiation dose modulation techniques in the multidetector CT era: from basics to practice. Radiographics 28:1451–1459
McCollough CH, Bruesewitz MR, Kofler JM (2006) CT dose reduction and dose management tools: overview of available options. Radiographics 26:503–512
Rizzo SMR, Kalra MK, Maher MM, Blake MA, Toth TL, Saini S (2005) Do metallic endoprostheses increase radiation dose associated with automatic tube-current modulation in abdominal-pelvic MDCT? A phantom and patient study. Am J Roentgenol 184:491–496
Sardanelli F, Hunink MG, Gilbert FJ et al (2010) Evidence-based radiology: why and how? Eur Radiol 20:1–15
Acknowledgments
The scientific guarantor of this publication is Prof. Francesco Sardanelli. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. One of the authors has significant statistical expertise. Institutional Review Board approval was not required because this is a phantom study. Methodology: Prospective, experimental, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Di Leo, G., Spadavecchia, C., Zanardo, M. et al. Should the automatic exposure control system of CT be disabled when scanning patients with endoaortic stents or mechanical heart valves? A phantom study. Eur Radiol 27, 2989–2994 (2017). https://doi.org/10.1007/s00330-016-4676-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4676-9